Abstract
Purpose
To determine the computed tomographic (CT) findings of acute pelvic inflammatory disease (PID).
Methods
This retrospective, single-institution case–control study was approved by our institutional review board, and the informed consent was waived owing to the retrospective nature of the study. CT images of 32 women with clinically proven acute PID and 32 control subjects with other conditions of similar presentation were retrospectively reviewed. Analysis of CT findings included hepatic capsular enhancement, pelvic fat haziness, complicated ascites, uterine serosal enhancement, tubal thickening, endometritis, and oophoritis. Comparison of CT findings was performed with the Chi square test or the Fisher exact test and logistic regression analysis was used to determine significant CT findings in predicting PID.
Results
The CT findings that showed a statistically significant difference were hepatic capsular enhancement on late arterial phase (p = 0.003), pelvic fat haziness (p = 0.045), and tubal thickening (p = 0.001). Subsequent multivariate logistic regression analysis revealed that the presence of hepatic capsular enhancement on late arterial phase and tubal thickening were significant predictors of PID (hepatic capsular enhancement on late arterial phase, p = 0.015, odds ratio [OR] = 4.8; tubal thickening, p = 0.005, OR = 10.5).
Conclusion
Diagnostic morphological CT findings in women with clinically proven PID and acute abdominal pain include hepatic capsular enhancement on late arterial phase and tubal thickening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pelvic inflammatory disease (PID) refers to ascending infection from the endocervix causing endometritis, salphingitis, oophoritis, tubo-ovarian abscess, and pelvic peritonitis. PID is the most frequent gynecologic cause of emergency room visits, approaching 350,000 per year in the United States [1] and thus, PID should be considered as a potential diagnosis when evaluating premenopausal women who present to emergency rooms with non-traumatic acute abdominal pain. Laparoscopy is the gold standard for the diagnosis of PID but its routine use is not justified on the basis of associated morbidity, cost, and the potential difficulty in demonstrating mild intra-tubal inflammation or endometritis [2, 3]. Because the clinical diagnosis of PID is imprecise due to the nonspecific nature of the presenting signs and symptoms [4], the women with PID often undergo CT scan to establish differential diagnosis including appendicitis, ovarian torsion, urinary tract infection, and constipation. In the absence of an established cause other than PID, suggestion of PID gives a great impact on the management of women with PID because PID is associated with significant morbidity, including infertility, ectopic pregnancy, tubo-ovarian abscess, pelvic adhesion, dyspareunia, and chronic pelvic pain [5] and early diagnosis of PID can be helpful in the prevention of those sequelae. Therefore, our study was designed to determine CT findings helpful for diagnosis of acute PID in women of reproductive age who present to emergency room with non-traumatic acute abdominal pain.
Materials and methods
Study population
This retrospective, single-institution case–control study was approved by our institutional review board, and the informed consent was waived owing to the retrospective nature of the study.
A total of 418 consecutive women of reproductive age, who (1) visited our emergency department due to non-traumatic acute abdominal pain (less than 1 week in duration) between April 2012 and March 2013, (2) underwent abdominopelvic CT examinations, and (3) returned for clinical follow-up, were enrolled in this retrospective analysis. Of these, 146 women underwent gynecologic evaluation under the clinical suspicion of PID and clinical diagnosis of PID was made in 32 women (mean age 31.3; age range 16–45 years). 114 women were excluded from the PID group with the following reasons: no record of bimanual pelvic examinations in 28 women, no cervical motion tenderness in 76 women, normal laboratory findings in 6 women, inappropriate CT protocols in 3 women, and recent termination of pregnancy in 1 woman. The criteria for diagnosis of PID included cervical motion tenderness/adnexal tenderness plus one or more of the followings: an oral temperature greater than 38.3 °C, abnormal cervical or vaginal mucopurulent discharge, elevated erythrocyte sedimentation rate (>20 mm/h), elevated C-reactive protein (>0.3 mg/dL), and laboratory documentation of a cervical infection with Neisseria gonorrhoeae or Chlamydia trachomatis [6–8].
For this retrospective case–control study, 32 control subjects of similar clinical and age characteristics were selected in the following way: (1) A list of 418 women enrolled in the study was generated with them sorted according to their age. (2) All patients suspected to have PID were then excluded from the list. (3) For each PID case, one of the researchers (M.H.L) manually selected the first woman with a similar age as a control subject. (4) The researcher then checked whether the diagnosis other than PID was made at the follow-up and, if the diagnosis other than PID was not made, the researcher selected the next suitable woman from the list. The same things were repeated to get 32 control subjects. Final diagnoses for selected 32 control subjects were as follows: acute appendicitis (n = 12), acute pyelonephritis (n = 5), acute gastroenteritis (n = 5), ruptured ovarian cyst (n = 3), ureter stone (n = 2), acute pancreatitis (n = 1), acute cholecystitis (n = 1), diverticulitis (n = 1), corpus luteal cyst (n = 1), and ovarian torsion (n = 1). The diagnosis was made by surgery in all women with acute appendicitis, acute cholecystitis, and ovarian torsion and in one of three women with ruptured ovarian cyst. Remaining 17 women including 2 women with ruptured ovarian cyst were diagnosed through a clinical follow-up.
