Abstract
Purpose
The aim of our study was to assess the performance value of magnetic resonance imaging (MRI) in the restaging of locally advanced rectal cancer after neoadjuvant chemoradiotherapy (CRT) and in the identification of good vs. poor responders to neoadjuvant therapy.
Materials and Methods
A total of 34 patients with locally advanced rectal cancer underwent MRI prior to and after CRT. T stage and tumor regression grade (TRG) on post-CRT MRI was compared with the pathological staging ypT and TRG. Tumor volume and the apparent diffusion coefficient (ADC) were measured using diffusion-weighted imaging (DWI) before and after neoadjuvant CRT; the percentage of tumor volume reduction and the change of ADC (ΔADC) was also calculated. ADC parameters and the percentage of tumor volume reduction were correlated to histopathological results. The diagnostic performance of ADC and volume reduction to assess tumor response was evaluated by calculating the area under the ROC curve and the optimal cut-off values.
Results
A significant correlation between the T stage and the TRG defined in DW-MRI after CRT and the ypT and the TRG observed on the surgical specimens was found (p = 0.001; p < 0.001). The mean post-CRT ADC and ΔADC in responder patients was significantly higher compared to non-responder ones (p = 0.001; p = 0.01). Furthermore, the mean post-CRT ADC values were significantly higher in tumors with T-downstage (p = 0.01).
Conclusion
DW-MRI may have a significant role in the restaging and in the evaluation of post-CRT response of locally advanced rectal cancer. Quantitative analysis of DWI through ADC map may result in a promising noninvasive tool to evaluate the response to therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neoadjuvant chemoradiotherapy (CRT) has long become the standard of treatment in patients with locally advanced rectal cancer [1], allowing improvement of local control of disease and a more widespread use of sphincter preservation procedures, suggesting in some studies an even better overall survival [1, 2].
Total mesorectal excision (TME) following neoadjuvant CRT has reduced historical figures of local recurrence rates from 25% to 30% to less than 10% [3, 4], with 10% to 30% of patients with locally advanced rectal cancer showing a complete pathologic response after neoadjuvant treatment [2, 5].
Patients with good local response have an excellent clinical outcome; therefore less invasive treatment, such as a local excision, could be considered as an alternative to more aggressive surgical treatment such as TME.
Some authors have proposed a ‘wait-and-see’ policy, avoiding surgery when a complete clinical response is obtained after CRT [6]. Accurate follow-up protocol is required in those instances.
In this scenario, an accurate assessment of the response to neoadjuvant CRT is essential, so that an effective patient-tailored treatment could be designed.
Magnetic resonance imaging (MRI) represents a valid tool for local staging of rectal cancer [7] and is also increasingly used for restaging following CRT [8]. Still, differentiation of residual viable tumor from diffuse fibrotic change is difficult [9].
Diffusion-weighted imaging (DWI) is a noninvasive method to assess biological features of a tissue, based on the diffusion properties of water molecules[10].
This provides tissue characterization and evaluation of intratumoral changes which are induced by chemoradiation therapy which is quantified by the apparent diffusion coefficient (ADC). This quantitative analysis has been shown to be useful in evaluating tumors and discriminating between benign and malignant lesions [10]. It has been suggested that areas exhibiting a low ADC values reflect dense cellular structures, and a recent study suggests that ADC values may reflect the aggressiveness of the tumor tissue profile [11].
The purpose of our study was to obtain performance values of MRI for restaging of locally advanced rectal cancer after neoadjuvant treatment. Moreover, in order to assess the ability of MRI to identify good vs. poor responders to CRT, we have used available imaging methods such as the ADC value and the volume reduction of the lesion, compared with the histopathological reference standards of ypT and tumor regression grade (TRG).
Materials and methods
Patient
This study was approved by our Institutional Review Board and informed consent was obtained from all patients.
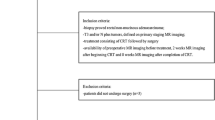
Between July 2013 and June 2015, 49 patients affected with biopsy-proven rectal cancer were considered for inclusion in this prospective study.
Inclusion criteria were (1) patients with locally advanced rectal cancer (≥T3) staged with MRI; (2) availability of staging MRI and post-CRT MRI performed about 6-8 weeks after neoadjuvant treatment, including DW images; (3) planned treatment consisting of CRT followed by surgery; and (4) availability of pathological reports of surgical specimens.
Exclusion criteria were as follows:
-
1.
mucinous adenocarcinoma, due to distinctive MRI characteristics which could potentially may introduce a bias in the study results [12];
-
2.
evidence of remote metastases or unresectable primary tumors.
