Abstract
Gastric cancer is a common deadly cancer worldwide. The tumor-node-metastasis (TNM) staging system is one of the most commonly used staging systems, and is accepted and maintained by the International Union against Cancer (UICC) and the American Joint Committee on Cancer (AJCC). The TNM system is well known to effectively predict the prognosis of gastric cancer patients. The latest revision of TNM staging was presented in the 7th edition of the AJCC in 2009. Multi-detector row CT (MDCT) is a powerful test for non-invasive evaluation and can assess metastatic and locoregional staging simultaneously. Current MDCT with isotropic imaging and 3D images has increased the accuracy of T and N staging in patients with gastric cancer. Multi-planar reformatted images permit the radiologist to select the optimal imaging plane to accurately evaluate tumor invasion depth of the gastric wall and perigastric infiltration to identify a fat plane between a tumor and adjacent organs, to avoid partial volume averaging effects, and to differentiate lymph nodes from small perigastric vessels. Thus, MDCT provides a useful all-in-one diagnostic method for the pre-operative evaluation of patients with known, or strongly suspected, gastric cancer according to the 7th AJCC TNM staging system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Gastric cancer is the 4th most common cancer and the 2nd leading cause of cancer-related deaths worldwide [1]. The incidence of gastric cancer is particularly common in eastern Asia [2]. In the past, the prognosis for gastric cancer patients was poor. The tumor-node-metastasis (TNM) staging system is well known as a prognostic factor which effectively predicts prognosis in gastric cancer patients [3]. The 5-year survival rate of patients with advanced gastric cancer (AGC) is 7%–27%, whereas the 5-year survival rate of patients with early gastric cancer (EGC) is 85%–100% [4–6]. The TNM staging system is renewed periodically in light of evidence and advances in understanding cancer prognosis. The latest revision of TNM was presented in the 7th edition of the American Joint Committee on Cancer (AJCC) in 2009 [7].
Current treatment options for gastric cancer vary from endoscopic mucosal resection (EMR) to pre-operative chemoradiotherapy, followed by gastrectomy, depending on tumor stage. In general, complete surgical resection provides the only chance for a cure. Therefore, appropriate pre-therapeutic staging by imaging techniques is critical in determining the optimal treatment.
There are several diagnostic methods to evaluate the extent of gastric cancer. Double-contrast barium studies have frequently been performed for the detection and anatomic localization of gastric tumors. Due to technical advances in endoscopy and EUS, the clinical use of barium studies has greatly diminished. Endoscopy provides direct visualization of lesions and an opportunity for biopsy. Endoscopic evaluation of gastric ulcers has a sensitivity of 98% for diagnosing gastric cancers of all stages [8] compared to barium studies with a sensitivity of only 14% in EGCs [9]. In the past, double-contrast barium examination and upper gastrointestinal endoscopy were used for the assessment of gastric cancer. Currently, the standard imaging methods for gastric cancer are endoscopic ultrasonography (EUS), computed tomography (CT), magnetic resonance imaging (MRI), and diagnostic laparoscopy. Each modality has strengths and weaknesses in diagnosing and staging disease for treatment planning. In the staging of gastric cancer, EUS has the ability to image five distinct wall layers with histologic correlates and to assess regional lymph node involvement in addition to local tumor infiltration [10]; however, EUS is not suitable for detecting distant metastases, including the liver and peritoneum. CT has been used for pre-operative staging work-ups, including assessment of liver metastases and distant spread after endoscopic evaluation with or without biopsies. Current multi-detector row CT with thin collimation provides isotropic imaging, which allows marked improvement of imaging resolution, especially in multi-planar reconstruction (MPR) images and virtual endoscopy. Some studies have reported that MDCT with MPR and three-dimensional (3D) images increases the accuracy of T staging in patients with gastric cancer [11, 12]. 3D virtual endoscopic images facilitate locating a lesion. Thus, MDCT with MPR images and 3D images is used as a routine protocol for gastric cancer staging. MRI has not been shown to provide higher diagnostic accuracy than CT for tumor staging or detection of lymph node metastases. Advances in MRI techniques, including the development of breath-hold sequences and the use of intravenous contrast agents, have also made MRI feasible for abdominal imaging, including imaging of the stomach [13, 14]; however, MRI is subject to artifacts secondary to respiratory and cardiac movement, and peristalsis in the thorax and abdomen; thus, the role of MRI for staging of gastric cancer is limited [15]. These imaging modalities have similar results with respect to diagnostic accuracy in T staging and a moderate degree of sensitivity and specificity in detecting lymph node metastases [16, 17]. Therefore, CT is most useful for pre-operative staging with respect to CT evaluation of simultaneous metastatic and locoregional staging.
