Abstract
Purpose
To evaluate the therapeutic impact of 18F-fluorocholine (FCH) PET/CT in biochemical recurrent prostate cancer (PC) and to investigate the value of quantitative FCH PET/CT parameters in predicting progression-free survival (PFS).
Methods
This retrospective study included 172 consecutive patients with PC who underwent FCH PET/CT for biochemical recurrence. Mean rising PSA was 10.7 ± 35.0 ng/ml. Patients with positive FCH PET were classified into three groups: those with uptake only in the prostatic bed, those with locoregional disease, and those with distant metastases. Referring physicians were asked to indicate the hypothetical therapeutic strategy with and without the FCH PET/CT results. Clinical variables and PET parameters including SUVmax, SUVpeak, SUVmean, total lesion choline kinase activity (TLCKA) and standardized added metabolic activity (SAM) were recorded and a multivariate analysis was performed to determine the factors independently predicting PFS.
Results
In 137 of the 172 patients, the FCH PET/CT scan was positive, and of these, 29.9 % (41/137) had prostatic recurrence, 42.3 % (58/137) had pelvic lymph node recurrence with or without prostatic recurrence, and 27.7 % (38/137) had distant metastases. The FCH PET/CT result led to a change in treatment plan in 43.6 % (75/172) of the 172 patients. Treatment was changed in 49.6 % (68/137) of those with a positive FCH PET/CT scan and in 20 % (7/35) of those with a negative FCH PET/CT scan. After a median follow-up of 29.3 months (95 % CI 18.9 – 45.9 months), according to multivariate analysis age <70 years, SAM ≥23 and SUVmean ≥3 were parameters independently predicting PFS. A nomogram constructed using the three parameters showed 49 months of PFS in patients with the best scores (0 or 1) and only 11 months in patients with a poor score (score 3).
Conclusion
This study indicates that a positive FCH PET result in PC patients with biochemical recurrence predicts a shorter PFS and confirms the major impact of the FCH PET result on the management of biochemical recurrent PC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most common malignancy in men in Europe and North America [1]. It is the third leading cause of cancer in Europe in men over 50 years [2]. Following initial curative treatment for PC, biochemical recurrence occurs in 20 % to 50 % of patients [3, 4], and this represents a considerable management challenge. Whilst PSA is a sensitive and specific biomarker for early diagnosis of recurrence, it does not assist in determining disease localization. Management depends on the site and spread of the disease: in patients with local recurrence, curative treatment would be proposed, whilst in patients with distant metastases, palliative treatment with hormone therapy or chemotherapy would be more appropriate [5]. Furthermore, new focal salvage treatments are being evaluated including high-intensity focused ultrasound (HIFU), cryotherapy, external beam radiation therapy and surgery, all of which require precise localization of the recurrent disease [6]. The reported sensitivity and specificity of current imaging methods such as bone scan, CT, and ultrasonography vary widely, especially in patients with a low PSA level [7]. In recent years, there has been an improvement in imaging techniques, including MRI with spectroscopic imaging and dynamic contrast-enhanced imaging [8].
PET/CT using 11C-choline or 18F-choline (FCH) has emerged as a promising tool for early detection of PC recurrence in patients with biochemical failure [9–11]. In biochemical recurrent PC, several studies have shown that the predictive factors for positive PET are trigger PSA, PSA velocity, PSA doubling time, age, initial lymph node (LN) status and initial disease stage [10–16]. FCH PET/CT seems to play a key role in the management of biochemical recurrence, even when PSA levels are low [17, 18].
The aim of this retrospective study was to investigate the value of FCH semiquantification in predicting progression-free survival (PFS) and to determine the impact of FCH PET/CT on the therapeutic strategy in biochemical recurrent PC.
