Abstract
Rectal adenocarcinoma with mucinous components is an uncommon type of rectal cancer with two distinct histologic subtypes: mucinous adenocarcinoma and signet-ring cell carcinoma. Mucin can also be identified as pattern of response after neoadjuvant treatment. On imaging modalities, mucin typically demonstrates high signal intensity on T2-weighted images, low attenuation on computed tomography, and may be negative on 18-fluorodeoxyglucose positron emission tomography. After neoadjuvant CRT, cellular and acellular mucin share similar imaging features, and differentiating them is currently the main challenge faced by radiologists. Radiologists should be aware of pros, cons, and limitations of each imaging modality in the primary staging and restaging to avoid misinterpretation of the radiological findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rectal adenocarcinoma with mucinous components is an uncommon type of rectal cancer with two distinct histologic subtypes: mucinous adenocarcinoma (MAC) and signet-ring cell carcinoma (SRCC). Mucin can also be identified as a pattern of response after neoadjuvant treatment. On imaging, these subtypes of mucin may demonstrate similar imaging features. When compared with classical adenocarcinoma, the subtypes MAC and SRCC are considered poorly differentiated tumors and are associated with young age, advanced tumor stage at presentation, higher rates of metastases, multiple metastatic sites, and worse clinical outcomes [1,2,3]. These differences are likely due to underlying differences in their molecular signature compared with that of classical rectal adenocarcinoma [4]. After neoadjuvant therapy, acellular mucin may indicate complete response to therapy, but cellular mucin does not. However, cellular and acellular mucin share similar imaging features, posing a major challenge to radiologists in clinical practice. Considering that, it is imperative for radiologists to be familiar with the concepts of mucinous rectal cancer and the challenges in its imaging to provide a proper staging. The aim of this study is to provide an overview of mucinous rectal cancer with emphasis on its main concepts and imaging challenges.
General concepts of mucinous rectal cancer
Mucinous adenocarcinoma
MAC is a histological subtype of rectal adenocarcinoma defined by the World Health Organization (WHO) as adenocarcinoma with pools of extracellular mucin in more than 50% of the tumor [1]. Different definitions exist regarding the proportion of mucin in MAC [2], and depending on the criteria used to define it, MAC represents 5%–20% of all colorectal cancers [5]. MAC is associated with a history of inflammatory bowel disease and pelvic radiotherapy [6].
MAC demonstrates specific molecular, genetic, and prognostic features distinct from the classical non-MAC. The risk of microsatellite instability (MSI) is increased in MAC [7]; accordingly, MAC is known to respond to checkpoint inhibitors, particularly in late-stage disease [8]. The presence of MSI is associated with hereditary cancer, such as Lynch syndrome, suggesting that the oncogenic pathway of MAC is different from conventional adenocarcinoma [9]. BRAF mutation is frequent in patients with MAC and is associated with infiltrative growth pattern. Additionally, MAC is associated with increased CpG island methylation phenotype and MUC-2 expression, which may play a role in promoting tumor development and therapy resistance [10]. On the other hand, MAC is associated with a decreased p53 expression and APC mutation [7].
The data regarding the prognosis and outcome of MAC are conflicting. Although some studies have demonstrated worse survival compared with non-MAC [2, 11], others did not show any significant difference [5, 12,13,14]. In terms of treatment, MAC is usually associated with a lower response to chemoradiotherapy (CRT) when compared with non-MAC [11, 15,16,17]. However, neoadjuvant CRT used in conjunction with standard total mesorectal excision for patients with MAC may be beneficial; using this approach, patients with MAC and patients with non-MAC have been shown to have similar survival outcomes [14].
Signet-ring cell carcinoma
SRCC is a subtype of adenocarcinoma with an even poorer outcome than MAC, and is defined by WHO as adenocarcinoma with more than 50% of tumor cells with prominent intracytoplasmic mucin [1]. It is a rare disease with a reported incidence of less than 1% [18]. Macroscopically, appears as a scirrhous infiltrative tumor similar in appearance to linitis plastica [19]. Histologically, SRCC is characterized by cells with an abundant mucin vacuole that fills the cytoplasm and displaces the nucleus peripherally; these tumor cells resemble signet rings, hence its name [1].