This review mainly discusses the diagnosis and pre-therapeutic staging of gastric cancer based on 7th edition of the AJCC TNM staging system with MDCT, including MPR images, and a brief review of the current management of gastric cancer.
Imaging Technique
Patient Preparation
All patients fasted for at least 6 h before the examination to empty the stomach. Each patient received an intramuscular or intravenous administration of scopolamine butylbromide (10 mg) 10–15 min before the study to decrease peristaltic bowel movement if there were no contraindications, such as a history of glaucoma, arrhythmias, or symptoms of urinary outflow obstruction.
Oral Contrast
When CT is performed for gastric disease, optimal gastric distention is necessary because a collapsed stomach can obscure disease or simulate pathology. Oral contrast has been categorized as positive [high density (diluted barium)], neutral [iso-density (water)], and negative [low density (air)]. Among these oral contrast agents, a positive contrast agent has not been used because it interferes with data manipulation during 3D imaging of the abdomen and evaluation of the enhancement pattern of the gastric wall. Thus, neutral (water) or negative (air) contrast agents are preferred to positive agents. With respect to virtual gastroscopic evaluation, negative oral contrast using effervescent granules is more effective. A recent study reported [18] that MDCT using the gas distention technique showed better detectability for the T staging of pre-operative gastric cancer comparable to that of the water distention technique.
Position
There are variable positions depending on the location of the lesion and oral contrast. When CT is performed using water, prone and supine positions are generally used. When CT is performed using air, a 30° left posterior oblique (LPO) position provides better gastric distention and less residual fluid in the lower part of the stomach. The right lateral decubitus position provides better gastric distention and less residual fluid in the upper part of the stomach, and is generally used to reduce potential obscuring of the lesion due to interference from the proper lumen–wall interface caused by the administration of effervescent agents and residual water on 3D imaging of the stomach [19]. At our institute, patients are placed in the left lateral decubitus position to shift the gastric contents from the lower two-thirds of the stomach to the fundus. Then, the patients are immediately placed on the scanning table in a 30° LPO position by placing a pillow under the back. If an earlier study had shown the lesion to be located in the upper one-third of the stomach, a right lateral decubitus position was used. An initial scout image was obtained to ensure adequate gastric distention.
Scanning Protocol
At our institution, MDCT is performed with a 64-detector row CT scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA). Two sets of two-phase CT scans were obtained. Unenhanced CT was not performed. For contrast-enhanced CT, a dose of 2 mg/kg of a non-ionic contrast agent (Iopromide, Ultravist; Schering, Berlin, Germany) is administrated intravenously through an 18-gauge angiographic catheter at a rate of 4 mL/s using a power injector (OptiVantage, Liebel-Flarsheim; Mallinckrodt, Neustadt, Germany). CT scanning was started 40 s after contrast material injection (in the arterial phase). The scanning range was from the xyphoid process to the lower end of the stomach. Then, CT scanning was started 70 s after contrast material injection (in the portal venous phase). The scanning range was from the xyphoid process to the lower end of the symphysis pubis. CT scanning parameters were as follows: 64 detector rows; beam collimation, 0.625–40 mm; pitch, 0.984; kVp/effective mA, 120/300; and gantry rotation time, 0.6 s. Isotropic raw data were acquired with a slice thickness of 1.25 mm and an interval of 1.25 mm at MDCT. Using this raw data, a transverse image was obtained with a slice thickness of 3.75 mm and an interval of 3.75 mm, then 3D CT gastrography and coronal and sagittal MPR images were reconstructed on a workstation. Each MPR image was obtained at 3-mm intervals with a slice thickness of 3 mm.