Materials and methods
Patients
From 2008 to 2013, we retrospectively studied 172 consecutive patients with a median age of 64 years (ranging 45 to 81 years). We obtained informed consent from all patients to allow the use of their clinical data for research purposes under a protocol approved in our institution. Patients were referred because of PC biochemical recurrence after negative or equivocal conventional imaging including bone scan, CT and/or MRI. In all patients, PSA values were recorded and PSA doubling time and velocity calculated [19]. Table 1 summarizes the patient characteristics.
The median time to biochemical recurrence after initial diagnosis was 3.49 years (range 0.1 – 16.25 years). Initial treatments included radiotherapy (35 %), radical prostatectomy (24 %), both modalities (29 %), androgen deprivation therapy alone (9 %), active surveillance (2 %), HIFU (0.5 %), and brachytherapy (0.5 %). Of the 172 patients, 77 (44.8 %) had a pelvic lymphadenectomy at initial staging, with positive results in 32.5 %. In 44 patients (25.6 %), the FCH PET/CT scan was performed during androgen deprivation therapy. The median rising PSA was 4.1 ng/ml (range 0.1 – 421 ng/ml). The median PSA doubling time and velocity were 6.8 months (range 0.3 – 1,049 months) and 2.7 ng/ml/year (range 0.1 – 193 ng/ml/year), respectively.
PET/CT imaging
18F-FCH was synthesized in Austria (IASOcholine; IASON GmbH) as described by Vassiliev et al. [20]. Two different PET/CT instruments were used for FCH PET/CT acquisitions. In 89 patients an integrated four-slice Discovery LS PET/CT system (GE Medical System, Waukesha, WI) was used consisting of a full-ring PET scanner with a 14.6-cm transverse field of view and an in-plane resolution of 4.8 mm at full-width at half-maximum. PET scans were acquired in two-dimensional mode and were reconstructed with a standard ordered-subsets expectation maximization iterative algorithm (two iterative steps). In 120 patients a Siemens Biograph mCT40 system (Siemens, Knoxville, TN) was used fitted with a 20-cm axial field of view, time-of-flight (TOF) feature and an in-plane resolution of 4.4 mm at full-width at half-maximum. Images were reconstructed with Ultra HD and TOF (three iterations, 21 subsets). All patients refrained from eating for at least 6 h before the FCH PET/CT scan. Acquisition was started 1 min after intravenous injection of 18F-FCH (3 MBq/kg) with dynamic PET images of the pelvis over 10 min (1 min/frame) to overcome the effect of bladder repletion. A whole-body PET/CT scan was performed 1 h after injection (six or seven bed positions) with an acquisition time of 3 min/bed position. A low-dose unenhanced CT scan was performed for localization and attenuation correction. Reformatted transverse, coronal and sagittal views were used for interpretation.
Image interpretation
FCH PET/CT images were scored as positive when focal tracer accumulation was greater than background activity (BG) or tracer physiological distribution. The diagnosis of malignant LNs following PET analysis was based on visual assessment of increased focal FCH uptake corresponding to LNs on the CT image, even if they were smaller than 10 mm. FCH uptake in the inguinal region was interpreted as reactive and was excluded from consideration as previously discussed in the literature [21, 22]. For bone lesions, any focus of nonphysiological uptake was considered as pathological. Patients with a positive FCH PET/CT scan were classified into three groups according to disease location: those with local recurrence with FCH uptake only in the prostatic bed, those with locoregional disease with FCH uptake in the pelvic LN only or also in the prostate area, and those with distant metastases with FCH uptake in bone, lung nodules or extrapelvic LN.
Tumour uptake and volumetric parameters
On the whole-body PET/CT scans, six indices were determined for the most intense tumour uptake considered as a positive target. These indices were:
-
1.
SUVmax: a volume of interest (VOI) covering the entire abnormal lesion was drawn.
-
2.
SUVpeak: a spherical region of interest of diameter 1 cm3 was defined. The region of interest location yielding the highest average SUV (SUVpeak) within the tumour was selected.
-
3.
SUVmean and metabolic volume derived by segmenting the tumour with a threshold of 40 %.
-
4.
