Abstract
Purpose
This prospective study aimed (1) to assess the non-small cell lung cancer (NSCLC) evolutive patterns to immunotherapy using FDG-PET and (2) to describe their association with clinical outcome.
Design
Fifty patients with metastatic NSCLC were included before pembrolizumab or nivolumab initiation. FDG-PET scan was performed at baseline and after 7 weeks of treatment (PETinterim1) and different criteria/parameters of tumor response were assessed, including PET response criteria in solid tumors (PERCIST). If a first PERCIST progressive disease (PD) without clinical worsening was observed, treatment was continued and a subsequent FDG-PET (PETinterim2) was performed at 3 months of treatment. Pseudo-progression (PsPD) was defined as a PERCIST response/stability on PETinterim2 after an initial PD. If a second PERCIST PD was assessed on PETinterim2, a homogeneous progression of lesions (termed immune homogeneous progressive-disease: iPDhomogeneous) was distinguished from a heterogeneous evolution (termed immune dissociated-response: iDR). A durable clinical benefit (DCB) of immunotherapy was defined as treatment continuation over a 6-month period. The association between PET evolutive profiles and DCB was assessed.
Results
Using PERCIST on PETinterim1, 42% (21/50) of patients showed a response or stable disease, most of them (18/21) reached a DCB. In contrast, 58% (29/50) showed a PD, but more than one-third (11/29) were misclassified as they finally reached a DCB. No standard PETinterim1 criteria could accurately distinguished responding from non-responding patients. Treatment was continued in 19/29 of patients with a first PERCIST PD; the subsequent PETinterim2 demonstrated iPDhomogeneous, iDR and PsPD in 42% (8/19), 26% (5/19), and 32% (6/19), respectively. Whereas no patients with iPDhomogeneous experienced a DCB, all patients with iDR and PsPD reached a clinical benefit to immunotherapy.
Conclusion
In patients with a first PD on PERCIST and treatment continuation, a subsequent PET identifies more than half of them with iDR and PsPD, both patterns being strongly associated with a clinical benefit of immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (immunotherapy) targeting programmed cell death-1 (PD-1) have been recently approved in locally advanced and metastatic non-small cell lung cancer (NSCLC), irrespectively of the histologic subtype [1].
One unresolved issue is how to identify patients that are more likely to benefit from immunotherapy, as early as possible [2, 3]. Although PD-L1 positivity enriches for populations with clinical benefit, PD-L1 testing alone appears to be insufficient for patient selection [4]. As there are currently no reliable biological markers for prediction or early identification of tumor response to immunotherapy, the therapeutic decision currently relies on imaging combined with a clinical evaluation [4]. But new challenges have been raised with the radiological assessment of immunotherapy efficiency. Conventional imaging criteria such response evaluation criteria in solid tumors (RECIST) v1.1, based on morphologic measurements on computed tomography (CT), or PET response criteria in solid tumors (PERCIST V1.0), based on glucose metabolic changes on 18F-fluoro-deoxy-glucose positron-emission tomography (18FDG PET), rely on the paradigm that the cytotoxic effect of chemotherapy translates into measurable tumor shrinkage and metabolism decrease, respectively [5, 6]. Yet, the mechanisms of action of immunotherapy are different from conventional chemotherapy; the lifting of the inhibition of the lymphocyte system induces a tumor infiltration and proliferation of immune cells that can lead to a transient increase in tumor burden on CT and metabolic activity on 18FDG PET [7,8,9]. Consequently, when using immunotherapy, new response patterns have been described such as a transient tumor increase in size and metabolism, or appearance of new lesions, followed by a delayed response or stability [7, 9]. This challenging and specific immune-related response pattern is termed “pseudo-progression” (PsPD) and may lead to misinterpretation of clinical images.