SRCC is associated with a worse prognosis than ordinary adenocarcinoma [20]. Patients with SRCC tend to be diagnosed at younger age, have a more advanced stage at diagnosis, and have a higher frequency of lymphovascular and perineural invasion than patients with ordinary adenocarcinoma [21]. Considering the evidence that rectal cancer is increasing in patients under 40 years, and that there is a higher incidence of SRCC in this age group, it is expected that there will be an increase in incidence in SRCC [22]. SRCC also tend to demonstrate a submucosal pattern of spread which may result in areas of narrowing with normal appearing mucosa seen on colonoscopy. This is a significant challenge as this may lead to the diagnosis of a colonic malignancy being missed. In addition, since the mucosa is relatively normal, biopsies are frequently negative and a biopsy of the deeper tissues i.e., submucosa are required to obtain the correct diagnosis. The radiologist has an important role in this situation, as the appearance on cross sectional imaging frequently reflects the submucosal and bowel wall abnormality that is not evident on the luminal aspect. This collaborative approach enables appropriately directed deep wall biopsies [23, 24].
Mucin after neoadjuvant therapy
Mucinous (or colloid) degeneration occurs when a non-mucinous tumor becomes mucinous after neoadjuvant CRT [25, 26]. However, on a histopathological level, the mucin may contain residual tumor cells (cellular mucin) or not (acellular mucin). The mechanism and clinical relevance of mucin response remains debatable; however, it has been demonstrated that this type of response is associated with an intermediate natural history when compared to other types of response [25, 27].
Acellular mucin (AM) is histologically defined as pools of mucin without residual or viable tumor within the specimen after neoadjuvant CRT. Therefore, it is not used to assign the T stage, and if it is located within lymph nodes, it is not considered a positive node [28]. Several studies have evaluated the clinical significance of acellular mucin in rectal cancer after CRT and demonstrated no adverse prognostic impact, including in overall survival, disease-free survival, and freedom from relapse [29,30,31,32,33]. However, considering the current insufficient data as demonstrated by Bhatti et al. in a meta-analysis, the management of the patients with AM should be individualized and a close follow-up (possibly with a low threshold for intervention) should be considered, particularly in situations where there is an AM on the margins of surgical resection [34]. In this setting of uncertainty of the significance of mucin pool post treatment, it is important on imaging to describe the complete extent of mucin pools on imaging as they are frequently infiltrative, to enable the surgeon to get a R0 resection.
Imaging evaluation of mucinous rectal cancer
Conventional imaging modalities
Computed tomography
MAC frequently shows large areas of low attenuation (> 2/3 of the tumor), heterogeneous enhancement, and intratumoral calcification on computed tomography (CT) when compared to non-mucinous tumors (Fig. 1) [35, 36]. Calcifications can be detected in both pre- and post-CRT settings.
A 59-year-old man with mucinous adenocarcinoma of the rectum (arrows) causing bowel obstruction. a, b Computed tomography on admission demonstrates a lesion in the upper rectum with heterogeneous enhancement and areas of low attenuation. The lesion causes upstream bowel obstruction (asterisks). Sagittal (c) and axial (d) T2WI shows the typical high signal intensity of the lesion, which was confirmed on fat-suppressed T2WI (e). Contrast-enhanced T1WI demonstrates heterogeneous enhancement that was more evident in the periphery of the tumor and less intense within the central areas of higher mucin content
SRCC tends to have more homogenous enhancement, and may demonstrate a “malignant” target appearance, with a higher frequency of peritoneal involvement [36]. Rectal scirrhous tumors, such as SRCC, may demonstrate imaging features similar to inflammatory and ischemic diseases, appearing as a long segment of thickening with target appearance (Fig. 2) [37]. In inflammatory conditions, the target sign tend to demonstrate a thicker hypoattenuating submucosa, while malignancies tend to show thicker and hyperattenuating mucosa and serosa [38]. Considering that SCRR is frequent in young patients, demonstrates imaging features similar to benign conditions, and false negative biopsies can occur due to its submucosal spread, negative endoscopic biopsies should be interpreted with caution.