Total lesion choline kinase activity (TLCKA): a parameter equivalent to total lesion glycolysis (TLG) for FDG, and calculated from the equation: TLCKA = SUVmean40% × volume40%.
-
5.
Standardized added metabolic activity (SAM) [23]. This figure-of-merit was defined as follows: a first VOI (VOI1) was drawn around the lesion and a second VOI (VOI2) was delineated. The borders of VOI1 were set at a reasonable distance from the tumour lesion in order to avoid the partial volume effect (PVE) and to ensure that no spill-over from the lesion to VOI2 occurred. Subsequently, the mean BG was calculated using the expression: mean BG = (SUV VOI2 − SUV VOI1)/(volume VOI2 − volume VOI1) where SUV is the product of the mean SUV and the respective volume. SAM was then calculated using the expression: SAM = SUV VOI1 − (mean BG × volume VOI1)
Follow-up
In most of the patients, the follow-up was the only method to validate the FCH PET/CT findings. The referring physicians were asked to indicate the hypothetical patient therapeutic strategy if no FCH PET/CT had been available, according to European Guidelines [5]. The final strategy was chosen by the referring physician based on the FCH PET/CT results in association with prognostic factors. A change in therapeutic strategy based on the FCH PET/CT results was defined as a different strategy, adaptation of the treatment plan, or the addition of a new strategy. Palliative treatment included chemotherapy and androgen deprivation therapy. Curative treatment included all local or locoregional treatments: surgery, external beam radiation therapy, HIFU or cryotherapy. Follow-up was performed by clinical examination and PSA measurements every 3 – 12 months until the date the patient was last known to be alive or had died.
PFS was considered as the interval between the PET/CT study and a new biochemical recurrence, or the last disease-free documented follow-up. Recurrence was defined as clinical evidence of recurrence or rising PSA. The clinical parameters recorded were: age, Gleason score, initial PSA, PSA doubling time, PSA velocity, androgen deprivation therapy during PET, and PET/CT parameters (number of hotspots, SUVmax, SUVpeak, SAM, TLCKA, and SUVmean40%).
Statistical analysis
The relationships among the qualitative and quantitative parameters and PFS were analysed using the log-rank test and univariate Cox regression analysis, respectively. The estimated hazard ratio (HR) of each parameter at the definite threshold was assessed using univariate Cox proportional hazards analysis. For each clinical or PET/CT parameter, different cut-off values were systematically studied and the most significant value was chosen. A p value <0.05 was considered statistically significant. From the results of the multivariate analysis a nomogram was constructed using the independent variables, and this score gave a measure of risk.
Results
FCH PET/CT results
Of the 172 FCH PET/CT scans, 137 (79.7 %) were analysed as positive. Median rising PSA was 4.1 ng/ml (range 0.1 – 421 ng/ml). One patient with low PSA (0.1 ng/ml) was referred because of bone pain and equivocal conventional imaging, before eventually undergoing radiation treatment. For the patient with the highest PSA (421 ng/ml), extensive conventional imaging was negative. He refused androgen deprivation therapy because of potential side effects. FCH PET/CT was proposed to target an eventual limited lesion and administer a new focal salvage treatment. Of the 44 PET/CT scans performed in patients on androgen deprivation therapy, 40 were positive (90.9 %). The median rising PSA and PSA velocity were, respectively, 5 ng/ml (range 0.1 – 421 ng/ml) and 3.5 ng/ml/year (range 0.2 – 193 ng/ml/year) in patients with a positive PET/CT scan, and 2.6 ng/ml (range 0.4 – 31.9 ng/ml) and 1.3 ng/ml/year (0.1 – 15.4 ng/ml/year) in those with a negative PET PET/CT scan (p < 0.001). The median PSA doubling time tended to be shorter in patients with a positive PET scan than in those with a negative PET scan (p = 0.061). No significant differences were found between the two groups regarding age at diagnosis, clinical stage, initial PSA, Gleason score, D’Amico risk group, or time of recurrence. Differences between the two groups are summarized in Table 2.