The limits of the RECIST criteria to assess tumor response in the setting of immunotherapy have led to new immunotherapy-adapted criteria: the iRECIST [9, 10]. Their key point is that because of a potentially delayed response to immunotherapy treatment, imaging assessment of disease progression should be made with two consecutive follow-up imaging studies performed at least 4 weeks apart, especially when a pseudo-progressive disease is suspected. When using FDG PET, no specific immune-related response criteria for solid tumors have been validated until now. The aims of this prospective monocentric study were:
- 1.
To investigate the accuracy of standard PET criteria, assessed 7 weeks after the initiation of immunotherapy, to identify patients who will clinically benefit from immunotherapy
- 2.
In patients with a first PERCIST progression on PET, to describe the different patterns of tumor metabolic evolution on a mandatory subsequent PET evaluation performed few weeks later, and their association with patients’ clinical outcome
Patients and methods
Population
From February 2017 to September 2018, 62 patients scheduled to initiate immunotherapy as their first or later systemic treatment for NSCLC were prospectively evaluated in this open, non-randomized, current care study in our institution. The study was approved by the ethics committee (CPP Sud Ouest et Outre Mer III) and regulatory agencies (FDG ECMI, n° ID-RCB: 2018-A02116-49), and a signed informed consent was obtained from all individual participants included in the study.
The inclusion criteria were the following: (1) pathologically proven locally advanced or metastatic NSCLC, irrespective of the histologic subtype; (2) an indication to start immune checkpoint inhibitors in first or later line; (3) performance status on the Eastern Cooperative Oncology Group corresponding to 0–2. The exclusion criteria were the following: (1) clinical or biological contraindication for immune checkpoints inhibitors, (2) evidence of concurrent cancer, (3) vulnerable patients as defined in Article L1121-5 to -8 of the French Public Health Code, (4) refusal of written consent, and (5) high glycaemia (> 9 mmol/l) before FDG PET scans.
Patients received one of two possible treatment regimens, either pembrolizumab administered intravenously at a standard dose of 2 mg/kg every 3 weeks or nivolumab at a standard dose of 240 mg every 2 weeks. If available, the pre-treatment PD-L1 tumor expression was recorded.
PET protocol
18FDG PET scans were performed before the start of immune checkpoints inhibitor (PETbaseline), after 7 weeks of treatment (PETinterim1) and after 3 months of treatment (PETinterim2).
All PET scans were performed using the same PET/CT system (Biograph mCT, Siemens Healthcare). Patients were instructed to fast for at least 6 h before the intravenous injection of 3 MBq/kg of 18F-FDG. Sixty minutes later, a low-dose attenuation CT acquisition was performed (50 kV, 50 mA, 5 mm slice thickness) followed by a static 3D PET acquisition with image duration of 150 s per bed position, an axial field of view of 13 cm, and a matrix of 256 × 256. Lastly, a diagnostic CT acquisition was done (auto-kV, auto-mA, 1 mm slice thickness) after a venous injection of iodinated contrast agent in the absence of allergy or renal impairment. PET images were reconstructed using the ordered subsets expectation maximization (OSEM 3D) iterative algorithm (2 iterations, 21 subsets), with point spread function and time-of-flight correction (ultra-HD PET). Peak-standardized uptake values, normalized either by body weight (SUVpeak) or by lean body mass (SULpeak), were calculated on the highest uptake site of the tumors at baseline and interim exams. Pathological hypermetabolic foci obviously deemed to be due to the therapy-related inflammation or immune activation (for example, symmetrical uptake in enlarged hilar/mediastinal lymph nodes, diffuse splenic uptake, colitis) were excluded of the lesion analyses.
PETinterim1 (after 7 weeks of treatment)
Different metabolic parameters were assessed:
PERCIST criteria were used to define complete metabolic response (CR), partial metabolic response (PR), stable metabolic disease (SD), or progressive metabolic disease (PD), considering the lesion with the highest FDG uptake (SULpeak) on each of these scans and the appearance of new hypermetabolic lesions [5].