Staging of Stomach Cancer
Gastric cancers may manifest as focal mural thickening with or without ulcerations, polypoid lesions, or diffuse mural thickening. EGC is usually defined as malignant invasion confined to the mucosa or submucosa, regardless of the presence of lymph node metastasis, while AGC invades the muscularis propria or beyond [20, 21]. EGCs are classified into three types, as follows: type I lesions are elevated and protrude >5 mm into the lumen; type IIa lesions are elevated, but protrude <5 mm into the lumen; type IIb lesions are essentially flat; type IIc lesions are slightly depressed, but do not penetrate the muscularis mucosae; and type III lesions are true mucosal ulcers that penetrate the muscularis mucosae, but not the muscularis propria [22]. AGCs can manifest as large, segmental, or diffuse wall thickening with or without ulceration or large, polypoid, and fungating lesions. Signet-ring cell cancer often manifests as obliteration of gastric folds and diffuse thickening of the gastric wall (linitis plastica) [21]. The TNM system is one of the most commonly used staging systems, and is accepted and maintained by the International Union against Cancer (UICC) and the AJCC. The latest revision of TNM presented in the 7th edition in 2009 (Table 1) [7].
T Staging
Pathologically, the stomach is composed of five layers (mucosa, submucosa, proper muscularis, subserosa, and serosa). However, the gastric wall is generally detected as three layers on CT; mucosal layers show high attenuation, submucosal layers show low attenuation, and musculoserosal layers show high attenuation [23]. Tumor size and invasion depth are independent factors for visibility. EGC is little visualized on MDCT with 2-dimensional imaging protocols [24]. The diagnostic performance of hydro-stomach CT to detect an EGC is not significantly different between blinded and unblinded analysis after knowing the location of the cancer [25]. In contrast, Kim et al. [26] reported that isotropic MDCT with MPR images, including coronal or sagittal reconstructions, can improve the accuracy of pre-operative T and N staging of gastric cancer. However, CT gastrography provides more effective detectablilty than hydro-stomach CT. Several studies have reported that hydro-stomach CT with 2D-based image analysis provides a 36%–48% EGC detection rate, while CT gastrography with 2D- and 3D-based image analysis provides a 73%–96% EGC detection rate [11, 26–28].
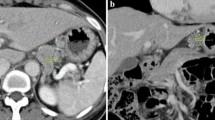
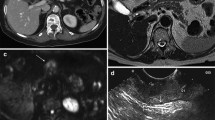
In the CT images demonstrating mural invasion of cancer in the gastric wall according to the 7th AJCC, T1a (Fig. 1) has a tendency not to be visualized on 2D CT images, and T1b frequently shows mucosal thickening with enhancement. In the differential point between T1b and T2 on CT images, T1b demonstrates a low-attenuation stripe at the base of the lesion corresponding to the submucosal layer, while T2 demonstrates a thickened gastric wall with loss or disruption of a low-attenuation stripe, but a clear and smooth outer gastric surface around the lesion [29] (Fig. 2). Previous CT criteria of T3 tumors have included nodular or irregular outer borders of the thickened gastric wall or perigastric fat infiltration [30]. Based on the new 7th AJCC T staging of gastric cancer, the differentiation of T3 and T4a on CT images is very difficult because the serosa of the gastric wall is not visible on CT images and subserosal adipose tissue is different from person-to-person (Figs. 3 and 4). In addition, the differentiation of perigastric infiltration from gastric cancer and perigastric inflammation or fibrosis on CT images can be difficult thus T2 tumors could be over-staged as T3 tumors or T4 tumors [30] (Fig. 5). Direct extension and invasion of tumor into a contiguous organ or structure on CT images is diagnosed as a T4b tumor.
A 58-year-old woman with early gastric cancer (type IIa + IIc) in the angle of the stomach, which was pathologically confirmed to be tubular adenocarcinoma and confined to the mucosa of the gastric wall (T1a). Transverse CT scans (A) and coronal MPR images (B) do not show any abnormalities of the stomach. However, 3-D reconstruction images (C) display a slightly elevated lesion (arrow) with ulceration on the gastric angle.
A 29-year-old woman with advanced gastric cancer in the lesser curvature of the antrum, which was pathologically confirmed to be signet-ring cell carcinoma invading the muscularis propria (T2). Transverse CT scans (A) and sagittal MPR images (B) show focal wall thickening (arrow) of the lesser curvature of the gastric antrum with mucosal enhancement and focal disruption of the low-attenuation stripe, but a smooth outer surface around the lesion.
