Abstract
Purpose
The aim of this study is to assess the added diagnostic value of Doppler ultrasound of the liver (DUL) performed within 3 days of contrast-enhanced CT (CECT) for the diagnosis of portal vein (PV) or hepatic vein (HV) thrombosis.
Methods
Adult patients were included if they underwent DUL within three days after a CECT of the abdomen in the emergency or inpatient setting. Retrospective review of clinical data and imaging reports was performed. In patients with discrepant or positive findings on CECT and/or DUL with respect to PV or HV thrombosis, image review was performed by three fellowship-trained abdominal radiologists in consensus.
Results
The final cohort consisted of 468 patients. Of these, 26 (5.6%) patients had equivocal findings for thrombosis on CECT, and DUL could make a confident diagnosis of positive or negative in 18 (69%) patients. Additionally, there were 2 (0.4%) patients with PV or HV thrombosis on DUL following a limited CECT, and 2 (0.4%) patients who developed interval PV thrombosis between CECT and DUL.
Conclusion
DUL after CECT added diagnostic value for PV and/or HV thrombosis in less than 5% of patients. The patency of PV and HV is often not explicitly mentioned in CECT reports at our institution, which may lead to uncertainty for the referring provider as to whether the PV and HV were adequately evaluated. Few CECT have false positive or missed or underreported findings, and a careful review of the original CECT should be performed if DUL is requested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Burgeoning healthcare costs are a major economic burden on the United States, with overall healthcare expenditures comprising nearly 18% of the gross domestic product [1]. In part, this can be explained by the increased utilization of advanced medical imaging, which has continually risen over the past couple decades [2]. Recent cuts in reimbursement have limited the impact of medical imaging on overall healthcare costs [3]. However, there is further opportunity to diminish medical imaging costs by reducing unnecessary and/or duplicative evaluations.
Doppler ultrasound of the liver (DUL) is an established imaging technique for evaluating the patency of the portal and hepatic veins [4]. Diagnosis of portal vein (PV) and hepatic vein (HV) thrombosis is of key importance, as these entities can progress to portal hypertension and acute hepatic insufficiency or cirrhosis, respectively, if untreated [5]. Intravenous (IV) contrast-enhanced computed tomography (CECT) of the abdomen is a commonly performed to identify acute intra-abdominal pathology in the emergency department (ED) and inpatient setting [6]. On CECT, the phase of contrast enhancement determines the adequacy of portal and hepatic vein evaluation. Our institutional experience suggests that routine CECT is sufficient in the most patients to diagnose or exclude PV or HV thrombosis. Despite this, DUL is frequently requested in the acute care setting and performed shortly after CECT, and the added diagnostic utility of DUL after CECT is unknown.
The aim of this study was to systematically assess the added diagnostic utility of DUL performed within 3 days of a CECT of the abdomen for the diagnosis of PV or HV thrombosis, among patients in the ED and inpatient setting.
Materials and methods
Study design
This retrospective study was performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA). The institutional review board (IRB) approved the protocol and waived the requirement for informed consent.
Study cohort
Patients were identified by query of our radiology database, which included all studies performed between June 1, 2010 through the date of our search on January 31, 2019. Sequential searching was performed by Current Procedural Terminology (CPT) code, which identified all patients who underwent CECT and a subsequent DUL within three days. Additional inclusion criteria included adult patients (age 18 or older) and initial CECT performed in either the emergency or inpatient setting. If a patient had more than one pair of CECT and DUL meeting criteria for inclusion, only the most recent pair of studies was selected for inclusion. Patients were excluded if they underwent abdominal surgery between the CECT and DUL. Our search criteria generated 472 patients, with our final cohort consisting of 468 patients (Fig. 1).
