Abstract
Segmental arterial mediolysis (SAM) is an uncommon, non-atherosclerotic, non-inflammatory arteriopathy that tends to affect the medium-sized splanchnic branches of the aorta along with renal, carotid, cerebral, and coronary arteries. The clinical presentation ranges from asymptomatic to severe, life-threatening intra-abdominal hemorrhage and shock. SAM overlaps clinically and radiologically with other inflammatory vasculitides. This article describes the pathologic–radiologic correlation, imaging findings, and the management of the disease. Radiologists should be familiar with this disease entity as imaging plays a crucial role in the diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Segmental arterial mediolysis (SAM) is an uncommon, non-atherosclerotic, non-inflammatory arteriopathy that tends to affect the medium-sized splanchnic and renal arteries in a ‘skip’ pattern. Carotid, cerebral, and coronary arteries can be affected less commonly. The disease affects patients in their fifth and sixth decade with a slight male predominance [1, 2]. Pulmonary arteries can rarely be involved [3]. Slavin and Gonzales, in 1976, first reported three autopsy cases of ruptured aneurysms which resulted in massive hemorrhage and death [4]. Slavin, at first, used the term “Segmental Mediolytic Arteritis” to describe this condition [4]. After lengthy histological studies, and to emphasize the absence of inflammatory changes, he renamed it to “Segmental Arterial Mediolysis” as a more appropriate term [2, 5]. SAM was initially thought to be rare, with only 19 cases reported in the literature until 1997 [1]. SAM reports are becoming more frequent, probably due to the increasing use of computed tomography (CT) and CT angiography (CTA) [6].
In this article, we discuss the pathology of SAM along with its imaging features including arterial dissections, intramural hematomas, aneurysms, and its end-organ complications of ischemia and hemoperitoneum. Imaging plays an important role in the evaluation, and follow-up of patients with SAM, while catheter angiography is performed for confirmation and management. Familiarity with the pathogenesis, imaging features, and treatment of SAM can aid radiologic diagnoses and guide appropriate patient management.
Pathogenesis
The pathology of SAM appears to be a dynamic process consisting of two phases: the initial injurious phase and the reparative phase [2, 5]. The initial phase begins with mediolysis, which is the pathologic hallmark of SAM [2]. Mediolysis involves varying sized vacuolar degenerations and lysis within the smooth muscle in the outer media of the arterial wall. Subsequently, a tear develops and separates the outer medial muscle from the adventitia layer (Fig. 1). This process may involve a sector of the arterial circumference or its entirety, and it exhibits a segmental distribution leaving normal arterial segments in between [2, 5, 7]. Eventually, arterial gaps develop in place of the destroyed internal elastic lamina and the intima layer. The resultant gaps loosen the arterial wall and render it more susceptible to dissections and aneurysms. The blood within the gaps progressively dissects and forms intramural hematoma and dissecting aneurysms. The shape of the aneurysm depends on the size of the arterial gaps. Saccular aneurysms develop if the gaps are small, while bigger gaps form fusiform aneurysms [2, 5]. After the initial arterial injury (mediolysis), the reparative phase starts with granulation tissue growing in the arterial gaps. Subsequently, fibrosis replaces the granulation tissue to heal the arterial wall and helps restore its shape [2, 5]. Unlike vasculitides, the process shows mild or no inflammatory cell infiltrate [8]. The extent and degree of the mediolysis and separation processes, along with their subsequent evolution in the reparative phase, is responsible for the variety of manifestations of SAM displays [5].
The pathogenesis is thought to be related to an underlying vasoconstrictive process. Many patients diagnosed with SAM, report a clinical history of a “vasoconstriction-related” event including migraine, stroke, hypertensive episode, pulmonary hypertension, hypoxemic event, or recent prior anesthesia. This observation has led to the belief that repetitive vasoconstrictive responses in the splanchnic bed may result in arterial mediolysis [6, 9, 10]. This vasoconstrictive-SAM correlation is further supported by the finding that arteries with chronic vasospasm have histologic features similar to SAM [6].