A 62-year-old man with advanced gastric cancer in the lesser curvature of the pre-pyloric antrum, which was pathologically confirmed to be tubular adenocarcinoma penetrating the subserosal connective tissue (T3). Transverse CT scans (A) and coronal MPR images (B) show segmental wall thickening (arrow) of the lesser curvature of the pre-pyloric antrum with enhancement through the gastric wall and loss of the low-attenuation stripe, irregular outer surface around the lesion, perigastric fat infiltration, and suspected tiny omental nodules. The pre-operative diagnosis of the lesion was T4 cancer with possible localized peritoneal seeding.
A 43-year-old woman with advanced gastric cancer in the greater curvature of the lower body, which was pathologically confirmed to be tubular adenocarcinoma penetrating the serosa (T4a). Transverse CT scans (A) and sagittal (B) and coronal (C) MPR images show segmental wall thickening (arrow) of the greater curvature of the lower body of the stomach with enhancement and loss of the low-attenuation stripe, nodular outer gastric surface around the lesion, and minimal perigastric fat infiltration. The pre-operative diagnosis of the lesion was T3 cancer.
A 76-year-old man with advanced gastric cancer in the greater curvature of the antrum, which was pathologically confirmed to be tubular adenocarcinoma invading the muscularis propria (T2). Transverse CT scans (A) and coronal MPR images (B) show segmental wall thickening (arrow) of the greater curvature of the gastric antrum with enhancement and disruption of the low-attenuation stripe, and a nodular outer surface around the lesion, but clear perigastric fat infiltration. The pre-operative diagnosis of the lesion was T3 cancer.
N Staging
Clinically, N staging is as important as T staging in deciding the appropriate surgical treatment and in determining the prognosis of gastric cancer. Previous studies [21, 31] have reported that regional lymph nodes are considered involved when the short axis diameter is >6 mm for perigastric lymph nodes and >8 mm for extra-perigastric lymph nodes. Other criteria for malignant involvement include a nearly round shape (longitudinal: transverse diameter ratio <1.5), loss of the normal fatty hilum, and marked or heterogeneous enhancement. However, nodal staging on CT images is inherently difficult independent of the technique used. In N staging of gastric cancer, the accuracy of previous reports has ranged from 51% to 83.8% [21, 26, 28, 32–35]. A recent study [26] with isotropic MDCT with MPR images, including coronal or sagittal reconstructions, achieved an improvement in the accuracy of pre-operative N staging of AGCs, while having little impact on the accuracy for EGCs. Therefore, the use of MPR imaging is expected to help the evaluation of pre-operative N staging. Although the size, CT attenuation values, and configuration of lymph nodes are used as criteria for nodal involvement, the reason for those poor results are considered to be caused by the lack of worldwide consensus regarding reliable CT criteria for metastatic lymph nodes. The wide ranges of sensitivity (62.5%–91.9%) in the literature demonstrate this problem of CT in nodal staging [17]. Although there is a clear correlation between lymph node size and cancer involvement, CT has significant inherent limitations in the nodal staging of gastric cancer because enlarged nodes may subsequently be proven to be inflammatory, whereas normal-sized nodes may be metastatic (microscopic nodal invasion).
M Staging
Distant metastases of gastric cancer could be categorized as hematogenous metastases, lymphatic metastases, and direct peritoneal metastases. Hematogenous metastases from gastric cancer most commonly involve the liver through the portal vein. Other less common sites of hematogenous spread include the lungs, adrenal glands, kidneys, bones, brain, and other regions of the gastrointestinal tract. In lymphatic metastasis, lymph node involvement, with the exception of the perigastric areas, is considered M1 disease. Hepatoduodenal nodes are considered distant metastatic nodes, but hepatoduodenal nodes are difficult to distinguish from common hepatic nodes. AGCs can develop peritoneal metastases which are correlated with tumor size and T staging [36]. The pre-operative diagnosis of peritoneal carcinomatosis is important to prevent unnecessary open surgery. CT findings suggesting peritoneal metastasis are as follows: ascites; the presence of soft tissue nodules or plaques on the peritoneal surface; small bowel wall thickening and nodularity; intra-abdominal fat stranding; and peritoneal thickening and/or enhancement [37] (Fig. 6). The presence of ascites on CT is the most important factor predicting peritoneal metastasis. Chang et al. [38] noted that when the estimated CT-defined ascitic volume was ≥ 50 mL, peritoneal carcinomatosis was identified in 75%–100% of patients. Yajima et al. [39] reported that positive ascites on CT predicted the presence of peritoneal metastasis with 51% sensitivity and 97% specificity. Therefore, when ascites is detected on CT images, we should search for seeded nodules in the peritoneal cavity.