ΔSUVpeak corresponding to the SUV percentage change of the most intense lesion (not necessarily the same) between baseline and interim PET, regardless of the appearance of new lesions.
-
The appearance of new hypermetabolic lymph node or visceral/bone lesion(s), regardless of SUVpeak changes.
Because of the investigational nature of PETinterim1 and the knowledge of the specific immune-related response pattern with CT, the result from this scan was not directly used to guide patient management. The treatment was continued even in the case of PD. Nonetheless, for patient security, the patient’s clinical status was assessed at each course of treatment and it could be stopped at any time in case of clinical worsening or toxicity.
PETinterim2 (after 3 months of treatment)
These scans were performed for patients with PD as assessed by PERCIST on PETinterim1. The PERCIST criteria were used for the interpretation of these scans but adding new evolutive patterns adapted to the issue of immunotherapy:
Pseudo-progression (PsPD): in the case of CR, PR, or SD according to PERCIST criteria, the nuclear physician concluded to PsPD, defined as a delayed tumor response or stability for tumor lesions that had initially been assessed as progressive. In this case, the oncologist could continue immunotherapy if no clinical worsening was observed.
In the case of FDG PET imaging showing a second and consecutive tumor progression according to PERCIST, two different progressive patterns were distinguished:
Immune homogeneous progressive disease (iPDhomogeneous)
Immune dissociated response (iDR) defined as concomitant decrease in certain hypermetabolic lesions associated with an increase in others lesions.
The decision to stop or continue the immunotherapy was then decided during a multi-disciplinary tumor board, confronting the patient’s clinical status and the PET results. Thus, the treatment could be continued in all of the 3 evolutive patterns on PETinterim2.
Follow-up and clinical endpoints
Patients were followed during a 6-month period with regular clinical evaluations and standard of care imaging (including follow-up brain MRI, 18FDG PET, and CT exams). The delay between the initiation of the treatment and the decision to stop it, as well as the reason of it, was recorded. If treatment was maintained at this 6-month follow-up endpoint, patients were considered having a durable clinical benefit (DCB) of immunotherapy, as done in previous studies [11, 12]. DCB was thus the primary endpoint. Overall survival (OS) was recorded and defined as the time from initial immunotherapy to death from any cause.
Statistical analyses
The required number of patient to be evaluated was calculated by considering the 95% confidence interval (CI) of sensitivity analysis between response and a durable clinical benefit. The hypotheses were the following: an expected sensitivity of 85% [12,13,14], alpha (bilateral) = 0.025. The estimated sample size of responding patients was calculated at 20 subjects. Taking into account that we expected a response rate of 40% [12, 14] and a 15% of patient’s loss to follow up, the inclusion of 60 patients satisfied the initial hypotheses.
Data entry and management were performed on the capture system (Ennov Clinical®). Qualitative data were presented as absolute frequencies and percentages. The quantitative data were presented in mean value and standard deviation, or median and extreme values. The sensibility, specificity, positive predictive value (PPV), negative predictive value (NPV), and Youden’s index (summarizing the performance of the diagnosis) of metabolic variables on PETinterim1 in their capacity to predict no-DCB of immunotherapy. The McNemar test was used for comparison of sensitivity and specificity of the different sets of criteria, and the Bonferroni adjustment was applied to counteract the problem of multiple comparisons (N = 6; P cutoff = 0.008). Exact McNemar test calculations were performed using R package exact2x2® version 3.2.2.
Results
Patients’ characteristics (Table 1)
Sixty-two patients were prospectively included. Seven patients were excluded because the treatment was stopped in the very first weeks of treatment, with the PETinterim1 being waved: 4/7 patients due to an early hyperprogressive disease (HPD) with severe clinical worsening and 3/7 due to severe treatment toxicity. Five patients were excluded for: lost to follow-up (N = 1), no PET target lesion due to concomitant radiotherapy on the only hypermetabolic lesion (N = 1), PETinterim1 cancelation or wrong timing (N = 3). Fifty remaining patients were therefore evaluated in this study. Mean patient age was 63.4 ± 10.2 years (range 42–83 years). All patients had a metastatic NSCLC: 20% (10/50) of squamous cell carcinoma and 80% (40/50) of adenocarcinoma. Forty-eight percent (24/50) of patients were treated with pembrolizumab, mostly in first line of the metastatic setting, and 52% (26/50) of patients were treated with nivolumab, mostly in second line of the metastatic setting.