Among the patients with a positive FCH PET/CT scan, 29.9 % (41/137) had prostatic bed recurrence (Fig. 1), 42.3 % (58/137) had pelvic LN recurrence with or without prostatic bed recurrence and 27.7 % (38/137) had distant metastatic recurrence (bone, lung, extrapelvic LN). However, none of the patients had isolated abdominal LN metastases. Initial PSA was higher in patients with distant metastatic recurrence (median 17 ng/ml) than in those with local recurrence (median 14 ng/ml), or locoregional disease (median 9.2 ng/ml; p = 0.04). No significant differences in age were observed among the three groups of patients (p = 0.54). Time to recurrence was significantly shorter in patients with metastatic recurrence (mean 44.4 months) than in those with local recurrence (mean 64.6 months; p = 0.01). PSA at the time of PET/CT examination was not significantly different among the three groups. PSA velocity was significantly higher (p = 0.007), and PSA doubling time shorter (p = 0.001) in patients with metastatic disease than in patients with local disease.
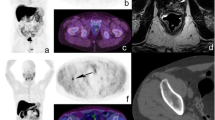
Axial PET (a), CT (b), and PET/CT (c) images show a single site of uptake in the prostate bed after initial external beam radiation therapy. Before FCH PET/CT, androgen deprivation therapy was planned in this patient. After FCH PET/CT this patient received salvage cryotherapy to the prostatic area. At the time of this report, PSA was below the limit of detection with 17 months since treatment recurrence
Therapeutic strategies
The FCH PET/CT results led to a change in the treatment plan in 43.6 % of patients (75/172). The PET/CT results led to a change in treatment in 20 % of patients (7/35) with a negative PET/CT scan and in 49.6 % of patients (68/137) with a positive PET/CT scan. Among these 68 patients, treatment was changed from curative to palliative in 5.9 % (4/68), from palliative to a different treatment in 41.2 % (28/68), and (due to limited disease) from palliative (androgen deprivation therapy or chemotherapy) to curative in 51.5 % (35/68), as shown in Fig. 2. Details of the different changes are presented in Table 3.
A 71-year-old patient had a PSA increase to 2.1 ng/ml 5 years after initial radical prostatectomy. FCH PET/CT imaging shows lymph node uptake in the pelvic area. Before FCH PET/CT, androgen deprivation therapy was planned. The PET/CT findings led to a change in therapeutic strategy to salvage radiation therapy extended to include the pelvic lymph nodes. After treatment, PSA decreased to 0.3 ng/ml, and at the time of this report he was still progression-free
Follow-up and PFS
The median follow-up was 29.3 months (95 % CI 18.9 – 45.9 months). Of the 172 patients, 49 (28.5 %) developed recurrence, and 123 (71.5 %) remained in remission following treatment guided by the FCH PET/CT findings. Two-year PFS was significantly higher in patients with prostate recurrence (66 %, 95 % CI 36 – 84 %) than in those with metastatic recurrence (42 %, 95 % CI 19 – 64 %; p < 0037; Fig. 3).
In the univariate analysis factors significantly associated with PFS were: age <70 years (HR 2.06, p = 0.035), rising PSA >9 ng/ml (HR 2.04, p = 0.041), PSA velocity >15 ng/ml/year (HR 3.31, p = 0.011), number of hotspots on FCH PET/CT three or more (HR 2.1, p = 0.019), SUVmax ≥5 (HR 3.09, p = 0.012), SUVpeak ≥6 (HR 2.11, p = 0.02), SUVmean40% ≥3 (HR 4.26, p = 0.003), and SAM ≥23 (HR 2.83, p = 0.003). In the multivariate analysis independent prognostic factors were: age <70 years (HR 2.12, p = 0.045), SAM ≥23 (HR 2.1, p = 0.039) and SUVmean ≥3 (HR 3.35, p = 0.02). The results regarding the prognostic and predictive values of all variables considered are presented in Table 4.