Clinical presentation and diagnosis
SAM is a relatively rare entity with a slight male predominance (M:F ratio of 1.5:1) [7]. It most commonly presents during the sixth decade in 33% of patients [2, 11]. Recently, SAM has been reported in all age groups [7]. The presentation of SAM ranges from an asymptomatic patient with incidental imaging features to patients who present with acute, life-threatening intra-abdominal or retroperitoneal hemorrhage. The most common clinical presentation is abdominal pain in 62% of cases [12]. Abdominal pain was followed by shock in 32%, abdominal distension in 13%, hematochezia in 11%, and stroke in 6%, and 9% of the patients were asymptomatic [13]. A smaller study reported abdominal pain in 50% of patients followed by headache, stroke, chest pain, flank pain, and suprapubic fullness (7% each) [14]. Arterial occlusion complicates SAM, often associated with an underlying dissection (Fig. 2). This can result in end-organ ischemia, most commonly renal infarcts, and bowel ischemia. Rarely, the vessels of the head and neck can be involved with SAM. To date, only 13 cases of intracranial arterial dissection coexisting with visceral involvement have been reported [15]. With intracranial involvement, patients may present with subarachnoid hemorrhage, and cerebral infarction [1]. It has been reported that hemoperitoneum may ensue following an episode of subarachnoid hemorrhage due to SAM in the perioperative period. Thus, abdominal CTA is recommended to screen the abdominal arteries in patients who present with multiple intracranial dissecting aneurysms [1].
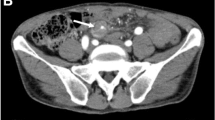
Superior mesenteric artery (SMA) dissection found incidentally on routine follow-up in a 43-year-old male after Roux-en-Y cystojejunostomy for a recurrent pancreatic pseudocyst. A Sagittal CTA shows SMA dissection with fusiform aneurysm formation, mural thrombosis, and middle colic occlusion (arrow). B Digital subtraction angiography (DSA) shows middle colic occlusion (arrow). C Coronal CTA shows focal left common iliac artery saccular aneurysm in the absence of atherosclerosis, presumably also related to SAM (arrow)
SAM may mimic other inflammatory vasculitides such as polyarteritis nodosa (PAN), Kawasaki disease, Takayasu arteritis, Behcet disease, allergic granulomatous angiitis, Wegener granulomatosis, Churg-Strauss syndrome, rheumatoid vasculitis, or systemic lupus erythematosus (SLE) [7]. As SAM is a non-inflammatory arteriopathy, its workup includes the exclusion of inflammatory and immune markers seen in these other vasculitides including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), rheumatoid factor (RF), and the presence of immune complexes. Other laboratory abnormalities used for exclusion include anemia, leukocytosis, thrombocytosis, reduced serum albumin concentration, and abnormal urinalysis results.
Connective tissue disorders such as Ehlers–Danlos syndrome, Marfan syndrome, Loeys–Dietz syndrome, and neurofibromatosis are excluded by detailed clinical history and genetic profile testing. Atherosclerosis, arterial trauma, and infection (mycotic aneurysms) should also be considered in the differential diagnosis.
A definitive diagnosis of SAM requires biopsy of the affected artery and pathologic evaluation. Even though it is highly recommended, biopsies and histological studies are rarely performed due the location of the involved arteries deep in the abdomen [16]. Instead, the clinical and imaging features of SAM are used for diagnosis. Three diagnostic criteria (clinical, radiological, and serological) can be used to diagnose SAM without the need for tissue sampling procedures to obtain a pathologic evaluation [17]. The diagnostic criteria include the following:
-
1.
Exclusion of connective tissue diseases (Ehlers–Danlos, Marfan, Loeys–Dietz), atherosclerosis, fibromuscular dysplasia (FMD), and other types of vasculitides.
-
2.