Flow chart for patient identification and image review. Patients were identified by query of the radiology database if they underwent Doppler ultrasound of the liver (DUL) within three days after contrast-enhanced CT (CECT). Clinical data and imaging reports were reviewed, which resulted in exclusion of four patients and a final study cohort of 468 patients. Tables show initial categorization with respect to portal vein (PV) and hepatic vein (HV) thrombosis. Imaging review and reclassification was performed in patients with discordant or positive findings on CECT and DUL (bolded)
Imaging protocols
CECT was performed according to standard protocols used at our institution. Most studies were performed using a single acquisition during the portal venous phase (i.e., 60–100 s scan delay) using an intravenous infusion rate of 1–3 mL/s. Additional protocols included those performed in the arterial phase according to an aortic dissection protocol or a multi-phase protocol which included non-contrast, arterial, and portal venous phases. DUL was performed by a trained sonographer according to a standard protocol. Additional scanning was performed by and at the discretion of the interpreting radiologist.
Patient characteristics and imaging findings
Retrospective review of the clinical data was performed by one of the study’s authors in the third year of radiology residency training (FWP) to obtain patient demographics, serum laboratory values, history of prior liver transplantation, indication for CECT and DUL, and intensive care unit (ICU) status at the time of the CECT and DUL. Imaging reports were reviewed to determine the presence of PV or HV thrombosis on CECT and DUL. Additionally, for DUL, the study was scored as limited for PV and/or HV thrombosis if the initial report described the limitation. Any study reported as equivocal (i.e., possible or probable) was initially scored as positive, in an attempt to be inclusive in our subsequent imaging review, which was performed in all patients with a positive finding on CECT or DUL. Data were collected on whether PV and/or HV patency was specifically mentioned in the imaging report. If no mention was made, these cases were considered negative for the purpose of this study. Additional data collected from the CECT imaging report included whether the report was initially generated by a resident physician or clinical fellow, and whether the attending radiologist was a member of the abdominal imaging section.
Imaging review
In patients with discrepant or positive findings on CECT and DUL with respect to PV or HV thrombosis, image review was performed by three fellowship-trained abdominal radiologists in consensus (DRL, ASS, MY), with 0, 5, and 7 years of post-fellowship experience, respectively. Image review was performed in 82 patients with positive or discrepant PV finding and 21 patients with positive or discrepant HV finding (Fig. 1). After review of the CECT and DUL images, the consensus interpretation of the reviewers was compared to the original imaging reports. On CECT, cases were reclassified into one of the following groups: (a) negative; (b) positive; (c) missed or unreported finding on CECT; (d) false positive; (e) equivocal finding (i.e., possible or probable thrombosis); and (f) limited evaluation (i.e., poor opacification of the PV or HV). On DUL, cases were reclassified as (a) negative; (b) positive; (c) equivocal (i.e., slow flow versus thrombosis); and c) limited. Specifically, an equivocal DUL was defined as a lack of signal on color flow Doppler without a correlate on grayscale images.
Statistical analysis
Continuous variables were summarized using means ± standard deviation (SD) or median ± interquartile range (IQR) for normally distributed and not normally distributed data, respectively. Categorical variables were summarized by frequency (percentage). Differences in categorical variables were assessed using the Pearson chi-squared test. P < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS Statistics Version 25 (International Business Machines Corp, Armonk, New York).
Results
Patient characteristics
The final cohort (N = 468) had a slight majority of male patients (N = 259; 55%) and an average age of 54.0 ± 14.5 years (Table 1). Of these, 65 (14%) had undergone prior liver transplantation. A significant minority were admitted to the ICU at the time of CECT (N = 80; 17%) or admitted to the ICU between CECT and DUL (N = 69; 15%). A large majority of CECT studies were performed in the portal venous phase (N = 457, 98%), with a minority performed in the arterial phase (N = 9, 2%) or according to multi-phase protocol (N = 2, 0.4%). The most common indications for CECT were abdominal pain (N = 204; 44%) and infection or inflammation (N = 146; 31%). On the other hand, the most common indications for DUL were vascular abnormality (N = 178; 38%) or abnormal laboratory values (N = 147; 31%).