A total of 34 patients (19 males, 15 females, age range 28–83 years, mean age 65 years) met the inclusion criteria.
Treatment protocol
Patients with locally advanced rectal cancer (cT3/T4 and/or cN+), with distal margin measured by rigid endoscopy ≤15 cm from the anal verge, were submitted to preoperative concomitant boost radiotherapy associated to chemotherapy when distant metastasis could be excluded.
Pre-treatment evaluation included patients’ history, physical examination, digital rectal examination, complete blood count, platelet count, renal and liver function, endorectal ultrasound, colonoscopy, and total body computed tomography (CT) scan. The performance status according to the Eastern Cooperative Oncology Group was 0–1 for all patients. Patients were staged according to the American Joint Committee for Cancer Staging System (AJCC) 2010 [13].
Radiation therapy (RT) was planned using a three-dimensional conformal technique based on the pre-treatment planning CT using a belly board device. The clinical target volume 1 (CTV1) included the entire macroscopic tumor, the mesorectum, internal iliac, and presacral lymph nodes. A margin of 1 cm in all directions was added to the CTV1 to generate the planning target volume 1 (PTV1), taking into account the individual organ motion and setup margin. The CTV2 included the macroscopic tumor and the corresponding mesorectum; the PTV2 was generated by adding a margin of 0.5–1 cm in the lateral–lateral and anterior–posterior direction, and a margin of 1.5–2 cm in superior–inferior direction. The total prescribed dose to the PTV1 was 45 Gy delivered in 25 fractions of 1.8 Gy per day for 5 days a week over 5 weeks. The concomitant boost prescribed dose to the PTV2 was 10 Gy in 10 fractions of 1 Gy twice a week over 5 weeks. RT was delivered by a linear accelerator using high-energy photons (15 MV).
All patients received concurrent chemotherapy consisting in oral administration of capecitabine at 825 mg m2 twice daily for 5 days a week. All underwent restaging MRI and a TME operation about 6–8 and 8–10 weeks after CRT completion, respectively. The possibility for sphincter-preserving surgery (anterior rectal resection) was considered based on the findings of restaging MRI and taking into account the local response and the distance from the anal verge.
MR imaging techniques
MRI was performed using a 1.5-Tesla scanner (Sonata Siemens, Erlangen, Germany), with a phased-array body surface coil.
To reduce intestinal motion artifacts, intramuscular injection of N-butyl scopolamine bromide, 20 mg, was administered, if not contraindicated, before MRI.
The standard imaging protocol consisted of axial Fast low-angle shot (FLASH) 2D T1 and axial Turbo Spin-Echo (TSE) T2-weighted images of the whole pelvis.
Thin-section (3-mm) Sagittal T2-weighted TSE images covering the entire length of the rectum were acquired and used to plan axial scans. Thin-section (3-mm) axial images through the rectal cancer were performed perpendicular to the long axis of the tumor.
For low rectal tumors, further oblique coronal T2-weighted TSE images along the long axis of the anal canal were acquired.
DWI was acquired using three increasing b values (50–400 to 800 s/mm2) with echo planar imaging sequence with respiratory triggering. The ADC maps were derived automatically on a voxel- by-voxel basis by using commercially available software (Syngo; Siemens Medical Solutions).
The parameters of the MR sequences are detailed in Table 1.
Image analysis
The terms ymrT (T stage on MRI images obtained after CRT), mrTRG (tumor regression grade on MRI images obtained after CRT), ypT (T stage on post-treatment histopathological examination of the resection specimen), and TRG (tumor regression grade on post-treatment histopathological examination of the resection specimen) are used to describe the data [14].
Two sets of MR images before and after CRT were prospectively read by two radiologists with experience in rectal cancer imaging, who evaluated ymrT, mrTRG, ADC values, and volume reduction of the lesion.
T stage post-CRT MRI
YmrT of the rectal cancer was defined on MRI performed after CRT, according to the TNM classification (AJCC 2010) [13]. Comparison was made with the pre-treatment images. Two separate sets of images were analyzed: T2-weighted images and subsequently combined T2-weighted images and DWI.
YmrT was compared with the pathological staging ypT performed on surgical specimens.
MrTRG was based on principles similar to the pathological TRG system, originally described by Mandard [15].
T2-weighted images, DWI, and ADC maps in the post-CRT MRI were reviewed to determine the degree of tumor replacement by post-actinic fibrous tissue. Table 2 illustrates the MR tumor regression grade system.