a A 42-year-old man with signet-ring cell carcinoma in the high rectum and sigmoid colon. Non-contrast enhanced computed tomography demonstrating a long segment of homogeneous thickening involving the upper rectum and sigmoid colon (arrows), with foci of calcifications (arrowhead). b A 45-year-old woman with signet-ring cell carcinoma in the rectum. Contrast-enhanced computed tomography shows thickening of the rectum with target appearance (dashed arrow)
Magnetic resonance imaging
Primary staging
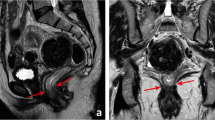
MAC typically shows high signal intensity on T2-weighted images (T2WI) due to the presence of mucin pools and it is generally defined as presence of > 50% of mucin within the tumor [39]. High signal intensity is defined as intensity similar to or brighter than the mesorectal fat. Atypically but sometimes, the signal intensity of the tumor is indistinct from the surrounding fat. In such cases, T2WI with fat suppression and a dynamic contrast-enhanced (DCE) series are helpful (Fig. 3), although it is not routinely recommended. If the T2WI with fat suppression if not available, the DWI b0 can be used. The European Society of Gastrointestinal and Abdominal Radiology suggested in their consensus paper that DCE may be useful to evaluate the conspicuity of mucinous tumors in some cases [40]. Despite the fact that mucinous tumors demonstrate less enhancement compared with non-mucinous tumors, the cellular portion may demonstrate heterogeneous enhancement, predominantly in the peripheral area [41]. On diffusion-weighted imaging (DWI), MAC demonstrates lower signal intensity than conventional adenocarcinoma and higher signal intensity on the apparent diffusion coefficient (ADC) map, owing to its low cellular density (Fig. 4) [42]. Some cases of MAC may also demonstrate a dark rim on T2WI surrounding the tumor similar to the muscularis propria and sphincter complex. In such cases, using the contour of the tumor may be helpful for proper staging (Fig. 5).
A 75-year-old woman with mucinous tumor in the lower rectum. Axial T2WI without fat suppression (a, c) and with fat suppression (b, d) demonstrating the lower rectal tumor with high signal intensity (arrows) infiltrating the sphincter complex (arrowhead) and a tumor deposit close to the mesorectal fascia (dashed arrows). Note that the sequence T2WI with fat suppression demonstrates mucin content with more conspicuity
A 44-year-old man with Crohn’s disease and perianal fistula who had mucinous rectal adenocarcinoma. 18-FDG PET/MRI on primary staging demonstrating a tumor in the lower rectum (arrow) with predominantly high signal intensity on T2WI (a). There is an infiltration of the right internal and external sphincters (arrow). On DWI (b) and ADC map (c) there is a focal area with diffusion restriction on the left which has low signal intensity on T2WI and is avid on FDG-PET (d dashed arrows), suggesting a more cellular component within the mucin pool. (e) PET/MRI fusion demonstrates that correlation. At 2 o’clock, an intersphincteric fistula with hyper metabolism is also demonstrated (arrowheads)
A 62-year-old woman with mucinous adenocarcinoma of the lower rectum without complete response after chemoradiotherapy on surgical specimen—cellular mucin. a Primary staging MRI demonstrating on T2WI the tumor with high signal intensity surrounded by a dark rim, which infiltrates the sphincter complex (arrows). b Restaging MRI demonstrates slight reduction in the size of the tumor with similar heterogeneous mucin content (dashed arrow). The patient underwent surgery and residual tumor was detected (cellular mucin)
The accuracy of magnetic resonance imaging (MRI) to detect MAC has been shown to be up to 97% [43]. On the other hand, endoscopic biopsy obtains only a superficial part of the tumor and might not contain a representative amount of mucin, leading to a misclassification in some cases [44]. MRI has a lower rate of false negatives in detecting MAC than biopsies [39], and the inter-reader agreement in assessing mucin on MRI is considerable [43]. The presence of mucin on MRI is a prognostic marker. On preoperative MRI, mucin has been demonstrated to be an independent biomarker for poor prognosis and worse response to neoadjuvant therapy [39, 45, 46]. Miyakita et al. showed in their study that mucin pools on MRI on the baseline scan was associated with large tumors and poor response to neoadjuvant CRT [46]. The possible causes of false positive mucin content on MRI are edema, congestion, abscess, or necrosis. False positives are particularly relevant in the posttreatment context because the normal rectal wall after CRT may demonstrate submucosal edema [47]. To avoid this common pitfall, it is extremely important to compare the restaging MRI with the baseline MRI to detect where the tumor was localized before the treatment. Mucinous metastatic lymph nodes, tumor deposits, and extramural vascular invasion demonstrate the same imaging features as that of the primary mucinous tumor (Figs. 3, 6).