A 73-year-old man with advanced gastric cancer in the gastric lower body to the antrum, which was pathologically confirmed to be tubular adenocarcinoma by endoscopic biopsy, and multiple peritoneal seeding (M1) by laparoscopy. Transverse CT scan (A) at the level of the stomach shows abnormal irregular thickening of the lower body to the antrum with enhancement and loss of the low-attenuation stripe, nodular outer surface around the lesion, and extensive perigastric fat infiltration. The other transverse CT scans (B–D) at the level of the pelvic cavity show increased peritoneal fat density in the right side and lower abdomen (arrows) (B), scanty pelvic ascites (arrow) (C), and minimal nodular peritoneal enhancing thickening (arrow) in the pelvis (D). The pre-operative diagnosis of the lesion was advanced gastric cancer with peritoneal seeding.
Management
The choice of treatment for patients with gastric cancer relies on the TNM stage of the disease, location and size of stomach, and condition of the patient. There are several available treatment options, including surgery, chemotherapy, or radiotherapy. Among the treatment options, complete surgical resection of the gastric cancer is generally considered the only effective curative treatment [40, 41]. An EGC < 2 cm in size with well- or moderately-differentiated histology limited to the mucosa and the superficial submucosa above the muscularis propria can be assessed by endoscopy, such as EMR and endoscopic submucosal dissection (ESD). Endoscopic treatments can only be curative when there is no lymph node metastasis at the time of treatment. Gotoda et al. [42] reported the en bloc resection rate of EGCs was 79%–100%, with local recurrence, bleeding, and perforation rates of 0%–1%, 1.7%–38%, and 0%–5%, respectively. The major advantages of non-surgical treatments are low morbidity and mortality with preservation of normal digestion and quality of life. Several centers [43, 44] have reported that cancer-free survival after endoscopic treatment is equivalent to that accomplished by surgical resection. A recent study [45] noted that 10-year survival rates of complete en bloc EMR for mucosal EGC is 99%.
Traditionally, radical surgery is recommended for patients with T1, T2, and T3 cancers with a relatively low lymph node burden. The aim of surgical resection is complete removal of the tumor. A tumor-free (R0) resection margin of at least 5 cm is usually required for proximal and distal margins of gastric cancer. Depending on the tumor location, a total gastrectomy for tumors in the proximal two-thirds of the stomach and a subtotal gastrectomy for tumors in the antrum or pylorus are performed. A randomized trial revealed the morbidity, mortality, and 5-year survival rates for patients with distal gastric cancer after subtotal gastrectomy were similar to total gastrectomy [46].
The extent of lymph node dissection for curative surgery is somewhat controversial. The draining lymph nodes of the stomach are categorized into 16 stations and these stations are grouped as three clusters (D1, D2, and D3). D1 includes perigastric lymph nodes, D2 includes nodes along the hepatic, left gastric, celiac, and splenic arteries, and the splenic hilum, and D3 includes nodes within the porta hepatic and peri-aortic region. For 20 years, several prospective randomized trials have not shown a statistically significant survival advantage of a D2 vs. D1 lymphadenectomy or a D3 vs. D2 lymphadenectomy [47–49]. A recent study reported complication rates of 17.9% and 12% with D2 and D1 dissections, respectively, and post-operative mortality rates of 2.2% and 3%, respectively [50]. Therefore, pancreas- and spleen-preserving D2 lymphadenectomies are recommended by the National Comprehensive Cancer Network.
Patients with AGCs with T4 disease involving adjacent structures that cannot be easily resected and patients with distant metastases should not undergo surgery. Therefore, effective chemotherapy and radiation regimens have been developed for use with surgical management, and a number of trials have been conducted or are on-going. The results from these treatment modalities have been modest, with little effect on overall survival. Schein et al. [51] revealed that the 4-year survival rate in patients with unresectable gastric cancer who received combined radiation and chemotherapy (18%) was higher than patients who received chemotherapy alone (6%). Macdonald et al. [52] showed a 52% overall survival rate with post-operative chemoradiotherapy compared to a 41% overall survival rate with surgery alone. A recent study reported that patients with operable gastric or lower esophageal adenocarcinomas who received peri-operative cisplatin-based chemotherapy had improved progression-free and overall survival [53]. Other treatment options for unresectable tumors and for patients with distant metastases are under development in a number of clinical trials that are underway.