Good MRI tumor regression grade was defined as grades 1 and 2 with poor defined as stages 3, 4, and 5. This binary division was chosen according to pathologist and prior studies [16].
MrTRG was compared with pathological TRG defined on histologic examination of the surgical specimen.
ADC values
Processed ADC measurements of tumor were analyzed on the pre- and post-CRT MR images.
After identification of a lesion on T2-weighted images and DWI, three non-overlapping regions of interest (ROI) were manually drawn on the ADC map. The average ADC was calculated for each tumor. When there was no residual tumor visible, the ROI was drawn on the normal residual rectum in the same area as that initially used in the pre-CRT MR imaging. The ADC changes were calculated using the formula: ΔADC = [post-CRT ADC (mm2/s)—pre-CRT ADC (mm2/s)] [17].
ADC values before and after neoadjuvant CRT and ΔADC were compared with TRG and ypT.
Volume reduction
The tumor volume before and after neoadjuvant CRT was calculated on DWI using T2-weighted images and ADC map as reference point. Lesion areas on each section were defined as regions of altered high signal intensity on DWI different from those of normal adjacent rectal wall. The cross-sectional area was acquired by manually tracing the lesion.
The lesion’s volumes were calculated automatically by using commercially available software (OSIRIX 6.5). To assess the tumor response to neoadjuvant CRT on MRI, the percentage of tumor volume reduction was calculated using the formula [18]:
Tumor volume reduction (%) = [pre-CRT volume (cm3) − post-CRT volume (cm3)] × 100/pre-CRT volume (cm3)].
In patients without residual tumor visible at the end of neoadjuvant CRT, the tumor volume and the percentage of tumor volume reduction were 0% and 100% respectively.
Analysis of the correlation between volume reduction and the TRG and between volume reduction and ypT was made.
Histopathological examination
Pathologic response was evaluated on resected specimens by a dedicated gastro-intestinal pathologist. Specimens were inked on the radial margin before sampling.
TRG was scored according to Mandard’s score system [15]: TRG1 (complete response), absence of residual cancer and fibrosis extending through the wall; TRG2, presence of rare residual cancer cells scattered through the fibrosis; TRG3, increased number of residual cancer cells, but still predominating fibrosis; TRG4 (minimal response), residual cancer outgrowing fibrosis; and TRG5 (no response), absence of regression changes. Neoplastic lesions were sampled and included entirely and the score was assigned after evaluation of all blocks. Distance between tumor maximum infiltration and radial inked margin was measured on slides under microscope visualization. ypT was assigned according to TNM staging system (AJCC 2010) [13].
Additional parameters not analyzed in this study were evaluated in the histopathological report: histological type, margin of resection, lymph nodes, and lymphovascular invasion.
Assessment of tumor response
To assess the tumor response to neoadjuvant CRT, each patient was categorized as a responder or non-responder according to the histopathological standard of reference.
For the parameter ypT, a responder was defined when a cancer staged as T3 or T4 on pre-CRT MR imaging converted to T2 or a lower stage on resected specimens (downstage group). A non-responder was defined as when a T3 or T4 cancer on pre-CRT MR imaging was stable to T3 or T4 (non-downstage group) [19].
For the parameter TRG, patients with TRG 1–2 scores were considered as responders to neoadjuvant treatment while patients with TRG 3–5 were classified as non-responders [16].
Statistical analysis
All statistical analyses were performed using software IBM-SPSS 22.0.
Continuous variables were analyzed using descriptive statistics. Student’s t test, with calculation of the relative p value, was used to compare mean ADCpre-CRT, ADCpost-CRT, ΔADC value, and volume reduction between responders and non-responders as well as between patients with and without downstaging. A p value <0.05 was considered statistically significant.
The diagnostic performance of these potential markers of response was evaluated by receiver operating characteristic curve (ROC). The area under the ROC curve (A z) was calculated. The A z was considered indicative of diagnostic accuracy. We used 95% confidence intervals to express the statistical precision of the results. Optimal cut-off values, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated.
Spearman correlation analysis was also used to assess the correlation between ymrT and ypT and mrTRG and TRG. A p value <0.05 was considered statistically significant.
Results
According to MRI pre-CRT, the T stage subtypes were as follows: 30 (88.2%) of 34 tumors were T3 and 4/34 (11.8%) tumors were classified as T4.
Comparing T stage on MRI pre-CRT with the pathological staging ypT performed on surgical specimens, 18 patients were classified as downstaged (Fig. 1) and 16 patients as non-downstaged.