Evidence of dissection and/or an aneurysm with or without organ infarction in one or more of the mesenteric or renal arteries.
-
3.
The absence of inflammatory markers such as erythrocyte sedimentation rate (ESR), antinuclear antibodies (ANA), and C-reactive protein (CRP). [17] (Table1).
Table 1 Kalva et al. criteria for non-invasive diagnosis of SAM [17]
All three criteria must be met to establish the diagnosis of SAM. These criteria are based on the reports from the literature, and they are the most used when diagnosing SAM. However, further studies are required to validate the diagnostic criteria [14].
SAM and FMD
Fibromuscular dysplasia (FMD) is the one vasculopathy that is most difficult to differentiate from SAM. However, they differ in demographics and distribution of the affected arteries and clinical symptoms. FMD presents in young to middle-aged women, is rarely painful, and is usually asymptomatic or associated with symptoms of occlusive disease or premature hypertension [7]. On the other hand, SAM presents at any age, has no gender predilection, and more associated with hemorrhage and arterial dissections [18]. In FMD patients, involvement of the renal and internal carotid arteries is more common than the visceral arteries (only 9%), direct opposite of SAM where visceral arteries are predominantly affected [6] (Fig. 3) (Table 2). Despite these differences, some authors assume that SAM is fundamentally a precursor of a variant of FMD, based on the observation that healed SAM lesions resemble FMD [10, 17].
Celiac and SMA dissecting aneurysms in a 63-year-old male with incidental findings during the workup of renal cell carcinoma. A CTA 3D-VRT shows celiac (red arrow) and superior mesenteric artery (yellow arrow) dissecting aneurysms. B CTA coronal MIP image shows irregular “beaded” appearance of the left renal artery. This case displays an overlap of findings also seen in fibromuscular dysplasia (FMD) with the beaded appearance of the left renal artery. SAM is the presumed diagnosis in this case given the predominant findings of splanchnic dissecting aneurysms
Imaging findings
This article focuses on the imaging features of SAM with visceral involvement, as well as appearances of end-organ ischemia and infarction. Similar features are seen with non-splanchnic involvement such as the carotid and cerebral vessels as the imaging features reflect the characteristic histological changes of the disease.
SAM most commonly affects the main aortic abdominal branches, and it rarely affects peripheral arteries not related to the aorta [6]. A study of 85 patients reported the involvement of the celiac axis and branches (55%), SMA and branches (38%), IMA and branches (13%), iliac arteries (5%), hepatic artery (27%), and renal arteries (25%), as well as the carotid and cerebral arteries (17.6%) [11]. A smaller study with pooled data showed the involvement of the splanchnic arteries in 76%, renal arteries in 7%, iliac arteries in 2.3%, extracranial cerebral arteries in 3.3%, and intracranial arteries in 4.5% [13].
Computed tomography angiography (CTA), magnetic resonance angiography (MRA), and catheter angiography are used to differentiate the imaging findings of SAM from those of small- to medium-sized vasculitides such as polyarteritis nodosa (PAN) and Wegner granulomatosis [6, 12]. Imaging features of SAM overlap broadly with those of vasculitides, and it is often a challenge to distinguish between both entities based solely on imaging appearances. Fortunately, inflammatory vasculitides can be diagnosed based on associated clinical manifestations and specific serological markers. However, imaging clues that help differentiate SAM include its distribution of involvement, its hallmark imaging features, and associated secondary findings [6]. DSA was the preferred modality to accurately diagnose SAM. A retrospective study by Michael et al. [6] compared the imaging features of DSA with those of CTA in four cases and reported similar findings. The study concluded that CTA provides sufficient evidence to diagnose SAM and substitutes DSA as a non-invasive modality to radiologically diagnose SAM and follow-up its progression (Fig. 2). MRA is equally sensitive in detecting SAM features without the risk of radiation, but it is limited by lengthy scan time and low spatial resolution [7]. Yoshida also proposed the use of maximum intensity projection (MIP) reformats of the CTA findings to vividly mark the imaging irregularities [19] (Fig. 3B).