Mention of PV and HV patency on CECT
PV patency was specifically mentioned in a slight majority of reports (N = 236; 53%), where HV patency was infrequently mentioned (N = 93; 20%) (Table 2). Resident or fellow generated reports mentioned the PV and HV at a similar frequency to those generated by an attending radiologist (p = 0.14 and 0.69 for PV and HV, respectively). Similarly, there was no difference in frequency among those interpreted by an abdominal radiologist compared to those interpreted by a non-abdominal radiologist (p = 0.83 and 0.28 for PV and HV, respectively).
Assessment of PV and HV thrombosis on CECT and DUL
Table 3 shows the final categorization for PV thrombosis by findings on CECT and DUL, whereas Table 4 shows the final categorization for HV thrombosis. The overall incidence of PV thrombosis was 40 (8.5%) and 38 (8.1%) by CECT and DUL, respectively. The incidence of HV thrombosis was substantially lower, at 4 (0.9%) and 4 (0.9%) by CECT and DUL, respectively. Among patients that were negative for thrombosis after adequate assessment on CECT, a new diagnosis of PV thrombosis was made on DUL in 2 (0.4%), indicative of interval thrombosis. No new diagnoses of HV thrombosis were made on DUL after a negative CECT.
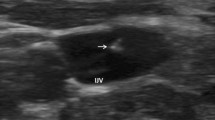
There were 16 (3.4%) and 10 (2.1%) patients who had equivocal findings on CECT for PV and HV thrombosis, respectively. Among those with equivocal PV thrombosis, 8 (50%) had a negative DUL, 1 (6%) had a positive DUL, and 7 (44%) had a limited DUL. Most patients with an equivocal HV thrombosis had a negative DUL (N = 9; 90%), whereas 1 (10%) patient had a limited DUL. Most equivocal findings on CECT represented flow-related artifacts (Fig. 2).
Two patients with equivocal findings of thrombosis on CT and a negative Doppler evaluation. In a 42-year-old man undergoing evaluation for nausea, vomiting, and weight loss, axial contrast-enhanced CT (a) shows a filling defect in the main portal vein which confirmed to be flow-related artifact on subsequent liver Doppler (b, c, arrows). Similarly, axial contrast-enhanced CT image (d) in a 32-year-old woman with endocarditis shows a filling defect in the right hepatic vein (arrowhead), which also had no correlate on subsequent liver Doppler (e, f, arrowheads), most consistent with flow artifact. Both cases were classified as equivocal on CT and negative on Doppler
DUL showed 1 (0.2%) and 1 (0.2%) PV and HV thrombosis, respectively, in patients with CECT limited by suboptimal opacification of the PV or HV. Similarly, there were 6 (1.3%) patients with a missed or underreported PV thrombosis which was identified on DUL, 5 (83%) of which were most likely chronic, as evidenced by the presence of cavernous collaterals (N = 1), occlusion by tumor (N = 2), or occlusion of a branch PV in the setting of a transjugular intrahepatic portosystemic shunt (N = 2). Additionally, there were 4 (0.9%) patients with PV thrombosis reported on CECT with a negative DUL, which were later classified as having a false-positive CECT. Finally, there were multiple cases (PV: N = 11, 2.4%; HV: N = 1, 0.2%) in which CECT showed thrombosis but the subsequent DUL was limited (Fig. 3).