From the results of the multivariate analysis, a PC nomogram was constructed using the independent negative prognostic variables (1 point for each of age <70 years, SUVmean ≥3 and SAM ≥23). PFS was shorter in patients with a score of 2 (p = 0.036) and score of 3 (p < 0.001) compared to patients with a score of 0. Patients with a score of 0 or 1 in the nomogram had a median PFS of 49 months, those with a score of 2 a median PFS of 23 months and those with a score of 3 a median PFS of 11 months. According to the nomogram scores, the probabilities of a PFS of 3 years were 65.2 % (95 % CI 41.9 – 81.0 %), 45.1 % (95 % CI 13.8 – 70.1 %) and 13.9 % (95 % CI 0.9 – 43.4 %) for scores 0 or 1, 2 and 3, respectively (Fig. 4).
PFS was significantly different between the patients with a negative PET/CT scan and those with a positive PET/CT scan with a score of 3 (p = 0.031). To understand the fate of all patients, a survival analysis of patients with a positive PET/CT scan and different scores and patients with a negative PET/CT scan is shown in Fig. 5.
Discussion
In recent years, many teams have evaluated PET/CT using choline radiolabelled with 11C (CCH) or 18F (FCH) in PC as attested by the growing number of studies and currently 116 listed publications [24]. Furthermore, CCH has been approved by the FDA, and FCH has been approved in Europe for the detection of bone metastases in PC [25, 26] which has led to a limited role assigned by oncological or urological societies [5]. However, choosing between local or locoregional salvage treatment or a systemic therapy in patients with biochemical recurrence of PC is one of the most difficult problems for urologists. This current limited recommendation can be explained by a lack of a common approach to histological confirmation in various studies in the literature, and this led Brogsitter at al. not to give FCH pooled specificity due to insufficient data [24].
Several studies with relevant results have focused on the impact of choline PET/CT on therapeutic decision making [3, 27]. Two retrospective studies investigated the prognostic implications of this imaging method on overall and disease-free survival, one using CCH in 195 patients with castrate-resistant PC [28] and the other using CCH in 302 hormone-naive patients with PC [29]. Breeuwsma et al. studied the correlation between the results of PET with CCH and disease-free survival in hormone-naive patients with recurrent PC after radical prostatectomy [30]. This retrospective study in 172 patients with biochemical recurrence of PC is the first study to our knowledge to look for prognostic factors corresponding to semiquantitative parameters derived from this imaging method. It is also the only study that has used semiquantitative data from FCH PET/CT to generate a nomogram to help clinicians in their treatment choice.
In PC recurrence after initial treatment or at the stage of castrate-resistance, overall survival and/or recurrence-free survival are relevant patient outcome data, and may be useful to guide and optimize treatment decisions. These data can be described by nomograms using clinical and biological parameters. Due to the disease heterogeneity in terms of prognosis, creating a prognostic model for overall or disease-free survival based on specific imaging data has a significant clinical value. In patients with castrate-resistant cancer, Giovacchini et al. using CCH PET found three independent prognostic factors: PET positivity, PSA level and Gleason score >7 [28]. In hormone-naive patients with biochemical failure after radical prostatectomy, the same team confirmed PET positivity as an independent prognostic factor associated with different clinical factors [29]. They constructed a nomogram that included CCH PET positivity and the decision to change treatment based on the PET results.
In this study, the multivariate analysis identified three independent prognostic factors, two of which are semiquantitative parameters of FCH PET: SAM index ≥23, SUVmean ≥3 and age <70 years. This is the first time to our knowledge that FCH semiquantitative parameters have been found to predict disease-free survival. In general, the SUVmean is rarely used because it requires precision and robustness to delineate the functional tumour volume and is influenced by the PVE [31]. This is most critical in therapeutic monitoring where the PVE may have a variable influence [32], but this was not the case in our study.