Mean delay between introduction of immunotherapy and PETinterim1 to evaluate response was 49 ± 5 days. Mean delay between introduction of immunotherapy and PETinterim2 was 92 ± 15 days.
Patients’ median follow-up was 11.5 months (95% CI = 11.1–13.8) for the whole cohort, 11.5 months (95% CI = 8.8–NA) for patients in the first-line setting, and 11.6 months (95% CI = 11.1–17.3) for patients in second or further line setting.
Results of PETinterim1 (Fig. 1)
PERCIST criteria
-
32% of patients achieved a CR or PR (N = 16/50)
Among them, 87.5% (14/16) reached a DCB, whereas treatment was stopped before the 6-month endpoint in the remaining 12.5% (2/16) patients due to tumor progression. None of these patients died during the 6-month follow-up.
-
10% of patients presented a SD (N = 5/50)
Eighty percent (4/5) of them reached a DCB. The treatment was stopped before 6 months in the only remaining patient due to severe toxicity. None of these patients died during the 6-month follow-up
-
58% of patients presented with a PD (N = 29/50)
37.9% (11/29) of them reached a DCB, whereas the treatment was stopped before 6 months in the remaining 62.1% (18/29) of these patients due to tumor progression with clinical worsening. The sensitivity, specificity, PPV, NPV, and Youden’s index of a first PERCIST PD to predict no-DCB were calculated at 85.7%, 62.1%, 62.1%, 85.7%, and 0.48, respectively. Among these patients with no-DCB, 50% (9/18) died during the 6-month follow-up.
ΔSUVpeak criteria
The mean ΔSUVpeak on PETinterim1 was − 3.3 ± 50 % with:
32% of patients (16/50) having a SUVpeak decrease (ΔSUVpeak ≤ − 30%)
46% of patients (23/50) having a SUVpeak stability (− 30% ≤ ΔSUVpeak ≤ + 30%)
22% of patients (11/50) having a SUVpeak increase (ΔSUVpeak ≥ + 30%)
A DCB was obtained in 93.7% (15/16) of patients with a SUVpeak decrease, 43.5% (10/23) with a SUVpeak stability, and 36.4% (4/11) with a SUVpeak increase.
The sensitivity, specificity, PPV, NPV, and Youden’s index of a ΔSUVpeak ≥ + 30% to predict no-DCB were 33.3%, 86.2%, 63.6%, 64.1%, and 0.2, respectively.
After multiple comparison correction, the ΔSUVpeak had a significantly lower sensitivity but significantly higher specificity than the PERCIST criteria (P = 0.0001 and 0.007, McNemar test).
New hypermetabolic lesion(s)
Half of the patients (25/50) showed at least one new hypermetabolic lesion on PETinterim1; 10% (N = 5) had only new lymph node(s) whereas 40% (N = 20) had also new visceral/bone lesion(s).
All types of new lesions (including lymph nodes)
80.0% (20/25) of patients without new lesions had a DCB, compared with only 36.0% (9/25) of patients with at least one new lesion.
0The sensitivity, specificity, PPV, NPV, and Youden’s index of the apparition of new lesion(s) on PETinterim1 to predict no-DCB were 76.2%, 69%, 64%, 80%, and 0.45, respectively.
After multiple comparison correction, the sensitivity and specificity were not significantly different from PERCIST criteria (P = 0.5 and 0.1, respectively).