Acquisition of CT images
All CT images were obtained in the emergency room with a 16-detector row CT scanner (LightSpeed; GE Healthcare, Milwaukee, WIs) by using the following parameters: detector configuration, 16 * 1.25 mm; tube voltage, 120 kVp; noise index, 12.35 with automatic exposure control (smart mA, GE Healthcare, Milwaukee, WIs); gantry rotation period, 0.6 s; pitch factor, 1.375; table speed, 27.5 mm per rotation, reconstructed section width, 3.75 mm; and reconstructed section interval, 3.75 mm. No oral contrast material was ingested for gastrointestinal tract opacification. Our standardized three-phase CT scan protocol for acute abdominal pain consisted of unenhanced and dual-phase (late arterial phase and portal venous phase) contrast material-enhanced CT images. After unenhanced images were obtained from the diaphragmatic dome to the symphysis pubis, nonionic iodine contrast material containing 350 mg of iodine per milliliter (iohexol, Omnihexol 350; Korea United Pharm Co, Seoul, Korea) was intravenously administered in a volume of 1.5 mL/kg by using a power injector (Optivantage DH; Mallinckrodt Imaging Solutions, Hazelwood, Mo) at a rate of 2–3 mL/s. The scanning delay for late arterial phase was 40 s after initiation of contrast material injection and 90 s for portal venous phase. The images of late arterial phase were obtained from the diaphragmatic dome to the iliac crest and those of portal venous phase were obtained from the diaphragmatic dome to the symphysis pubis.
Image analysis
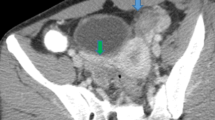
CT images were reviewed retrospectively by consensus by two genitourinary radiologists (M.H.M., with 12 years of experience; C.K.S., with 15 years of experience) with a picture archiving and communication system (PACS) software (Maroview 5.4, Infinitt, Seoul, Korea). The categories for image analysis were as follows: hepatic capsular enhancement, pelvic fat haziness, complicated ascites, uterine serosal enhancement, tubal thickening, endometritis, and oophoritis. Hepatic capsular enhancement was defined as intense enhancement along the surface of the liver (Fig. 1). This was distinguished from beam hardening artifact by the adjacent rib and linear hyperattenuated hepatic capsules due to diffuse fatty liver. The presence of hepatic capsular enhancement was determined on late arterial and portal venous phase scans. Pelvic fat haziness refers to increased attenuation and stranding of the pelvic fat, compared to the retroperitoneal fat (Fig. 2). Complicated ascites was defined as ascites with enhancement of the adjacent peritoneal membrane and uterine serosal enhancement was defined as linear enhancement along the uterine serosa. Tubal thickening was defined as perceptible tubal structure with an axial diameter of above 5 mm in the adnexa (Fig. 3) [9] and the presence of tubo-ovarian abscess/complex was also classified as having tubal thickening (Fig. 4). Endometritis was defined as abnormal endometrial enhancement with fluid collection in the endometrial cavity and oophoritis was defined as an enlarged ovary (short axis diameter > 3 cm) with polycystic appearance [9, 10].
A 38-year-old woman with abdominal pain. Portal venous axial (A) and coronal (B) scan shows pelvic fat haziness (arrows) in the pelvic cavity. Pelvic fat haziness is seen as increased attenuation and stranding of the pelvic fat, compared to the attenuation and stranding of the retroperitoneal fat (curved arrows in A).
A 21-year-old woman with right flank pain. Portal venous axial scan of the pelvic cavity (A) shows tubular structure arising from the left uterine cornus (arrowheads in A). On image (B) obtained cranial to A, the cross section of the tubular structure appears as ovoid mass (arrowheads in B) with the left ovary (curved arrow in B) separated from the tubular structure.
A 30-year-old woman with abdominal pain. Contrast-enhanced CT scan (A) shows thick-walled, multiloculated cystic mass (arrowheads in A) in the right adnexa. The mass has thick mural enhancement with multilayered appearance. On the image (B) obtained caudal to A, pelvic fat haziness is also seen (curved arrow in B).