Slavin et al. described six possible angiographic appearances of SAM as (1) arterial dilation, (2) single aneurysm, (3) multiple aneurysms, (4) dissecting hematomas, (5) arterial stenosis, and (6) arterial occlusion [4]. The principle imaging hallmark of SAM is dissecting aneurysms [6] (Fig. 4, 5). CTA helps demonstrate the classical appearance of arterial wall irregularity with perivascular inflammation and alternating aneurysms and stenosis in a “beading” pattern involving the aortic abdominal arteries, which may be elongated and kinked [20]. Other imaging findings include segmental arterial wall thickening and arterial occlusions (Fig. 2). Dissections may be fusiform or saccular in shape, depending on the size of the arterial gaps [6, 21]. A study by Inada et al. found dissections in 78% and multiple aneurysms in 33% of cases [7]. Single or multiple aneurysms may be seen in an artery in a segmental, skip pattern with circumferential involvement or involvement of only a portion of the arterial wall [6]. Lesions may affect one or more arteries simultaneously or at different times [6, 9]. The presence of a peripheral isolated arterial dissection unrelated to the aorta is uncommon and should suggest the diagnosis of SAM [6].
SMA dissection in a 44-year-old male with acute onset abdominal pain. A Sagittal CTA image shows SMA dissection with a fusiform aneurysm (arrow). B Axial CTA image shows the SMA dissection with surrounding fat stranding (arrow). Follow-up axial (C) and Coronal (D) CTA images 18 months after the initial presentation show a new celiac artery dissection and aneurysm formation (arrows). Note the new periceliac inflammation and resolution of the soft tissue hue surrounding the SMA dissection on the coronal view (arrow)
Mild surrounding inflammatory response can be seen in acute cases, but the more typical perivascular feature is a rind of wall “cuffing.” This finding is presumably due to the reparative fibrosis seen in cases of chronic SAM resulting in vessel remodeling and restoration of a smooth arterial wall [14] (Fig. 6).
The most feared complication of SAM is aneurysmal rupture in one or multiple arteries and subsequent intra-abdominal hemorrhage. CT is the gold standard in detecting omental, mesenteric, or retroperitoneal hematomas [6]. Retroperitoneal hematoma is frequently due to bleed from the renal arteries or its branches [5]. Intra-abdominal hematoma is illustrated as a collection of free or loculated fluid collection. The fluid collection is of higher density compared with bland ascites, consistent with clotted blood [22] (Fig. 7A). Colonic wall thickening may be detected. CT also offers the advantage of detecting the features of end-organ injury such as pneumatosis, pancreatitis, and renal or splenic infarction. Areas of infarction are demonstrated as interspersed areas of hypodensity suggestive of chronic infarction [6, 20, 23] (Fig. 7B).
Despite the increasing use of CTA studies in evaluating SAM and the advancement of using a multi-detector row CT with three-dimensional (3D) reconstruction, catheter angiography still plays an important role in the diagnosis and particularly the management of SAM [6]. Angiography helps with intervention and endovascular treatment at the time of diagnosis. Also, it has more sensitivity in visualizing the rare complication of SAM, arteriovenous fistula [6].
Treatment
SAM is usually a self-limited disease [16]. The mainstay management strategy of SAM is primarily supportive with pain control, antihypertensive regimens, and antiplatelet therapy. Intervention is reserved for patients that are hemodynamically unstable, developing significant end-organ ischemia or at high morbidity and mortality risk. Acute hemorrhagic emergencies are life threatening, and immediate surgical or vascular intervention is indicated [8, 12, 15, 16]. Patients with aneurysms may need to be treated depending on size or evidence of growth on interval imaging. Occasionally, intervention is warranted in patients with an arteriovenous fistula. Angiography can confirm the imaging diagnosis of SAM and be a guide for endovascular therapies. In a study involving 85 patients, intervention was reported in 24 cases (28%). The most common endovascular technique used was coil embolization of aneurysms (79%). Stenting was another technique reported (Fig. 8, 9). The intervention was successful in 88% of cases, with 3 cases requiring open surgical management [1]. Open surgery was most commonly described in the form of emergency exploratory laparotomy, with ligation of bleeding vessels and/or resection of aneurysmal portions, often with vascular reconstruction.