A 55-year-old man with locally advanced rectal cancer, T-downstaging, and partial response after CRT (TRG3). (A) Pre-CRT axial T2-weighted MR image shows a mrT3 rectal cancer, hyperintense on DWI (B). (C) Post-CRT axial T2-weighted image demonstrates low signal intensity fibrosis with residual tumoral tissue within the muscular wall clearly depicted on DWI (D) and corresponding ADC map (E) (mean ADC 1.18 × 10−3 mm2/s) (mr-T2; mr-TRG3). (F) Residual adenocarcinoma glands are present in muscularis propria (ypT2) (arrow) surrounded by fibrosis that partly extends in the adipose perirectal tissue. Before neoadjuvant treatment the neoplasm extended to adipose tissue. CRT, chemoradiotherapy; TRG, tumor regression grade; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient
According to histological analysis of the surgical specimen, the TRG subtypes were as follows: 7 (20.5%) of 34 tumors were TRG1 (Fig. 2), 4/34 (11.7%) tumors were classified as TRG2, 11/34 (32.3%) tumors were classified as TRG3 and 12/34 (35.2%) tumors were classified as TRG4 (Fig. 3). No tumors were classified as TRG5.
MR images of a 68-year-old man with a complete response after CRT (TRG1). (A) Pre-CRT axial T2-weighted image shows a lesion of the distal rectum, that spread through the rectal wall into the perirectal fat (T3). (B) Pre-CRT DWI (b = 800 s/mm2) demonstrates a focal high signal intensity area in the corresponding tumor. (C) Post-CRT axial T2-weighted image shows rectal wall thickening with low signal intensity tissue as fibrotic change; no residual tumor mass is clearly detectable. (D) No residual high signal intensity in the primary tumor location is shown in post-CRT DWI (b = 800 s/mm2) (mr-T0; mr-TRG1). (E) Post-CRT ADC map at the same level: the ADC value was 1.25 × 10−3 s/mm2. (F) At the histopatholocial exam no carcinoma glands were observed in the context of fibrosis. An acellular mucus containing lake is evident in the submucosa (arrow). (Hematoxylin/EOSIN staining 5X). CRT, chemoradiotherapy; TRG, tumor regression Grade; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient
MR images of a 74 years old man with no response to CRT (TRG4). (A) Pre-CRT T2-weighted axial image shows neoplastic tissue in the middle rectum with diffuse infiltration of the rectal wall and mesorectal fat (T3). (B) Pre-CRT DWI (b = 800 s/mm2) image reveals high signal within the tumor consistent with restricted diffusion. (C) Post-CRT T2-weighted axial image shows intermediate signal intensity tissue that spread through the rectal wall with persistent high signal intensity area on DWI (b = 800 s/mm2) (D) (MR-T3; MR-TRG4). (E) The ADC value is 1.03 × 10−3 s/mm2 on ADC map. (F) Histopathological exam on surgical specimen demonstrates extensive infiltration of carcinoma glands in the rectal wall and the perirectal adipose tissue (arrow) (Hematoxylin/Eosin staining 5X). CRT, chemoradiotherapy; TRG, tumor regression Grade; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient
Therefore histopathologic responders and non-responders included 11 patients (32.3%) and 23 (67.7%) patients, respectively.
To investigate the correlation between ymrT stage and ypT and between mrTRG and TRG, the Spearman’s rank test was used, considering statistically significant when the p value was <0.05.
The correlation between ypT and ymrT defined in T2-weighted images was validated with a ρ = 0.457 and a p value of 0.007, indicative of direct linear correlation. A more significant relationship was found between ypT and ymrT defined in the combined T2-weighted images and DWI with a ρ = 0.611 and a p value = 0.001.
The correlation between MR-TRG and TRG was validated and significant with a ρ = 0.700 and a p value <0.001, indicative of direct linear correlation.
In all patients of this study, a downsizing of the rectal cancer was observed after neoadjuvant CRT.
The mean percentage of tumor volume reduction was 87% ± 15.8% in responders and 73% ± 20.7% in non-responders. There was no significant difference in downsizing in the responders and non-responders (p = 0.07).
The mean percentage of tumor volume reduction after neoadjuvant CRT was 78.2% ± 14.8 in the downstage group and 77.9% ± 24.4% in non-downstage group. There were no significant differences between the two groups of patient (p = 0.970).
The downsizing of tumor showed no accuracy in prediction of the treatment response (A z = 0.300) and downstaging (A z = 0.438).