Statistical analysis
All statistical analyses were performed with IBM SPSS version 20 (IBM Software Inc.). A p value of less than 0.05 was considered to indicate a statistically significant difference. Comparison of CT findings between the PID group and the control group was performed with the Chi square test or the Fisher exact test. Univariable logistic regression analysis was used to determine the significance of each CT finding in predicting PID by odds ratio [OR] evaluation. Multivariable stepwise logistic regression analysis with backward elimination was used to determine significant CT findings in predicting PID by adjusted OR evaluation. Variables with a p value of <0.05 according to the univariable analysis were used as input variables for multivariable stepwise logistic regression analysis and the removal of variables was based on likelihood ratio statistics with a probability of 0.10.
Results
Table 1 summarizes the results of the CT findings for the PID group and the control group. The CT findings that showed a statistically significant difference between the PID group and the control group were hepatic capsular enhancement on late arterial phase (p = 0.003), pelvic fat haziness (p = 0.045), and tubal thickening (p = 0.001). However, pelvic fat haziness was not a significant predictor of PID in univariate logistic regression analysis and thus pelvic fat haziness was not used as input variables for multivariate stepwise logistic regression analysis. Multivariate logistic regression analysis revealed that the presence of hepatic capsular enhancement on late arterial phase and tubal thickening were significant predictors of PID (hepatic capsular enhancement on late arterial phase, p = 0.015, OR = 4.8; tubal thickening, p = 0.005, OR = 10.5). Table 2 shows the results of univariate and multivariate logistic regression analysis of CT findings. If the CT findings were considered to be suggestive of PID when CT showed either hepatic capsular enhancement on late arterial phase or tubal thickening, we could achieve a sensitivity, specificity, and accuracy of 71.9%, 81.3%, and 76.6%, respectively.
Discussion
A variety of CT findings have been described as a predictor of PID, including endometrial enhancement with fluid collection suggestive of endometritis, tubal thickening suggestive of salpingitis, pyosalpinx, or tubo-ovarian abscess, polycystic appearance of the ovary suggestive of oophoritis, and pelvic fat haziness/complicated free fluid collection suggestive of pelvic peritonitis. Although those CT findings have been documented frequently in the literature dealing with PID [10, 11], only a few attempts have been made to test their diagnostic performance for diagnosis of PID [9].
In the present study, tubal thickening, pelvic fat haziness, and hepatic capsular enhancement on late arterial phase showed a statistically significant difference between the PID group and the control group (p < 0.05). However, endometrial enhancement with fluid collection, polycystic appearance of the ovary, and complicated fluid collection were not reliable CT findings of acute PID. These results were not surprising and actually expected, because CT plays little role in the diagnosis of endometritis or oophoritis [9, 12, 13] and complicated free fluid collection can be also frequently seen in women with pathologies other than PID such as ovarian cyst rupture and acute appendicitis [9].
Given that pelvic peritonitis is major pathologic change of PID, pelvic fat haziness is expected to be one of helpful CT imaging findings for diagnosis of PID and, in the present study, pelvic fat haziness was seen in 21 of 32 women with PID (65.6%). The result is similar to the previous report by Jung et al. [9] who showed that pelvic fat haziness was seen in 41.6%–60.4% of the women with acute PID. However, in routine clinical practice, diagnostic performance of pelvic fat haziness is not as satisfactory as expected. We assume that this might be due to the fact that any other gastrointestinal, genitourinary, and gynecologic conditions could be associated with pelvic peritonitis. This is the reason why pelvic fat haziness (OR = 2.8) was not as strong as tubal thickening (OR = 11.7) or perihepatitis (OR = 5.4) as predictor of acute PID.
The intraperitoneal spread of PID can cause perihepatitis that is inflammation of the peritoneal covering of the liver. In women with PID, perihepatitis associated with right upper abdominal pain is known as Fitz–Hugh–Curtis syndrome (FHCS) [14–17]. In the present study, hepatic capsular enhancement implying perihepatitis was found in 16 of 32 women with PID (50%). This relatively high rate of perihepatitis is beyond the reported incidence of FHCS from 5% to 37% [18–20] and furthermore, only 5 of 16 women (31%) with hepatic capsular enhancement complained of right upper abdominal pain suggestive of FHCS. The results are in agreement with the study by Kim et al. [21], in which hepatic capsular enhancement was seen in 59% of women with PID and 27 of 55 women (49%) with hepatic capsular enhancement had right upper abdominal pain. These results suggest that hepatic capsular enhancement on late arterial phase can be one of the useful CT imaging findings for diagnosis of acute PID, regardless of association with FHCS.
Our study has several limitations. First, we used clinical criteria as standard of reference for the diagnosis of PID. Although laparoscopy is the gold standard for the diagnosis of PID, its routine clinical use is not justified on the basis of associated morbidity, cost, and the potential difficulty in identifying mild intra-tubal inflammation or endometritis. Therefore, we think that this limitation is unavoidable when conducting retrospective observational study for women with acute PID. Second, only women who underwent CT examinations were enrolled in this retrospective study. This might result in a selection bias because more symptomatic women or more clinically difficult cases were more likely to be imaged with CT. The nature of this retrospective case–control study may also have led to a selection bias, with small number of study population. Further prospective studies with a larger number of cases are needed to assess the value of CT imaging findings in predicting PID in women of reproductive age who present to emergency room with non-traumatic acute abdominal pain.