Extensive abdominal and cerebral arterial involvement in a 58-year-old female. Axial CTA of A aneurysmal dilation of the celiac artery (arrow) and B dissection of the proximal SMA with contrast extending into both the true and false lumens (arrow). C, D CTA and 3D reconstruction showing beaded appearance of the right renal artery (arrows). E, F, G Axial CTA and 3D reconstruction showing aneurysmal dilatation of both common iliac arteries and focal dissection within the left external iliac artery (arrows). H, I MR angiogram showing aneurysm at the right posterior communicating artery origin and endovascular coil therapy afterward (arrows)
Hepatic artery aneurysm found incidentally in a 53-year-old female. A Disruption of the smooth muscle in the media (arrows) with associated mucoid intimal changes. Note the lack of significant inflammation (H&E, 200X). B Loss of the internal elastic lamina (between arrowheads) of the same small hepatic artery (Van Gieson, 400X). C Disordered smooth muscle in the media (smooth muscle actin immunohistochemical stain, 200X). D Axial CTA image shows the largest 2.4-cm aneurysm (yellow arrow) and right hepatic perfusion anomaly (red arrow). E Subsequent hepatic arteriogram shows multiple hepatic artery (HA) aneurysms (arrows) with fistulous connection to the portal vein (PV). F Following coil embolization of the two largest hepatic artery aneurysms improved HA branch flow and decreased PV shunting. 2.5-month follow-up CTA showed persistent anterior right PV arterioportal shunting. Therefore, repeat angiogram was performed. G After further embolization with 500–700 micron PVA performed, no residual PV shunting was present. Ultrasound-guided liver biopsy was performed during the same session as the hepatic arteriogram. Although this patient did not present with clinical symptoms, the multiple large saccular aneurysms are high risk for rupture. Furthermore, the arterioportal shunting is likely to result in portal hypertension if not treated
Prognosis
An overall mortality of 25% has been reported with SAM [11], but advanced endovascular treatment options along with close imaging follow-up and optimization of medical therapy will likely continue to improve morbidity and mortality in these patients. Three outcomes are associated with the disease: progression (worsening), stabilization, and improvement (resolution). Progression refers to either extended involvement of a previously existing aneurysm or dissection or newly evolved abnormality in a different location that was not present at the initial presentation (Fig. 10).
Celiac artery dilation and dissection in a 66-year-old male. A, B Sagittal and Axial CTA images show 1.8-cm aneurysmal dilation of the celiac trunk (arrows), along with a dissection involving the SMA (arrow) (C). D, E 11-month follow-ups of Axial CTA and Angiogram show worsening (progression) of the disease by extension of the disease to involve the common hepatic, proper and right hepatic arteries including intrahepatic branches (arrows)
Conclusion
SAM is a systemic disease with life-threatening complications if not detected early and managed properly. SAM most commonly affects the splanchnic and renal arteries with dissections, aneurysms, beading, or occlusion. Radiologists should be familiar with the imaging findings of SAM as imaging plays a large role in the diagnosis of this disease.