Before neoadjuvant CRT, the mean ADC values (0.935 ± 0.158 × 10−3mm2/s) of responders group were higher than those of non-responders group (0.870 ± 0.097 × 10−3 mm2/s), although differences were not statistically significant (p = 0.151).
After neoadjuvant CRT, the mean ADC values of responders and non-responders was 1.429 ± 0.268 × 10−3 and 1.164 ± 0.147 × 10−3 mm2/s, respectively. The mean post-CRT ADC values of responders were significantly higher than those of non-responders (p = 0.001).
When a post-CRT ADC value of 1.23 × 10−3 mm2/s was used as the cut-off value for discriminating between responders and non-responders, the following diagnostic predictive values were observed: sensitivity, 90.9%; specificity, 82.6%; PPV, 90%; and NPV, 82%. The prediction of the favorable responders showed good accuracy (Az = 0.854).
The mean ΔADC was 0.493 ± 0.299 × 10−3 mm2/s in responders and 0.293 ± 0.135 × 10−3 mm2/s in non-responders. The ΔADC was significantly higher in responders than in non-responders (P = 0.01).
When a ΔADC value of 0.375 × 10−3 mm2/s was used as the cut-off value to evaluate the treatment response, the following diagnostic predictive values were observed: sensitivity, 72%; specificity, 82%; PPV, 72%; and NPV, 82%. The prediction of the favorable responders showed fair accuracy (A z = 0.743).
The mean pre-CRT ADC values (0.920 ± 0.143 × 10−3 mm2/s) of downstage group were higher than those of non-downstage group (0.898 ± 0.076 × 10−3 mm2/s), although the differences were not statistically significant (p = 0.268).
The mean post-CRT ADC values were 1.338 ± 0.237 × 10−3 mm2/s in downstage group and 1.150 ± 0.175 × 10−3 mm2/s in non-downstage group. The post-CRT ADC values were significantly higher in downstage group (p = 0.01).
A post-CRT ADC cut-off value of 1.160 × 10−3 mm2/s allowed to differentiate the two groups of patient with a sensitivity of 89%, a specificity of 57%, a PPV of 88%, and NPV of 56%.
The mean ΔADC in downstage group (0.407 ± 0.253 × 10−3 mm2/s) was higher than in non-downstage (0.302 ± 0.165 × 10−3 mm2/s), although the differences were not statistically significant (p = 0.168).
Tables 3 and 4 summarize ADC values and percentage of volume reduction in responders/non-responders and downstaged/non-downstaged.
Discussion
Neoadjuvant CRT is the actual standard of care in the treatment of locally advanced rectal cancer [1]. The benefits of neoadjuvant CRT have been well documented with improvement in disease local control, and include tumor regression and downstaging. At present, the assessment of tumor response after chemoradiation is fundamentally determined by clinical restaging based on digital rectal examination and rectoscopy, although MRI is acquiring an important role.
Accurate restaging following CRT is crucial to indicate the optimal surgical strategy, such as planning of sphincter-saving procedure for low-lying tumors or local excision in lesions confined to the rectal wall [20]. Furthermore, transanal endoscopic microsurgery has been adopted in patients who otherwise would have undergone abdominoperineal resection [21].
Actually, there is no agreement whether surgery could be avoided in patient with complete response to RT. A potential “wait-and-see” approach with careful follow-up in complete responders is still controversial, although interesting results have been presented [22].
Accurate imaging restaging is fundamental in the decision making process, involving sphincter preservation as well as local excision vs. a complex more radical TME procedure. Of course, other parameters should be taken into account, such as pre-CRT staging, anal sphincter function, patient age, and surgical risk.
In our study, the MRI re-evaluation of T stage and the mrTRG assessment showed a consistent relationship both with ypT and TRG histopathological parameters.
Furthermore, our results demonstrated that the relationship between ymrT defined in the combined T2WI and DWI and histopathology staging was stronger than ymrT defined in T2WI alone. High-resolution T2WI sequences represent the core of rectal cancer staging [23]. However, changes induced by RT on the tumoral tissue, such as fibrosis, cell swelling, and necrosis make the detection of residual viable tumor very difficult.
DWI has several potential benefits in the assessment of tumor localization and restaging [24].
Kim et al. reported that diagnostic accuracy in the evaluation of tumor response after CRT significantly increased when DWI was added to conventional MR imaging [9]. These findings are consistent with our results. A better accuracy was observed by the addition of DWI to standard MRI, especially when a fibrotic thickening of rectal wall was present. DWI in combination with T2WI could, therefore, lead to more accurate detection of viable tumor post-CRT.