The women with PID often undergo CT scan to establish differential diagnosis responsible for her acute abdominal pain and suggestion of PID to those women give a great impact on the management of women with PID. Our study showed that CT findings of hepatic capsular enhancement on late arterial phase and tubal thickening were significant predictors of PID with sensitivity, specificity, and accuracy of 71.9%, 81.3%, and 76.6%, respectively. We hope that our results will be helpful in the management of women with PID who present to emergency room with non-traumatic acute abdominal pain.
References
Curtis KM, Hillis SD, Kieke BA Jr, et al. (1998) Visits to emergency departments for gynecologic disorders in the United States, 1992–1994. Obstet Gynecol 91(6):1007–1012
Bevan C, Johal B, Mumtaz G, Ridgway G, Siddle N (1995) Clinical, laparoscopic and microbiological findings in acute salpingitis: report on a United Kingdom cohort. BJOG 102(5):407–414
Morcos R, Frost N, Hnat M, Petrunak A, Caldito G (1993) Laparoscopic versus clinical diagnosis of acute pelvic inflammatory disease. J Reprod Med 38(1):53
Goyal M, Hersh A, Luan X, et al. (2013) National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health 53(2):249–252
Trent M, Haggerty CL, Jennings JM, et al. (2011) Adverse adolescent reproductive health outcomes after pelvic inflammatory disease. Arch Pediat Adolesc Med 165(1):49–54
Peipert JF (2003) Clinical practice. Genital chlamydial infections. N Engl J Med 349(25):2424–2430
Paavonen J (1998) Pelvic inflammatory disease. From diagnosis to prevention. Dermatol Clin 16(4):747–756, xii
Banikarim C, Chacko MR (2004) Pelvic inflammatory disease in adolescents. Adolesc Med Clin 15(2):273–285, viii
Jung SI, Kim YJ, Park HS, Jeon HJ, Jeong KA (2011) Acute pelvic inflammatory disease: diagnostic performance of CT. J Obstet Gynaecol Res 37(3):228–235
Sam JW, Jacobs JE, Birnbaum BA (2002) Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics 22(6):1327–1334
Siddall KA, Rubens DJ (2005) Multidetector CT of the female pelvis. Radiol Clin North Am 43(6):1097–1118, ix
Urban BA, Fishman EK (1995) Spiral CT of the female pelvis: clinical applications. Abdom Imaging 20(1):9–14
Grossman J, Ricci ZJ, Rozenblit A, et al. (2008) Efficacy of contrast-enhanced CT in assessing the endometrium. AJR Am J Roentgenol 191(3):664–669
Nishie A, Yoshimitsu K, Irie H, et al. (2003) Fitz–Hugh–Curtis syndrome. Radiologic manifestation. J Comput Assist Tomogr 27(5):786–791
Tsubuku M, Hayashi S, Terahara A, Furukawa T, Ohmura G (2002) Fitz–Hugh–Curtis syndrome: linear contrast enhancement of the surface of the liver on CT. J Comput Assist Tomogr 26(3):456–458
Joo SH, Kim MJ, Lim JS, Kim JH, Kim KW (2007) CT diagnosis of Fitz–Hugh and Curtis syndrome: value of the arterial phase scan. Korean J Radiol 8(1):40–47
Cho HJ, Kim HK, Suh JH, et al. (2008) Fitz–Hugh–Curtis syndrome: CT findings of three cases. Emerg Radiol 15(1):43–46
Peter NG, Clark LR, Jaeger JR (2004) Fitz–Hugh–Curtis syndrome: a diagnosis to consider in women with right upper quadrant pain. Clevel Clin J Med 71(3):233–239
Onsrud M (1980) Perihepatitis in pelvic inflammatory disease—association with intrauterine contraception. Acta Obstet Gynecol Scand 59(1):69–71
Paavonen J, Saikku P, von Knorring J, Aho K, Wang SP (1981) Association of infection with Chlamydia trachomatis with Fitz–Hugh–Curtis syndrome. J Infect Dis 144(2):176
Kim JY, Kim Y, Jeong WK, Song SY, Cho OK (2009) Perihepatitis with pelvic inflammatory disease (PID) on MDCT: characteristic findings and relevance to PID. Abdom Imaging 34(6):737–742
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, M.H., Moon, M.H., Sung, C.K. et al. CT findings of acute pelvic inflammatory disease. Abdom Imaging 39, 1350–1355 (2014). https://doi.org/10.1007/s00261-014-0158-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0158-1