A 51-year-old man with Crohn’s disease and primary sclerosing cholangitis status post orthotopic liver transplantation three years earlier, with extensive portomesenteric thrombosis. Axial and coronal contrast-enhanced CT images (a, b, respectively) show a non-occlusive thrombus within the main portal vein (solid arrow) and occlusive thrombosis of the superior mesenteric vein and a proximal jejunal branch (arrowheads). Note the associated mesenteric edema (asterisks). Contemporaneous grayscale and color Doppler ultrasound images (c, d, respectively) show a patent distal portal vein (dashed arrows). Evaluation of the proximal portal vein was limited by the extensive shadowing attributable to overlying bowel (plus signs). This case was classified as CT positive and Doppler limited
Discussion
DUL is commonly performed at our institution in the acute care setting shortly following CECT. In most patients, DUL was duplicative and did not provide additional information regarding the diagnosis of PV or HV thrombosis. A small subset of 26 (5.6%) patients had equivocal findings for PV or HV thrombosis on CECT, and among these DUL was sufficient to make a confident diagnosis of positive or negative in 18 (69%) patients. Additionally, there were 2 (0.4%) patients with PV or HV thrombosis on DUL following a CECT with limited PV or HV opacification, and 2 (0.4%) patients who developed interval PV thrombosis between CECT and DUL. Thus, DUL may have a defined role shortly following CECT in a small proportion of patients (< 5%) with equivocal findings on CECT, limited PV or HV opacification on CECT, or an acute change in clinical status with suspected new thrombosis.
Specific mention of PV and HV patency is often not explicitly described on CECT reports at our institution, which may lead to uncertainty for the referring clinician as to whether the PV and HV were adequately evaluated. Specifically in the setting of PV thrombosis, there may be a perception that DUL is more sensitive than CECT, in part due to longstanding practice patterns and recommendations in older literature [5, 7]. However, most recent American Association for the Study of Liver Diseases (AASLD) guidelines recommended CECT over DUL as the initial test of choice for the diagnosis of acute PV thrombosis [8]. CECT often provides superior evaluation of the mesenteric veins, which may be difficult to visualize with DUL. Additionally, CECT can simultaneously evaluate for alternative or unsuspected diagnoses. In certain circumstances, such as those in which IV contrast cannot be administered or the patient is unstable for transport to the radiology department, DUL may be preferable over CECT.
A prior study showed a high rate (i.e., > 40%) of repeat abdominal imaging examinations a tertiary care hospital, in particular in the ED and inpatient setting [9]. Our study was not designed to assess the overall rate of DUL shortly after CECT, which is likely substantially lower than repeat abdominal imaging in general. Regardless, it would be prudent to implement strategies to reduce duplicative DUL at the time the order is placed, such as clinical decision support tools and/or best practice advisories [10, 11]. In practice, when we are aware of these orders before the DUL is performed, we speak directly with the referring provider after reviewing the CT to determine if the clinical question can be adequately addressed from the CT alone. Additionally, specific mention of PV and HV patency on CECT reports, when adequately assessed, may alleviate uncertainty on the part of the referring clinicians.
A small number of patients (N = 13; 2.8%) had a missed or underreported finding on CECT or a false-positive CECT, most of which were reported as such on subsequent DUL. Diagnostic errors in this context are likely more common when a CECT is more complex (i.e., multiple important findings), prior studies are not available for comparison, and the clinical questions is unclear at the time of the interpretation [12]. Multiple studies have highlighted the added value of ‘double reading’ in specific clinical scenarios [13, 14]. A request for DUL after recent CECT suggests a heighted clinical suspicion for PV or HV thrombosis, and an opportunity to carefully review of the original CECT for additional or false-positive PV or HV findings.
Our data have multiple limitations, most notably in that our imaging review did not include cases that were reported as negative on CECT and negative or limited on DUL. It is possible that our imaging review would have uncovered additional missed or unreported CECT findings among those with a limited DUL. Furthermore, some cases that were reported as negative on CECT may have had underreported equivocal findings and a negative DUL, underestimating the added value of DUL after CECT. Additionally, DUL images are representative, and retrospective review is not a reliable substitute for real-time evaluation. Next, we used PV or HV thrombosis as the endpoint rather than a specific clinical outcome or a change in patient management, such as decision to treat with anticoagulation. Finally, we assessed for PV or HV thrombosis in a binary fashion and did not assess for progression (i.e., non-occlusive to occlusive) or propagation, which may have been the DUL indication in many of the DUL studies after a positive CECT.