A 56-year-old man with mucinous rectal adenocarcinoma. Axial (a), sagittal (b), and coronal (c) T2WI demonstrate the tumor in the upper rectum with high signal intensity (arrows) and a positive mesorectal lymph node (dashed arrow), which also demonstrates high signal intensity on T2WI due to mucin content
Regarding SRCC, MRI features are similar to the CT features described above.
In summary, MRI on primary staging can accurately detect mucinous tumor and has high inter-reader agreement and higher accuracy than biopsy; additionally, it is also a prognostic marker.
Restaging
Currently, MRI is the modality of choice to assess treatment response in patients with rectal cancer after CRT; however, there is no consensus in the literature regarding the assessment of treatment response in mucinous rectal cancer. Some studies have demonstrated that mucinous tumors show minimal change in their volume and very few morphologic changes after CRT [48]. Nevertheless, it can be due to lack of response to treatment or mucin pools without viable tumor cells (acellular mucin) [48, 49].
The assessment of treatment response of mucinous tumors is challenging, given that both cellular and acellular mucin show high signal intensity on T2WI, which may be homogeneous or heterogeneous (Figs. 5, 6, 7) [50]. DWI and DCE sequences cannot differentiate between cellular and acellular mucin. Thus, in general the patient will undergo surgery or close imaging followup [51]. Park et al. proposed a TRG system for mucinous tumor using T2WI and volumetric analysis based on baseline MRI and restaging MRI. In this study, mucin was defined as tumor components with high signal intensity on T2WI, soft tissue (viable tumor) was the areas of intermediate T2WI signal intensity, and fibrosis was the regions of low signal intensity on T2WI. The TRG score was defined as follows: TRG 1, no identifiable residual lesion; TRG 2, no residual soft tissue, only pure mucin and/or fibrosis; TRG 3, good response of soft tissue; TRG 4, all tumors that do not meet criteria to TRG 1–3; and TRG 5 no response or progression. In their population, the majority of patients categorized as responders (TRG 1 or 2) were responsive on pathology. However, the interobserver agreement in the assessment of MRI TRG was moderate [49].
A 54-year-old man with mucinous adenocarcinoma of the lower rectum with complete response after chemoradiotherapy on surgical specimen—acellular mucin. a Primary staging MRI demonstrates on T2WI the tumor with high signal intensity infiltrating (arrow). b Restaging MRI demonstrates no significant change in the tumor, which maintains a heterogeneous mucin content (dashed arrow). The patient underwent surgery and no residual tumor was detected (acellular mucin) and he was classified as having complete pathological response
In this scenario, cases of mucinous tumor after CRT should be discussed individually considering its rarity and the lack of data in the literature (Fig. 8) [50].
Two patients with mucinous degeneration post chemoradiotherapy, one with cellular (a, b) and the other with acellular mucin (c, d) detected on surgical specimen. a, b A 49-year-old man with non-mucinous tumor on primary staging MRI (a, arrow). On restaging MRI after chemoradiotherapy, the tumor demonstrated a significant reduction in size and mucin was detected on the tumor bed (b dashed arrow); however, on histopathological evaluation, cellular mucin was detected. c, d A 67-year-old man with non-mucinous tumor on initial MRI (c arrow). Restaging MRI did not show a significant reduction in tumor size, but there was mucinous degeneration of the tumor and a heterogeneous mucin content was demonstrated within the treated area (d dashed arrow). After surgery, no tumor was detected and the mucin content was acellular
Mucinous tumor in chronic perianal fistula
Chronic perianal fistula may be complicated by malignancies including mucinous carcinoma. Considering that mucinous tumors demonstrate imaging features similar to fistulas and abscesses, i.e., high signal intensity on T2WI and peripheral enhancement, an early diagnosis of malignancy is challenging. Radiologists should suspect mucinous tumors in cases of heterogeneous internal mesh-like enhancement, very high signal intensity on T2WI, and mass effect (Fig. 4) [52]. Nevertheless, in cases of doubt, biopsy should be performed.