Exclusively visceral/bone new lesion(s)
80.0% (24/30) of patients without new visceral/bone lesions had a DCB, compared with only 25.0% (5/20) of patients with at least one new visceral/bone lesion.
The sensitivity, specificity, PPV, NPV, and Youden’s index to predict no-DCB were 71.4%, 82.8%, 75%, 80%, and 0.54, respectively.
After multiple comparison correction, the sensitivity and specificity were not significantly different from PERCIST criteria (P = 0.06 and 0.25, respectively).
Results of PETinterim2
Among the patients presenting with a first PERCIST progression on PETinterim1, PETinterim2 was waved in 34% (10/29) due to a major clinical worsening and treatment stop. Therefore, 19/29 patients underwent PETinterim2:
31.6% (6/19) of patients had a PsPD. They corresponded to patients with a metabolic CR (1/19), PR (2/19), or SD (3/19) on PETinterim2 after an initial PD on PETinterim1. All of these patients had a DCB.
68.4% (13/19) of patients had a second and consecutive PERCIST PD on PETinterim2. Among them:
26.3% (5/19) had iDR (Figs. 2 and 3). In all these patients, the treatment was continued (due to an improved, or at least stable, clinical status according to the tumor board) and they all achieved a durable clinical benefit of immunotherapy.
42.1% (8/19) had iPDhomogeneous. All of them stopped the treatment and did not have a durable clinical benefit of immunotherapy. Three of them died during this 6-month follow-up.
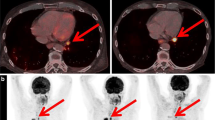
A 76-year-old man with metastatic squamous cell carcinoma treated with a second line of nivolumab. On PETinterim1 at 7 weeks of treatment (4 cycles), the maximum intensity projection image (MIP) showed a major metabolic progression of bone and liver metastases. PETinterim2, at 3 months of treatment (8 cycles), showed an immune-dissociated response (iDR) with disappearance of most liver and bone lesions but appearance of a new hypermetabolic cervical lymph node (black arrow). The patient had a durable clinical benefit and, at the last follow-up (12 months of treatment), was still treated with nivolumab
A 68-year-old women with metastatic adenocarcinoma treated with pembrolizumab. On PETinterim1 at 7 weeks of reatment (3 cycles), the maximum intensity projection image (MIP) showed a metabolic progression of adrenal and mediastinal lymph nodes and showed a stability of pulmonary lesion. PETinterim2, performed at 3 months of treatment (5 cycles), showed an immune-dissociated response with disappearance of most mediastinal lymph nodes, stability of pulmonary lesion, and progression of adrenal lesion. Because, the clinical status of the patient remained stable, the treatment was continued and the patient finally obtained a durable clinical benefit of immunotherapy
Correlation between the number of previous treatment line(s), PD-L1 tumor expression, and patients’ response to treatment
No significant differences of PET response patterns or DCB rates were assessed between patients in first or further treatment lines (Table 2).
No significant differences of PET response patterns or DCB rates were assessed according to the tumor PD-L1 expression (under or over 50%) (Table 3).
Discussion
Because tumor metabolic changes due to chemotherapy occur earlier than morphologic changes, 18FDG PET is widely used in oncology for monitoring response to conventional chemotherapy [15]. Nonetheless, few data are available concerning tumor response monitoring with 18FDG PET in metastatic lung cancer treated with anti-PD-1 or anti-PDL-1-based immunotherapies [14, 16, 17]. Based on limited patients’ cohort, new immune-related PET criteria have been proposed, mainly focusing on melanoma or lymphoma [13, 14, 18, 19]. Before robust and validated criteria can be defined by expert consensus, there is a strong need to firstly describe the imaging PET patterns of tumor metabolic changes to immunotherapy, and their association with patients’ clinical outcome. Because the issue of pseudo-progression has deeply changed the paradigm for tumor response evaluation; the prospective design of the present study is crucial. It guarantees a homogenized imaging protocol, image interpretation, and clinical decision in the case of a tumor progression on 18FDG PET.