Conclusion
The technical advances involving MDCT have broadened the role of CT for evaluating patients with gastric cancer. MDCT in conjunction with MPR images of the stomach can enhance the performance of CT in the evaluation of patients who have gastric cancer. Despite the limitations of CT staging, such as an inability to identify metastatic nodes with certainty and an inability to determine the exact depth of tumor invasion through the gastric wall (particularly T3 and T4a according to the 7th AJCC TNM staging system), MDCT has provided detailed evaluation of gastric morphology that can not only identify primary tumors, but also provide pre-operative staging, including regional and distant metastases. On the basis of these considerations, we believe that MDCT is a useful all-in-one diagnostic method for the pre-operative evaluation of patients with known, or strongly suspected, gastric carcinoma based on the 7th AJCC TNM staging system. There are several treatment options available, such as endoscopic resection, surgery, chemotherapy, or radiotherapy. The selection of treatment options relies on the TNM stage of the disease, and location and size of the stomach. Complete resection of gastric cancer is generally considered the only proven effective curative treatment despite the availability of other treatment options.
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Moore MA, Eser S, Igisinov N, et al. (2010) Cancer epidemiology and control in North-Western and Central Asia—past, present and future. Asian Pac J Cancer Prev 11(Suppl 2):17–32
Ichikura T, Tomimatsu S, Uefuji K, et al. (1999) Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer 86:553–558
Martin RC II, Jaques DP, Brennan MF, Karpeh M (2002) Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg 236:159–165
Nitti D, Marchet A, Mammano E, et al. (2005) Extended lymphadenectomy (D2) in patients with early gastric cancer. Eur J Surg Oncol 31:875–881
Carter KJ, Schaffer HA, Ritchie WPJr (1984) Early gastric cancer. Ann Surg 199:604–609
Sobin L, Gospodarowisz M, Wittekind C (2009) TNM classification of malignant tumours. New York: Wiley
Graham DY, Schwartz JT, Cain GD, Gyorkey F (1982) Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 82:228–231
Longo WE, Zucker KA, Zdon MJ, Modlin IM (1989) Detection of early gastric cancer in an aggressive endoscopy unit. Am Surg 55:100–104
Byrne MF, Jowell PS (2002) Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology 122:1631–1648
Kim HJ, Kim AY, Oh ST, et al. (2005) Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology 236:879–885
Chen CY, Hsu JS, Wu DC, et al. (2007) Gastric cancer: preoperative local staging with 3D multi-detector row CT–correlation with surgical and histopathologic results. Radiology 242:472–482
Semelka RC, Balci NC, Op de Beeck B, Reinhold C (1999) Evaluation of a 10-minute comprehensive MR imaging examination of the upper abdomen. Radiology 211:189–195
Keogan MT, Edelman RR (2001) Technologic advances in abdominal MR imaging. Radiology 220:310–320
Motohara T, Semelka RC (2002) MRI in staging of gastric cancer. Abdom Imaging 27:376–383
Kwee RM, Kwee TC (2007) Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol 25:2107–2116
Kwee RM, Kwee TC (2009) Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 12:6–22
Park HS, Lee JM, Kim SH, et al. (2010) Three-dimensional MDCT for preoperative local staging of gastric cancer using gas and water distention methods: a retrospective cohort study. AJR Am J Roentgenol 195:1316–1323
Kim SH, Lee JM, Han JK, et al. (2005) Effect of adjusted positioning on gastric distention and fluid distribution during CT gastrography. AJR Am J Roentgenol 185:1180–1184
Katai H, Sano T (2005) Early gastric cancer: concepts, diagnosis, and management. Int J Clin Oncol 10:375–383
Ba-Ssalamah A, Prokop M, Uffmann M, et al. (2003) Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics 23:625–644
Sleisenger MH, Fordtran J (1993) Gastrointestinal disease. In: Davis GR (ed) Neoplasms of the stomach. Philadelphia, PA: Saunders, pp 763–792
Minami M, Kawauchi N, Itai Y, et al. (1992) Gastric tumors: radiologic-pathologic correlation and accuracy of T staging with dynamic CT. Radiology 185:173–178
Yu JS, Choi SH, Choi WH, et al. (2007) Value of nonvisualized primary lesions of gastric cancer on preoperative MDCT. AJR Am J Roentgenol 189:W315–W319