References
Shenouda M, et al. (2014) Segmental arterial mediolysis: a systematic review of 85 cases. Ann Vasc Surg 28(1):269–277
Tabassum A, et al. (2013) Segmental arterial mediolysis of left gastric artery: a case report and review of pathology. BMC Clin Pathol 13(1):26
Alturkustani M, Ang LC (2013) Intracranial segmental arterial mediolysis: report of 2 cases and review of the literature. Am J Forensic Med Pathol 34(2):98–102
Slavin RE (2009) Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation. Cardiovasc Pathol 18(6):352–360
Obara H, et al. (2006) Reconstructive surgery for segmental arterial mediolysis involving both the internal carotid artery and visceral arteries. J Vasc Surg 43(3):623–626
Michael M, et al. (2006) Segmental arterial mediolysis: CTA findings at presentation and follow-up. AJR Am J Roentgenol 187(6):1463–1469
Pillai AK, et al. (2014) Segmental arterial mediolysis. Cardiovasc Interv Radiol 37(3):604–612
Hall ET, et al. (2016) Segmental arterial mediolysis and fibromuscular dysplasia: what comes first, the chicken or the egg? Cardiovasc Pathol 25(2):113–115
Ryan JM, Suhocki PV, Smith TP (2000) Coil embolization of segmental arterial mediolysis of the hepatic artery. J Vasc Interv Radiol 11(7):865–868
Slavin RE, Gonzalez-Vitale JC (1976) Segmental mediolytic arteritis: a clinical pathologic study. Lab Investig 35(1):23–29
Chao CP (2009) Segmental arterial mediolysis. Semin Interv Radiol 26(3):224–232
Tameo MN, Dougherty MJ, Calligaro KD (2011) Spontaneous dissection with rupture of the superior mesenteric artery from segmental arterial mediolysis. J Vasc Surg 53(4):1107–1112
Slavin RE, et al. (1995) Segmental arterial mediolysis: a precursor to fibromuscular dysplasia? Mod Pathol 8(3):287–294
Soulen MC, et al. (2004) Segmental arterial mediolysis: angioplasty of bilateral renal artery stenoses with 2-year imaging follow-up. J Vasc Interv Radiol 15(7):763–767
Shinoda N, et al. (2015) Segmental arterial mediolysis involving both vertebral and middle colic arteries leading to subarachnoid and intraperitoneal hemorrhage. World Neurosurg 88:694
Slavin RE, Cafferty L, Cartwright J Jr (1989) Segmental mediolytic arteritis. A clinicopathologic and ultrastructural study of two cases. Am J Surg Pathol 13(7):558–568
Kalva SP, et al. (2011) Segmental arterial mediolysis: clinical and imaging features at presentation and during follow-up. J Vasc Interv Radiol 22(10):1380–1387
Kimura Y, et al. (2015) Successful hybrid treatment for huge visceral artery aneurysms with contained rupture complicating segmental arterial mediolysis. Interact Cardiovasc Thorac Surg 21(6):814–816
Yoshida H, et al. (2013) A case report of segmental arterial mediolysis in which computed tomography angiography was useful for diagnosis. Clin J Gastroenterol 6(6):447–453
Frauenfelder T, et al. (2004) Nontraumatic emergent abdominal vascular conditions: advantages of multi-detector row CT and three-dimensional imaging. Radiographics 24(2):481–496
Inada K, Maeda M, Ikeda T (2007) Segmental arterial mediolysis: unrecognized cases culled from cases of ruptured aneurysm of abdominal visceral arteries reported in the Japanese literature. Pathol Res Pract 203(11):771–778
Rengstorff DS, et al. (2004) Intra-abdominal hemorrhage caused by segmental arterial mediolysis of the inferior mesenteric artery: report of a case. Dis Colon Rectum 47(5):769–772
Wang JJ, Huang TW (1994) Ischemic colitis caused by an isolated dissecting aneurysm of the left colic artery: a presumed case of segmental mediolytic arteriopathy. J Formos Med Assoc 93(8):715–720
Filippone EJ, et al. (2011) Segmental arterial mediolysis: report of 2 cases and review of the literature. Am J Kidney Dis 58(6):981–987
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Alhalabi, K., Menias, C., Hines, R. et al. Imaging and clinical findings in segmental arterial mediolysis (SAM). Abdom Radiol 42, 602–611 (2017). https://doi.org/10.1007/s00261-016-0887-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0887-4