In the setting of immunotherapy monitoring, the recent iRECIST recommendations have introduced a new metabolic response category in the case of a first tumor progression, termed “indeterminate progression” [9, 10]. This new classification provides the flexibility to have patients continue with treatment despite a first tumor progression if clinically stable. A subsequent evaluation is then required a few weeks later to confirm or deny true PD. When using 18FDG PET, the risk of falsely concluding to treatment failure after a first tumor progression is even higher than with CT because of its high sensitivity in detecting metabolic lesion activation, linked with the immune infiltrate. In the present study, new visceral/bone lesion(s) was the best surrogate criteria of treatment failure on PETinterim1 but still misclassified a high number of responding patients (PPV = 75%). As previously suggested in melanoma and lymphoma, it supports the need to introduce the “indeterminate response” PET category in the case of a first PERCIST progression that should be termed iUPD (immune unconfirmed progressive disease), as for iRECIST [19].
Pseudo-progression (PsPD) and progression
PsPD to immunotherapy is defined as a transient increase followed by a decrease in total tumor burden [7, 9, 20]. In the literature, pseudo-progression is assessed with CT in up to 10% of patients with melanoma but is very rare in NSCLC (0–5%) [9, 12, 20,21,22,23]. In our cohort, using 18FDG PET, pseudo-progression was observed in 12% (6/50) of patients, which is close from the only other PET retrospective study in lung cancer [14]. We found that PsPD is a common finding with PET and a surrogate of good tumor response to immunotherapy as all these patients obtained a DCB.
But the present study found a more unexpected finding: the immunotherapy was finally maintained in many patients with two consecutive PD according to PERCIST, and all these patients exhibited a durable clinical benefit of immunotherapy. The reasons that led clinicians to continue the immunotherapy were based both on the good clinical status and on a dissociated tumor evolution on PET, with concomitant progressive and responding lesions. Contrary to iRECIST, two consecutive PD on PET is not a robust criterion to confirm treatment failure to immunotherapy. The pattern of a dissociated progressive disease on PETinterim2 is strongly associated with a clinical benefit of immunotherapy and we propose to retermed it “immune dissociated response” (iDR). Using CT, Tazdait et al. have also described a dissociated pattern of tumor response (7.5% of patients vs 10% in our cohort) which was associated with significantly longer median OS than patients with confirmed progression [12]. Using PET, the retrospective study of Goldfarb et al. did not describe this dissociated evolutive pattern [14]. It may mainly be explained by our prospective design, leading to a higher proportion of patients treated beyond a first PERCIST progression and benefiting of a subsequent PETinterim2 (around 66% vs 30%).
Thus, our study demonstrates a new paradigm; pseudo-progression and dissociated-response are both very common findings when using 18FDG PET to monitor NSCLC response to immunotherapy and are surrogates of good tumor response. If the clinical condition of the patient is not worsening, these two atypical evolutive patterns should systematically be considered when assessing a first tumor progression with PET. Indeed, not to stop an effective treatment is a main issue in this critical setting. Based on these findings, an algorithm could be proposed for the use of 18FDG PET to monitor response of NSCLC to immunotherapy (Fig. 4).
Proposed algorithm for the use of 18FDG PET to monitor response of NSCLC to immunotherapy. Response categories according to PERCIST criteria: CR, complete metabolic response, PR, partial metabolic response; SD, stable metabolic disease; PD, progressive metabolic disease. New immune-adapted response categories adapted to immunotherapy: PsPD, pseudo-progression; iDR, immune-dissociated response (heterogeneous evolution of lesions on the subsequent PETinterim); iPDhomogeneous, immune-confirmed progressive disease (homogeneous progression of lesions on the subsequent PETinterim)
Response and stability on PETinterim1
Seven weeks after the initiation of immunotherapy, a PERCIST response (CR or PR) was observed in nearly a third of patients and appears as a reliable surrogate of a durable clinical benefit of the treatment. Indeed, immunotherapy was continued over the 6-month follow-up period in 87.5% (14/16) of these patients.