With FDG, easily applicable and straightforward methodologies for assessing TLG while avoiding PVE and assessing tumour volume by thresholding, and with less count dependence, are of major interest [33]. SAM with FDG is a marker derived from TLG which takes into account metabolic activity as well as lesion volume [34]. In contrast to TLG, the calculation of SAM includes background subtraction, assuming that tumour tissue develops on top of normal tissue. As such, the SAM method also avoids the problems of lesion segmentation. Moreover, when the necessary VOIs are placed at a sufficient distance from the actual tumour border, PVE are also avoided, which makes accurate response assessment possible in small lesions [23]. In the literature, this parameter is described as being superior to the widely used SUV, as it is related to the total specific activity in the object rather than the concentration of activity. This means that it is independent of image resolution [35]. Therefore, the SAM index has the advantages of potential independence from the determination of the active metabolic tumour volume and being less influenced by background noise than SUVmax [23].
The combination of our three independent prognostic factors allowed construction of a prognostic nomogram for predicting PFS. Patients had very different PFS depending on their score: 49 months in patients with the best scores (score 0 or 1), and only 11 months in patients with a poor score (score 3). This system allows the clinician to adopt the best therapeutic approach for the patient. As stated by the speaker in the SNMMI Highlights lecture: “This study is an example of the ways in which we are acquiring similar tools in molecular imaging and therapy that are helpful in patient guidance in oncology” [36].
This score is relevant in hormone-naive patients with castrate-resistant PC with positive PET/CT results, and is a new tool that is more informative than qualitative visual analysis. An interesting finding was that the estimated PFS in patients with a negative PET/CT result was not better than the estimated PFS in patients with positive PET results. This finding is contrary to that of Giovacchini et al. in hormone-naive patients with PC [29]. In this study, the outcome in patients with a negative PET choline scan was not different from that in patients with a positive choline PET scan with a score of 0 or 1 or 2, but was less unfavourable than in patients with a positive choline PET scan and a score of 3. This could be explained by the fact that clinicians were seemingly more likely to adopt a “wait-and see” approach when no focus was identified before choosing between a systemic or a locoregional treatment. Nevertheless, in this situation of biochemical recurrence, the natural history seems to be progression, which ultimately leads to an event in the course of the patient’s disease. Our population was heterogeneous (hormone-naive and castrate-resistant disease) including patients with different stages of disease (early recurrence or more advanced disease), in contrast to the study by Giovacchini et al. that included patients with first recurrence, i.e. less advanced disease.
The impact of choline PET/CT on the choice of therapy in patients with biochemical recurrence of PC has been studied by several groups [3, 27]. In a retrospective series of 156 patients, Soyka et al. found that FCH PET/CT led to a change in treatment offered in 48 % of patients [3]. Ceci et al. found that CCH PET/CT led to a treatment change in 46.7 % of patients [27]. In our study, FCH PET/CT led to a treatment change in 43.6 % of patients. Whilst slightly lower than in the previous studies, this value would have been in accordance with those in the previous studies if we had included PET-positive patients (49.6 %). In our study, a treatment change was also observed in patients who had negative FCH PET (20 %). In the majority of these patients, urologists chose surveillance (57 %) or carried forward the planned treatment or preferred a localized radiation therapy (43 %), which is in agreement with previous reports [3, 27]. In 51.5 % of patients with a positive FCH PET scan treatment was switched between palliative treatment and focused curative therapy (pelvic lymphadenectomy, stereotaxic radiotherapy, cryotherapy or HIFU), which is similar to previously reported findings [3].
Patients with positive CCH or FCH PET scans often benefit from recently developed focused treatments. These imaging studies are of particular interest given that these treatments, that delay the use of systemic therapy, are now available. Finally, the impact of these treatment changes on progression of the disease needs to be considered. Ceci et al. described this aspect in some patients, in whom a major clinical impact was observed, with a complete or partial response in 71.4 % [27]. Soyka et al. observed a complete or partial response in 69.2 % of patients who underwent a therapeutic change [3]. In our study, 71.5 % of patients were in remission without further recurrence. In these three studies, the outcome of treatment changes dictated by the choline PET results appears promising with a high rate of complete or partial responses.