Positron emission tomography (PET)
Mucinous tumors have been shown to demonstrate low 18-fluorodeoxyglucose (FDG) uptake in both PET/CT and PET/MRI. The tumor FDG avidity on PET inversely correlates with the overall amount of mucin, which may result in false negative cases (Fig. 8) [53]. Some recent studies found no significant differences in metabolic 18-FDG PET parameters between mucinous and non-mucinous rectal cancer [54, 55]. In our experience, it is not uncommon for the PET to be negative in the setting of mucinous tumors, thus a negative PET should be interpreted with caution.
Imaging challenges and future directions
Currently, the main imaging challenge in the context of mucinous rectal tumor is to provide an early diagnosis of SRCC, and to accurately differentiate cellular from acellular mucin, particularly in the context of mucin degeneration. Up to now, there is no imaging method able to diagnosis complete response after CRT in rectal mucinous tumor. Some advances in technology are promising, including radiomics in which quantitative texture analysis may be extracted from images and correlated with tumor characteristics that are inaccessible to the naked eye. These textural features may allow the assessment of cellularity and perfusion, for example [56]. The use of radiomics in MRI to evaluate mucinous tumor is promising.
Conclusion
Mucinous rectal cancer is a distinct entity with clinical and histopathological characteristics. On imaging modalities, mucin typically demonstrates high signal intensity on T2WI, low attenuation on CT, and may be negative on FDG-PET. MRI has a high diagnostic performance for detecting mucinous tumors, higher than presurgical biopsy. After neoadjuvant CRT, cellular and acellular mucin share similar imaging features, and differentiating them is currently the main challenge faced by radiologists. Radiologists should be aware of pros, cons, and limitations of each imaging modality in the primary staging and restaging, to avoid misinterpretation of the radiological findings.
References
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System: World Health Organization, International Agency for Research on Cancer, 2010.
Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012;65(5):381-388. https://doi.org/10.1136/jclinpath-2011-200340
Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, Slotta-Huspenina J, Käser SA, Michalski CW, Janssen KP, Friess H, Rosenberg R, Bader FG. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013;258(5):775-782; discussion 782-773. https://doi.org/10.1097/sla.0b013e3182a69f7e
Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol 2016;13(6):361-369. https://doi.org/10.1038/nrclinonc.2015.140
Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol 2009;34(4):1109-1115.
Hugen N, van Beek JJ, de Wilt JH, Nagtegaal ID. Insight into mucinous colorectal carcinoma: clues from etiology. Ann Surg Oncol 2014;21(9):2963-2970. https://doi.org/10.1245/s10434-014-3706-6
Song GA, Deng G, Bell I, Kakar S, Sleisenger MH, Kim YS. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol 2005;26(3):745-750.