Moreover, our data demonstrated a significant statistical correlation between mrTRG and TRG (p < 0.001 and ρ = 0.700). There are several TRG systems (e.g., those proposed by Dworak et al. [25], Mandard et al. [15], and Ryan et al. [26]), all based on the relative proportion of fibrosis and viable tumor in the resected specimen. In our study, TRG was scored according to Mandard’s system [15]. Applying similar evaluation criteria with MRI (Table 2), we showed that it is possible to assess tumor regression before surgery, by providing a promising tool to optimize treatment in patients with a good response to neoadjuvant treatment.
Tumor downsizing after neoadjuvant CRT was observed in all patients. Our results showed that the tumor volume decreases in responders as well as in non-responders and in downstage group as well as in non-downstage group. The difference in the tumor downsizing rate between the two groups was not statistically significant.
Many studies in literature have evaluated the relationship between MR tumor volume reduction on T2WI and tumor response to neoadjuvant treatment, showing conflicting results [27–30].
Kang et al. [28] found a significant correlation between complete response and tumor volume reduction rate (>75%), while Kim et al. and Genovesi et al. [29, 30] observed no significant difference between responders and non-responders.
In accordance with Kim et al. and Genovesi et al., the results of our study could not demonstrate a correlation between tumor volume reduction and response to treatment. The pathology examination of resected specimens demonstrated that a significant amount of cancer cells can be observed even in markedly reduced tumor mass.
DW-MRI was investigated as a potential tool to predict tumor response.
Previous studies have suggested that quantitative interpretation of the ADC can be used as a biomarker for treatment response in several tumors [31–33]. However, this matter is still controversial in rectal cancer [17, 30, 34].
The focus of this study was to determine the diagnostic performance of the pre- and post-CRT ADC values, and their absolute changes (ΔADC), to assess the response, by correlating histopathological parameters for response.
Our data demonstrated no difference in the pre-CRT distribution of ADC values between non-responders and responders, and between downstage group and non-downstage showing the inability of pre-treatment ADC to predict response to therapy. However, the literature shows conflicting results regarding the role of pre-CRT ADC as a predictor of therapeutic response in rectal cancer [17, 30, 34].
In our study, the post-CRT ADC values were higher in all patients compared with the pre-treatment ADC values. Furthermore, it is interesting that post-CRT ADC value and Δ-ADC in responders were significantly higher than in non-responder.
An explanation was proposed by a recent study [35]: oncologic treatment results in loss of cell membrane integrity, increased extracellular space, and therefore, an increase in water diffusion. Increased ADC has been histologically correlated with areas of necrotic tissue and reduced cell density with a variable degree of edema, fibrosis, and inflammation [10, 11, 31]. Therefore, a greater increase of the ADC may be related to response to therapy.
Our study showed that using the cut-off value of post-CRT ADC, the area under the curve was 0.854. Our best mean post-CRT ADC cut-off values were 1.23 × 10−3 mm2/s using ROC curve analysis. Therefore, according to our analysis, all those tumors that show a post-CRT ADC value >1.23 × 10−3 mm2/s have the potential to reveal a tumor response after CRT with a sensitivity of 90.9%, a specificity of 82.6%, a PPV of 90%, and a NPV of 82%.
One of the clinical applications of the cut-off ADC value of 1.23 × 10−3 mm2/s showing a good NPV in our study is that it can be used for proper selection of the non-responders in which TME should be performed.
The diagnostic performance of ADC cut-off values (range 1.2–1.4 × 10−3 s/mm2) in rectal cancer is reported ranging from 46% to 100% for sensitivity and from 56% to 84% for specificity and significant differences are also found for PPV (27–86%) [9, 34, 36]. Differences between the results in these previous studies are probably related to differences in the definition of the standard of reference, image analysis and ADC measurements adopted.
In most published studies, a variety of methods for ROI placement in quantitative analysis of DWI have been performed [9, 34, 37]. It remains unclear whether ROIs for ADC measurements should incorporate the entire tumor volume, or a single tumor slice or small tumor samples [38]. In our study, three non-overlapping ROI were manually drawn by two radiologists in consensus, on the ADC map. Probably, further studies are needed to standardize a method of quantitative analysis of DWI in order to find out a definitive clinical application of this technique.
In our experience, although pre-CRT ADC could not reliably be used as a tool to discriminate responder from non-responder, post-CRT ADC resulted to be correlated with tumor response.