Around 10% of patients demonstrated a stable PERCIST disease, equivalent to the rate of SD observed with CT [12]. Most of these patients achieved a durable clinical benefit of immunotherapy.
Endpoints of the study
Due to the issue of pseudo-progression, traditional endpoints such as progression-free survival may not be a good surrogate of treatment efficacy of anticancer immunotherapies. Consequently, early metabolic changes observed at PETinterim1 and PETinterim2 were analyzed for their capacity to predict a durable clinical benefit, which we defined as continuation of treatment and maintained patients’ clinical status over 6 months after the initiation of immunotherapy. A durable clinical benefit was also the main endpoint in other recent studies researching predictive biomarkers for immunotherapy [11,12,13, 18]. Nonetheless, overall survival remains the major endpoint and a longer patient follow-up will be necessary in future studies.
The early identification of patients that will achieve a durable clinical benefit from immunotherapy may provide major information to the clinician for patient follow-up. In responding patients, it will allow not to stop an effective treatment mistakingly in cases with atypical PET evolutive patterns actually linked to treatment efficiency. In non-responding patients, it will allow an earlier treatment discontinuation. The benefits will then be the early stop of an inefficient treatment with possible adverse effects, the earlier start of a more efficient therapeutic modality for the patient, and the cost reduction of immunotherapy for society.
Limits of the study
In NSCLC, Ferrara et al. classified 13.8% of patients as having hyperprogressive disease and a poor prognosis [24]. In our study, 4/62 patients were excluded due to a severe clinical degradation and treatment stop before PETinterim1. Determining the molecular mechanisms involved, as well as seeking for baseline biomarkers of HPD, remain an important challenge.
In the present study, 20% (10/50) of patients had a PD on PETinterim1, concomitant with a significant clinical worsening. These patients were considered having an early treatment failure and the immunotherapy was stopped before PETinterim2. Among these patients, 6/10 died during the 6-month follow-up. Nonetheless, we cannot be sure that the remaining 4/10 patients would not have showed a delayed tumor response. Indeed, previous case reports described pseudo-progressions accompanied with worsening of clinical symptoms, followed by delayed and drastic treatment response to immunotherapy [25].
Compared with CT, the limit of 18FDG PET/CT to monitor tumor response to immunotherapy is the lack of specific guidelines/criteria for response assessment, such as iRECIST [10]. Robust studies comparing the concordance between RECIST, iRECIST, and PERCIST in this setting are also needed. Moreover, brain is a common site of metastatic spread in patients with NSCLC. The gold standard for the diagnosis of brain metastases is MRI. 18FDG PET/CT has indeed a lower sensitivity in detecting brain metastases [26]. Thus, when using PET/CT for the initial staging of NSCLC, brain MRI cannot be withdrawn. During the course of their disease, some patients with NSCLC will develop brain metastases. Because some of them may be detected by 18FDG PET/CT [27], the brain should be included in the PET acquisition field, and iodinated contrast agent should be injected if there are no contraindications. Nonetheless, new brain metastases will still be missed if no brain MRI is performed in the follow-up.
In conclusion, a PERCIST response assessed on FDG PET performed 7 weeks after beginning immunotherapy may accurately identify NSCLC patients with a durable clinical benefit of immunotherapy. To identify treatment failure, new visceral/bone hypermetabolic lesion is not a good criterion as it does not avoid misclassification of many responding patients. In patients with a first PD on PERCIST and treatment continuation, a subsequent PET identifies more than half of patients with an atypical response pattern, either pseudo-progression or immune dissociated response (iDR), both being strongly associated with a favorable clinical outcome.
References
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer [Internet]. https://doi.org/10.1056/NEJMoa1504627. 2015 [cited 2019 Mar 6]. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1504627.