Berry J, Vreeland T, Trappey A, Hale D, Peace K, Tyler J, Walker A, Brown R, Herbert G, Yi F, Jackson D, Clifton G, Peoples GE. Cancer vaccines in colon and rectal cancer over the last decade: lessons learned and future directions. Expert Rev Clin Immunol 2017;13(3):235-245. https://doi.org/10.1080/1744666x.2016.1226132
de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, Bednarski B, Messick CA, Skibber JM, Feig BW, Lynch PM, Vilar E, You YN. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol 2016;34(25):3039-3046. https://doi.org/10.1200/jco.2016.66.6826
Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta 2011;1815(2):224-240. https://doi.org/10.1016/j.bbcan.2011.01.001
Kim TG, Park W, Choi DH, Park HC, Kim SH, Cho YB, Yun SH, Kim HC, Lee WY, Lee J, Park JO, Park YS, Lim HY, Kang WK, Chun HK. Clinical significance of mucinous rectal adenocarcinoma following preoperative chemoradiotherapy and curative surgery. Tumori 2016;102(1):114-121. https://doi.org/10.5301/tj.5000439
Tarantino I, Hüttner FJ, Warschkow R, Schmied BM, Diener MK, Ulrich A. Prognostic Relevance of Mucinous Subtype in a Population-based Propensity Score Analysis of 40,083 Rectal Cancer Patients. Ann Surg Oncol 2016;23(5):1576-1586. https://doi.org/10.1245/s10434-015-5029-7
Kaneko K, Kawai K, Kazama S, Murono K, Sasaki K, Yasuda K, Ohtani K, Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Ishihara S, Morikawa T, Fukayama M, Watanabe T. Clinical significance of mucinous components in rectal cancer after preoperative chemoradiotherapy. Surg Today 2017;47(6):697-704. https://doi.org/10.1007/s00595-016-1419-0
Hugen N, van de Velde CJ, Bosch SL, Fütterer JJ, Elferink MA, Marijnen CA, Rutten HJ, de Wilt JH, Nagtegaal ID. Modern Treatment of Rectal Cancer Closes the Gap Between Common Adenocarcinoma and Mucinous Carcinoma. Ann Surg Oncol 2015;22(8):2669-2676. https://doi.org/10.1245/s10434-014-4339-5
Catalano OA, Sahani DV, Forcione DG, Czermak B, Liu CH, Soricelli A, Arellano RS, Muller PR, Hahn PF. Biliary infections: spectrum of imaging findings and management. Radiographics 2009;29(7):2059-2080. https://doi.org/10.1148/rg.297095051
Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 2005;16(8):1305-1310. https://doi.org/10.1093/annonc/mdi244
Sengul N, Wexner SD, Woodhouse S, Arrigain S, Xu M, Larach JA, Ahn BK, Weiss EG, Nogueras JJ, Berho M. Effects of radiotherapy on different histopathological types of rectal carcinoma. Colorectal Dis 2006;8(4):283-288. https://doi.org/10.1111/j.1463-1318.2005.00934.x
Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48(6):1161-1168. https://doi.org/10.1007/s10350-004-0932-1
Belli S, Aytac HO, Karagulle E, Yabanoglu H, Kayaselcuk F, Yildirim S. Outcomes of surgical treatment of primary signet ring cell carcinoma of the colon and rectum: 22 cases reviewed with literature. Int Surg 2014;99(6):691-698. https://doi.org/10.9738/intsurg-d-14-00067.1
Ciarrocchi A. Rectal versus non-rectal primary signet ring cell carcinoma of the colorectum: a retrospective survival analysis controlled for confounders. J Gastrointest Cancer 2014;45(3):312-318. https://doi.org/10.1007/s12029-014-9604-0
Liang Z, Yan D, Li G, Cheng H. Clinical Analysis of Primary Colorectal Signet-Ring Cell Carcinoma. Clin Colorectal Cancer 2018;17(1):e39-e44. https://doi.org/10.1016/j.clcc.2017.06.010
Tawadros PS, Paquette IM, Hanly AM, Mellgren AF, Rothenberger DA, Madoff RD. Adenocarcinoma of the rectum in patients under age 40 is increasing: impact of signet-ring cell histology. Dis Colon Rectum 2015;58(5):474-478. https://doi.org/10.1097/dcr.0000000000000318
Chen JS, Hsieh PS, Hung SY, Tang R, Tsai WS, Changchien CR, Lin PY, Wang JY, Yeh CY. Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis 2004;19(2):102-107. https://doi.org/10.1007/s00384-003-0515-y
Gopalan V, Smith RA, Ho YH, Lam AK. Signet-ring cell carcinoma of colorectum--current perspectives and molecular biology. Int J Colorectal Dis 2011;26(2):127-133. https://doi.org/10.1007/s00384-010-1037-z
Rullier A, Laurent C, Vendrely V, Le Bail B, Bioulac-Sage P, Rullier E. Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma. Am J Surg Pathol 2005;29(5):602-606.