The relationship between post-CRT ADC values and tumor response could allow to select patients for a less aggressive surgery or for “wait-and-see” approach. But, applying this criterion, there is a risk of selecting not only patients with complete response but also patients with post-CRT near-complete response. Kim et al. [34] showed a poor PPV of post-CRT ADC in the identification of complete responder, probably due to the presence of a considerable overlap of ADC values between patient with complete response and near-complete response. In fact, it seems difficult to obtain a precise assessment of the underlying histopathological findings at a microscopic level with DW imaging. Detection of microscopically small cluster of residual viable tumor cells remains challenging. Further studies are warranted to investigate this issue.
Our study found that Δ ADC was significantly higher in responders than in non-responders. This finding suggests a change from higher cellularity on pre-CRT tissue to lower cellularity and higher necrosis on post-CRT tissue. When a ΔADC of 0.375 × 10−3 mm2/s was used as the cut-off value for discriminating between the responders and non-responders, the sensitivity was 72% and specificity was 82%. Because diagnostic performance of post-CRT ADC (A z = 0.854) in the evaluation of the favorable responders resulted to be significantly higher than that of ΔADC (A z = 0.743), we considered the mean post-CRT ADC as a more reliable predictor of tumor response.
Another promising aspect of our study is that post-CRT ADC value was significantly higher also in downstage group. Tumor downstaging after preoperative CRT is an important prognostic factor in tumor local recurrence rate and 5-year survival rate for patients with primary rectal neoplasm [2]. Therefore, post-CRT ADC values may be used as a tool in the assessment of the prognosis and outcome of the patient.
A wait-and-see approach or local excision in patients with a good response after chemoradiation is, at present, still debatable [39, 40]. In our study, we did not evaluate the complete responders; therefore we aimed to provide a tool to identify those patients with a good response to treatment, eligible for a less invasive surgery. Our goal was to assess whether MR imaging can be beneficial in this regard. We particularly evaluated the potential of DW images. DWI is a noninvasive technique; it can easily be added to the standard MRI protocol and provides a functional imaging.
We have showed that the quantitative analysis of DWI with ADC values allows a differentiation between responders and non-responders. Furthermore, this quantitative analysis of DWI allows us to evaluate the response to CRT treatment in terms of downstage of the lesion, an important factor in the prognosis of the patient.
Our results suggest that MRI with DWI can evaluate the restaging and treatment response of the colorectal cancer and therefore can significantly sustain clinical tools (digital examination, endoscopy, and biopsy) in a more precise selection of patients eligible to undergo minimally invasive treatments.
This study shows some limitations. First, it involved a small sample, even if the group of rectal cancer, evaluated in a single center, was homogeneous. Second, the identification of tumor downstaging was based on a comparison between initial mrT staging and pathological staging, which could have induced an inadvertent bias. Although MRI is known to be a reliable modality for rectal cancer staging, it may underestimate or overestimate the tumor.
Third, a limitation of T stage with MRI it is to distinguish true mesorectal tumor invasion from desmoplastic and inflammatory reaction; therefore T2 rectal cancers with inflammation may have been included in this study.
In conclusion, our study highlights the significant role of MRI with DWI in the restaging and in the evaluation of post-CRT response of locally advanced rectal cancer. Furthermore, quantitative analysis of DWI through ADC map may result in a promising noninvasive tool to evaluate the response to therapy. Post-CRT ADC values showed the highest diagnostic performance compared to ΔADC. Our results show that MRI with DWI may potentially help the surgeon in a more precise selection of patients who are eligible to undergo less invasive treatments. Correlation with oncologic outcomes balanced with patient life expectancy is necessary to further validate any local treatment protocol.