Conclusion
DUL after CECT added diagnostic value for PV and HV thrombosis in less than 5% of patients, which included those with equivocal findings on CECT, and few with limited opacification of the PV or HV on CECT or interval PV thrombosis. DUL may have a defined role in the period shortly following CECT as a problem-solving tool after equivocal CECT or the setting of an acute change in clinical status and suspected new thrombosis.
Specific mention of PV and HV patency, when adequately assessed, may reduce uncertainty for referring clinicians and result in fewer requests for DUL after CECT. A small number of CECT have false-positive or missed or underreported findings, and a careful review of the original CECT should be performed if DUL is requested.
References
National Health Expenditure Data | CMS. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/. Accessed 8 Oct 2020
Smith-Bindman R, Kwan ML, Marlow EC, et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000-2016. JAMA 2019;322:843
Lee DW, Duszak R, Hughes DR. Comparative Analysis of Medicare Spending for Medical Imaging: Sustained Dramatic Slowdown Compared With Other Services. American Journal of Roentgenology 2013;201:1277–1282
McNaughton DA, Abu-Yousef MM. Doppler US of the Liver Made Simple. RadioGraphics 2011;31:161–188
Sabol TP, Molina M, Wu GY. Thrombotic Venous Diseases of the Liver. Journal of Clinical and Translational Hepatology 2015;3:189–194
Zhang X, Kim J, Patzer RE, Pitts SR, Chokshi FH, Schrager JD. Advanced diagnostic imaging utilization during emergency department visits in the United States: A predictive modeling study for emergency department triage. PLOS ONE 2019;14:e0214905
Sacerdoti D, Serianni G, Gaiani S, Bolognesi M, Bombonato G, Gatta A. Thrombosis of the portal venous system. J Ultrasound 2007;10:12–21
DeLeve LD, Valla D-C, Garcia-Tsao G. Vascular disorders of the liver. Hepatology 2009;49:1729–1764
Ip IK, Mortele KJ, Prevedello LM, Khorasani R. Repeat Abdominal Imaging Examinations in a Tertiary Care Hospital. The American Journal of Medicine 2012;125:155–161
Adenaw N, Wen J, Pahwa AK, Sheth S, Johnson PT. Decreasing Duplicative Imaging: Inpatient and Emergency Medicine Abdominal Ultrasound Within 72 Hours of Abdominal CT. Journal of the American College of Radiology 2020;17:590–596
Khorasani R, Hentel K, Darer J, et al. Ten Commandments for Effective Clinical Decision Support for Imaging: Enabling Evidence-Based Practice to Improve Quality and Reduce Waste. American Journal of Roentgenology 2014;203:945–951
Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of Diagnostic Error in Imaging. RadioGraphics 2018;38:1845–1865
Geijer H, Geijer M. Added value of double reading in diagnostic radiology,a systematic review. Insights Imaging 2018;9:287–301
Banaste N, Caurier B, Bratan F, Bergerot J-F, Thomson V, Millet I. Whole-Body CT in Patients with Multiple Traumas: Factors Leading to Missed Injury. Radiology 2018;289:374–383
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
DRL: conceptualization, data curation, formal analysis, investigation, writing—original draft. FPIII: conceptualization, investigation, writing—review & editing. ASS: conceptualization, investigation, writing—review & editing. MY: conceptualization, investigation, writing—review & editing, supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Ethical approval
The institutional review board (IRB) approved the protocol.
Informed consent
The IRB waived the requirement for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ludwig, D.R., Petraglia, F.W., Shetty, A.S. et al. Limited added value of Doppler ultrasound of the liver after recent contrast-enhanced computed tomography. Abdom Radiol 46, 2567–2574 (2021). https://doi.org/10.1007/s00261-021-02950-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-02950-y