Park KJ, Lee MW, Koo JH, et al. (2011) Detection of early gastric cancer using hydro-stomach CT: blinded vs unblinded analysis. World J Gastroenterol 17:1051–1057
Kim YN, Choi D, Kim SH, et al. (2009) Gastric cancer staging at isotropic MDCT including coronal and sagittal MPR images: endoscopically diagnosed early vs. advanced gastric cancer. Abdom Imaging 34:26–34
Kim JH, Eun HW, Choi JH, et al. (2007) Diagnostic performance of virtual gastroscopy using MDCT in early gastric cancer compared with 2D axial CT: focusing on interobserver variation. AJR Am J Roentgenol 189:299–305
Yang DM, Kim HC, Jin W, et al. (2007) 64 multidetector-row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr 31:98–103
Lee IJ, Lee JM, Kim SH, et al. (2010) Diagnostic performance of 64-channel multidetector CT in the evaluation of gastric cancer: differentiation of mucosal cancer (T1a) from submucosal involvement (T1b and T2). Radiology 255:805–814
Kim AY, Kim HJ, Ha HK (2005) Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging 30:465–472
Husband JE, Reznek R (2004) Imaging in oncology. In: Mclean A (ed) Gastric cancer. London: Taylor and Francis, pp 189–215
Ziegler K, Sanft C, Zimmer T, et al. (1993) Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 34:604–610
Cho JS, Kim JK, Rho SM, et al. (1994) Preoperative assessment of gastric carcinoma: value of two-phase dynamic CT with mechanical iv. injection of contrast material. AJR Am J Roentgenol 163:69–75
Fukuya T, Honda H, Hayashi T, et al. (1995) Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology 197:705–711
Kim HS, Han HY, Choi JA, et al. (2001) Preoperative evaluation of gastric cancer: value of spiral CT during gastric arteriography (CTGA). Abdom Imaging 26:123–130
Kim SJ, Kim HH, Kim YH, et al. (2009) Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology 253:407–415
Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH (1993) Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 72:1631–1636
Chang DK, Kim JW, Kim BK, et al. (2005) Clinical significance of CT-defined minimal ascites in patients with gastric cancer. World J Gastroenterol 11:6587–6592
Yajima K, Kanda T, Ohashi M, et al. (2006) Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am J Surg 192:185–190
Gore RM (1997) Gastric cancer. Clinical and pathologic features. Radiol Clin North Am 35:295–310
Kim JP (1999) Surgical results in gastric cancer. Semin Surg Oncol 17:132–138
Gotoda T, Yamamoto H, Soetikno RM (2006) Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 41:929–942
Green D, Ponce de Leon S, Leon-Rodriguez E, Sosa-Sanchez R (2002) Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. Am J Clin Oncol 25:84–89
Bonenkamp JJ, Sasako M, Hermans J, van de Velde CJ (2001) Tumor load and surgical palliation in gastric cancer. Hepatogastroenterology 48:1219–1221
Uedo N, Iishi H, Tatsuta M, et al. (2006) Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer 9:88–92
Bozzetti F, Marubini E, Bonfanti G, et al. (1999) Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 230:170–178
Sano T, Sasako M, Yamamoto S, et al. (2004) Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy–Japan Clinical Oncology Group study 9501. J Clin Oncol 22:2767–2773
Hartgrink HH, van de Velde CJ, Putter H, et al. (2004) Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22:2069–2077
Bonenkamp JJ, Hermans J, Sasako M, et al. (1999) Extended lymph-node dissection for gastric cancer. N Engl J Med 340:908–914
Degiuli M, Sasako M, Ponti A (2010) Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 97:643–649
Schein PS et al. (1982) A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Gastrointestinal Tumor Study Group. Cancer 49:1771–1777
Macdonald JS, Smalley SR, Benedetti J, et al. (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Cunningham D, Allum WH, Stenning SP, et al. (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Acknowledgement
This study was supported by the Samsung Biomedical Research Institute grant, #SBRI C-A9-241-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, M.H., Choi, D., Park, M.J. et al. Gastric cancer: Imaging and staging with MDCT based on the 7th AJCC guidelines. Abdom Imaging 37, 531–540 (2012). https://doi.org/10.1007/s00261-011-9780-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-011-9780-3