References
Valentini V, Aristei C, Glimelius B, et al. (2009) Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol 92:148–163
Janjan NA, Crane C, Feig BW, et al. (2001) Improved overall survival among responders to preoperative chemoradiation therapy for locally advanced rectal cancer. Am J Clin Oncol 24:107–112
Nesbakken A, Nygaard K, Westerheim O, Mala T, Lunde OC (2002) Local recurrence afte rmesorectal excision for rectal cancer. Eur J Surg Oncol 28:126–134
Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. (2001) Preoperative radio-therapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 346:638–646
van Gijn W, Marijnen CA, Nagtegaal ID, et al. (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582
Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 15(88):822–828
Iannicelli E, Di Renzo S, Ferri M, et al. (2014) Accuracy of high-resolution MRI with lumen distention in rectal cancer staging and circumferential margin involvement prediction. Korean J Radiol 15:37–44
Kim DJ, Kim JH, Lim JS, et al. (2010) Restaging of rectal cancer with MR imaging after concurrent chemotherapy and radiation therapy. Radiographics 30(2):503–516
Kim SH, Lee JM, Hong SH, et al. (2009) Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 253:116–125
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Curvo-Semedo L, Lambregts DM, Maas M, et al. (2012) Diffusion-weighted MRI in rectal cancer: Apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 35:1365–1371
Nasu K, Kuroki Y, Minami M (2012) Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region: comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn J Radiol 30(2):120–127
Byrd, Compton CC, Fritz AG, et al. (2010) AJCC cancer staging manual, vol. 649. New York: Springer
Moran B, Brown G, Cunningham D, et al. (2008) Clarifying the TNM staging of rectal cancer in the context of modern imaging and neo-adjuvant treatment: ‘y’‘u’ and ‘p’ need ‘mr’ and ‘ct’. Colorectal Dis 10:242–243
Mandard AM, Dalibard F, Mandard JC, et al. (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680–2686
Vecchio FB, Valentini V, Minsky B, et al. (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 62:752–760
Jung SH, Heo SH, Kim JW, et al. (2012) Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: Diffusion-weighted 3 tesla MR imaging. J Magn Reson Imaging 35:110–116
Jemal A, Siegel R, Ward E, et al. (2007) Cancer statistics 2007. CA Cancer J Clin 57:43–66
Barbaro B, Fiorucci C, Tebala C, et al. (2009) Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 250:730–739
Barbaro B, Vitale R, Leccisotti L, et al. (2010) Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics 30:699–716
Buess G (1991) Transanal microsurgery [in German]. Langenbecks Arch Chir Suppl Kongressbd 1991:441–447
Habr-Gama A, Perez RO, Nadalin W, et al. (2004) Operative versus nonoperative treatment for stage distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240:711–717
Brown G, Radcliffe AG, Newcombe RG, et al. (2003) Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 90:355–364
Patel UB, Blomqvist LK, Taylor F, et al. (2012) MRI after treatment of locally advanced rectal cancer: how to report tumor response—the MERCURY experience. AJR Am J Roentgenol. 199:W486–W495
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23
Ryan R, Gibbons D, Hyland JM, et al. (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47:141–146
Torkzad MR, Lindholm J, Martling A, et al. (2007) MRI after preoperative radiotherapy for rectal cancer; correlation with histopathology and the role of volumetry. Eur Radiol. 17:1566–1573
Kang H, Kim YC, Kim H, et al. (2010) Tumor volume changes assessed by three-dimensional magnetic resonance volumetry in rectal cancer patients after preoperative chemoradiation: the impact of the volume reduction ratio on the prediction of pathologic complete response. Int J Radiat Oncol Biol Phys. 15(76):1018–1025
Kim NK, Baik SH, Min BS, et al. (2007) A comparative study of volumetric analysis, histopathologic downstaging, and tumor regression grade in evaluating tumor response in locally advanced rectal cancer following preoperative chemoradiation. Int J Radiat Oncol Biol Phys 67:204–210
Genovesi D, Filippone A, Cefaro GA, et al. (2013) Diffusion-weighted magnetic resonance for prediction of response after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: preliminary results of a monoinstitutional prospective study. Eur J Surg Oncol 39:1071–1078
Patterson DM, Padhani AR, Collins DJ (2008) Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol 5:220–233
Koh DM, Scurr E, Collins D, et al. (2007) Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol 188:1001–1008
Niwa T, Ueno M, Ohkawa S, et al. (2009) Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy. Br J Radiol 82:28–34
Kim SH, Lee YJ, Lee JM, Han JK, Choi BI (2011) Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 21:987–995
Moffat BA, Hall DE, Stojanovska J, et al. (2004) Diffusion imaging for evaluation of tumor therapies in preclinical animal models. MAGMA 17:249–259
Semedo LC, Lambregts DM, Maas M, et al. (2011) Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260:734–743
Sun YS, Zhang XP, Tang L, et al. (2010) Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254:170–178
Lambregts D, Beets G, Maas M, et al. (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Habr-Gama A, Perez RO, Proscurshim I, et al. (2006) Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 10:1319–1328
Lezoche G, Baldarelli M, Guerrieri M, et al. (2008) A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc 22:352–358
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Iannicelli, E., Di Pietropaolo, M., Pilozzi, E. et al. Value of diffusion-weighted MRI and apparent diffusion coefficient measurements for predicting the response of locally advanced rectal cancer to neoadjuvant chemoradiotherapy. Abdom Radiol 41, 1906–1917 (2016). https://doi.org/10.1007/s00261-016-0805-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0805-9