Patel UB, Blomqvist LK, Taylor F, George C, Guthrie A, Bees N, Brown G. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol 2012;199(4):W486-495. https://doi.org/10.2214/ajr.11.8210
Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol 2004;204(2):183-192. https://doi.org/10.1002/path.1621
Washington MK, Berlin J, Branton PA, Burgart LJ, Carter DK, Fitzgibbons PL, Frankel WL, Jessup JM, Kakar S, Minsky B, Nakhleh RE, Compton CC, Cancer Committee CloAP. Protocol for the examination of specimens from patients with primary carcinomas of the colon and rectum. Arch Pathol Lab Med 2008;132(7):1182-1193. https://doi.org/10.1043/1543-2165(2008)132%5b1182:pfteos%5d2.0.co;2
Smith KD, Tan D, Das P, Chang GJ, Kattepogu K, Feig BW, Skibber JM, Rodriguez-Bigas MA. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg 2010;251(2):261-264. https://doi.org/10.1097/sla.0b013e3181bdfc27
Shia J, McManus M, Guillem JG, Leibold T, Zhou Q, Tang LH, Riedel ER, Weiser MR, Paty PB, Temple LK, Nash G, Kolosov K, Minsky BD, Wong WD, Klimstra DS. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol 2011;35(1):127-134. https://doi.org/10.1097/pas.0b013e318200cf78
Lim SB, Hong SM, Yu CS, Hong YS, Kim TW, Park JH, Kim JH, Kim JC. Prevalence and clinical significance of acellular mucin in locally advanced rectal cancer patients showing pathologic complete response to preoperative chemoradiotherapy. Am J Surg Pathol 2013;37(1):47-52. https://doi.org/10.1097/pas.0b013e3182657186
de Campos-Lobato LF, Dietz DW, Stocchi L, Vogel JD, Lavery IC, Goldblum JR, Skacel M, Pelley RJ, Kalady MF. Clinical implications of acellular mucin pools in resected rectal cancer with pathological complete response to neoadjuvant chemoradiation. Colorectal Dis 2012;14(1):62-67. https://doi.org/10.1111/j.1463-1318.2010.02532.x
Cienfuegos JA, Baixauli J, Rotellar F, Arredondo J, Sola JJ, Arbea L, Pastor C, Hernández-Lizoáin JL. Clinical significance of cellular and acellular mucin pools in rectal carcinoma following preoperative chemoradiotherapy. Clin Transl Oncol 2016;18(7):714-721. https://doi.org/10.1007/s12094-015-1422-8
Bhatti ABH, Zaheer S, Shafique K. Prognostic Role of Acellular Mucin Pools in Patients with Rectal Cancer after Pathological Complete Response to Preoperative Chemoradiation: Systematic Review and Meta-Analysis. J Coll Physicians Surg Pak 2017;27(11):714-718.
Ko EY, Ha HK, Kim AY, Yoon KH, Yoo CS, Kim HC, Kim JC. CT differentiation of mucinous and nonmucinous colorectal carcinoma. AJR Am J Roentgenol 2007;188(3):785-791. https://doi.org/10.2214/ajr.06.0476
Li ZH, You DY, Gao DP, Yang GJ, Dong XX, Zhang DF, Ding YY. Role of CT scan in differentiating the type of colorectal cancer. Onco Targets Ther 2017;10:2297-2303. https://doi.org/10.2147/ott.s131008
Kim HJ, Ha HK, Cho KS, Yu E, Kim JC, Yoo CS, Kim HC, Yang SK, Jeong HY, Auh YH. CT features of primary colorectal signet-ring cell carcinoma. J Comput Assist Tomogr 2001;25(2):225-230.
Gollub MJ, Schwartz MB, Shia J. Scirrhous metastases to the gastrointestinal tract at CT: the malignant target sign. AJR Am J Roentgenol 2009;192(4):936-940. https://doi.org/10.2214/ajr.08.1152
Yu SK, Chand M, Tait DM, Brown G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer 2014;50(5):920-927. https://doi.org/10.1016/j.ejca.2013.12.007
Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Correction to: Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28(6):2711. https://doi.org/10.1007/s00330-017-5204-2
Hussain SM, Outwater EK, Siegelman ES. Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging. Radiology 1999;213(1):79-85. https://doi.org/10.1148/radiology.213.1.r99se3879
Nasu K, Kuroki Y, Minami M. Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region: comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn J Radiol 2012;30(2):120-127. https://doi.org/10.1007/s11604-011-0023-x
Kim MJ, Park JS, Park SI, Kim NK, Kim JH, Moon HJ, Park YN, Kim WH. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr 2003;27(1):48-55.