Catacchio I, Scattone A, Silvestris N, Mangia A. Immune prophets of lung cancer: the prognostic and predictive landscape of cellular and molecular immune markers. Transl Oncol. 2018;11:825–35.
Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non–small cell lung cancer. Lung Cancer Amst Neth. 2016;99:79–87.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–51.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med Off Publ Soc Nucl Med. 2009;50(Suppl 1):122S–50S.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy : report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46:238–50.
Shields AF, Jacobs PM, Sznol M, Graham MM, Germain RN, Lum LG, et al. Immune modulation therapy and imaging: workshop report. J Nucl Med. 2018;59:410–7.
Carter BW, Bhosale PR, Yang WT. Immunotherapy and the role of imaging. Cancer. 2018;124:2906–22.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Leiserson MDM, Syrgkanis V, Gilson A, Dudik M, Gillett S, Chayes J, et al. A multifactorial model of T cell expansion and durable clinical benefit in response to a PD-L1 inhibitor. PLoS ONE [Internet]. 2018 [cited 2019 Mar 8];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6312275/.
Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47.
Sachpekidis C, Anwar H, Winkler J, Kopp-Schneider A, Larribere L, Haberkorn U, et al. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45:1289–96.
Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9:8.
Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med. 2007;48:36S–44S.
Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66.
Higuchi M, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, et al. FDG-PET in the evaluation of response to nivolumab in recurrent non-small-cell lung cancer. World J Surg Oncol [Internet]. 2016 [cited 2019 Mar 8];14. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5011843/.
Cho SY, Lipson EJ, Im H-J, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–8.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–96.
Wang GX, Kurra V, Gainor JF, Sullivan RJ, Flaherty KT, Lee SI, et al. Immune checkpoint inhibitor cancer therapy: spectrum of imaging findings. Radiogr Rev Publ Radiol Soc N Am Inc. 2017;37:2132–44.
Hodi FS, Hwu W-J, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7.
Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer [Internet]. 2016 [cited 2019 Mar 8];4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5168591/.
Hammer M, Bagley S, Aggarwal C, Bauml J, Nachiappan AC, Simone CB, et al. Thoracic imaging of non-small cell lung cancer treated with anti-programmed death receptor-1 therapy. Curr Probl Diagn Radiol. 2019;48:142–7.
Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52.
Vrankar M, Unk M. Immune RECIST criteria and symptomatic pseudoprogression in non-small cell lung cancer patients treated with immunotherapy. Radiol Oncol. 2018;52:365–9.
Deuschl C, Nensa F, Grueneisen J, Poeppel TD, Sawicki LM, Heusch P, et al. Diagnostic impact of integrated 18F-FDG PET/MRI in cerebral staging of patients with non-small cell lung cancer. Acta Radiol Stockh Swed 1987. 2017;58:991–6.
Nia ES, Garland LL, Eshghi N, Nia BB, Avery RJ, Kuo PH. Incidence of brain metastases on follow-up 18F-FDG PET/CT scans of non-small cell lung cancer patients: should we include the brain? J Nucl Med Technol. 2017;45:193–7.
Acknowledgements
The authors would like to thank Colin Debaigt for protocol submission to regulatory agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
O. Humbert, N. Cadour, M. Paquet, R. Schiappa, M. Poudenx, D. Chardin, D. Borchiellini, D. Benisvy, M.J. Ouvrier, C. Zwarthoed, A. Schiazza, H. Ghalloussi, P.M. Koulibaly, J. Darcourt, and J. Otto declare that they have no conflict of interest. M. Ilie reports personal fees from AstraZeneca, Bristol-Myers Squibb, Roche, Boehringer-Ingelheim, and Merck & Co outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Chest
Rights and permissions
About this article
Cite this article
Humbert, O., Cadour, N., Paquet, M. et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging 47, 1158–1167 (2020). https://doi.org/10.1007/s00259-019-04573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04573-4