Younes M, Katikaneni PR, Lechago J. The value of the preoperative mucosal biopsy in the diagnosis of colorectal mucinous adenocarcinoma. Cancer 1993;72(12):3588-3592.
Oberholzer K, Menig M, Kreft A, Schneider A, Junginger T, Heintz A, Kreitner KF, Hötker AM, Hansen T, Düber C, Schmidberger H. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys 2012;82(2):842-848. https://doi.org/10.1016/j.ijrobp.2010.08.057
Miyakita H, Sadahiro S, Ogimi T, Saito G, Okada K, Tanaka A, Suzuki T, Kajiwara H, Yamamuro H, Akiba T. Mucinous components assessed by magnetic resonance imaging in primary rectal cancer tissue before and after chemoradiotherapy and tumor response. Int J Colorectal Dis 2018;33(8):1135-1138. https://doi.org/10.1007/s00384-018-3047-1
Aker M, Boone D, Chandramohan A, Sizer B, Motson R, Arulampalam T. Diagnostic accuracy of MRI in assessing tumor regression and identifying complete response in patients with locally advanced rectal cancer after neoadjuvant treatment. Abdom Radiol (NY) 2018. https://doi.org/10.1007/s00261-018-1627-8
Grillo-Ruggieri F, Mantello G, Berardi R, Cardinali M, Fenu F, Iovini G, Montisci M, Fabbietti L, Marmorale C, Guerrieri M, Saba V, Bearzi I, Mattioli R, Bonsignori M, Cascinu S. Mucinous rectal adenocarcinoma can be associated to tumor downstaging after preoperative chemoradiotherapy. Dis Colon Rectum 2007;50(10):1594-1603. https://doi.org/10.1007/s10350-007-9026-1
Park SH, Lim JS, Lee J, Kim HY, Koom WS, Hur H, Park MS, Kim MJ, Kim H. Rectal Mucinous Adenocarcinoma: MR Imaging Assessment of Response to Concurrent Chemotherapy and Radiation Therapy-A Hypothesis-generating Study. Radiology 2017;285(1):124-133. https://doi.org/10.1148/radiol.2017162657
Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol 2007;188(2):442-451. https://doi.org/10.2214/ajr.05.1967
Network NCC. Clinical Practice Guidelines in Oncology (NCCN Guidelines) Colon Cancer. Version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Published 2018.01.30.2018.
Hama Y, Makita K, Yamana T, Dodanuki K. Mucinous adenocarcinoma arising from fistula in ano: MRI findings. AJR Am J Roentgenol 2006;187(2):517-521. https://doi.org/10.2214/ajr.05.0011
Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol 2000;174(4):1005-1008. https://doi.org/10.2214/ajr.174.4.1741005
Dos Anjos DA, Habr-Gama A, Vailati BB, Rossi CB, Coturel AE, Perez RO, São Julião GP, de Sousa JB, Buchpiguel CA. (18)F-FDG uptake by rectal cancer is similar in mucinous and nonmucinous histological subtypes. Ann Nucl Med 2016;30(8):513-517. https://doi.org/10.1007/s12149-016-1089-4
Barbaro B, Leccisotti L, Vecchio FM, Di Matteo M, Serra T, Salsano M, Poscia A, Coco C, Persiani R, Alfieri S, Gambacorta MA, Valentini V, Giordano A, Bonomo L. The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. Br J Radiol 2017;90(1069):20150836. https://doi.org/10.1259/bjr.20150836
Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278(2):563-577. https://doi.org/10.1148/radiol.2015151169
Funding
This work was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horvat, N., Hope, T.A., Pickhardt, P.J. et al. Mucinous rectal cancer: concepts and imaging challenges. Abdom Radiol 44, 3569–3580 (2019). https://doi.org/10.1007/s00261-019-02019-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02019-x