A possible limitation of our study was its retrospective and single-centre design and the new use by urologists of FCH PET data for the care of their patients which could have led to bias. Favourable results of the therapeutic changes evaluated in this study must be compared with those of a prospective multicentre study. In addition, follow-up was short in some of our patients. However, we do not think there was significant bias in this study: patient management complied with guidelines recommended in patients with PC with biochemical recurrence. The fact that only a few lesions detected by FCH PET were analysed pathologically may be another limitation of the study data. However, it would not have been ethical or practical to perform several biopsies in the same patient to verify the nature of the abnormalities revealed by PET imaging. Studies using radiolabelled choline PET/CT in patients with biochemical recurrence have also encountered this problem with pathological confirmation in only 4.5 % to 10 – 15 % of patients [14, 28]. The problem was addressed in these studies by monitoring patients as in our study. Because of the absence of deaths during follow-up, evaluation of the impact of imaging on overall patient survival was not possible in this study. FCH PET was requested most often in patients with very moderate biochemical recurrence and monitoring was probably not long enough compare to the low frequency of deaths at this stage of the disease.
Conclusion
This study indicated that not only positive qualitative FCH PET/CT results predict a short PFS in PC patients with biochemical recurrence, but a semiquantitative approach also allows individualizing different patient subpopulations and enables clinicians to tailor available salvage treatments for personalized medicine. Further research is warranted to confirm these findings in a larger population, as well as to explore how FCH PET imaging might guide treatment in PC patients with biochemical recurrence.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Malvezzi M, Bertuccio P, Levi F, Vecchia CL, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24(3):792–800.
Soyka JD, Muster MA, Schmid DT, Seifert B, Schick U, Miralbell R, et al. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39(6):936–43.
Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol. 2011;59(1):51–60.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79.
Punnen S, Cooperberg MR, D’Amico AV, Karakiewicz PI, Moul JW, Scher HI, et al. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64(6):905–15.
Kane CJ, Amling CL, Johnstone PAS, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61(3):607–11.
Sciarra A, Panebianco V, Salciccia S, Cattarino S, Lisi D, Gentilucci A, et al. Modern role of magnetic resonance and spectroscopy in the imaging of prostate cancer. Urol Oncol. 2009;29(1):12–20.
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [11C] choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2009;37:301–9.
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50(9):1394–400.
Kwee SA, Coel MN, Lim J. Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med. 2012;26(6):501–7.
Marzola MC, Chondrogiannis S, Ferretti A, Grassetto G, Rampin L, Massaro A, et al. Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin Nucl Med. 2013;38(1):e26–32.
Giovacchini G, Picchio M, Parra RG, Briganti A, Gianolli L, Montorsi F, et al. Prostate-specific antigen velocity versus prostate-specific antigen doubling time for prediction of 11C choline PET/CT in prostate cancer patients with biochemical failure after radical prostatectomy. Clin Nucl Med. 2012;37(4):325–31.
Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54(6):833–40.
Detti B, Scoccianti S, Franceschini D, Cipressi S, Cassani S, Villari D, et al. Predictive factors of [18F]-Choline PET/CT in 170 patients with increasing PSA after primary radical treatment. J Cancer Res Clin Oncol. 2013;139(3):521–8.
Rybalov M, Breeuwsma AJ, Leliveld AM, Pruim J, Dierckx RA, de Jong IJ. Impact of total PSA, PSA doubling time and PSA velocity on detection rates of 11C-Choline positron emission tomography in recurrent prostate cancer. World J Urol. 2013;31(2):319–23.
Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38(1):55–63.
Graute V, Jansen N, Ubleis C, Seitz M, Hartenbach M, Scherr MK, et al. Relationship between PSA kinetics and [18F] fluorocholine PET/CT detection rates of recurrence in patients with prostate cancer after total prostatectomy. Eur J Nucl Med Mol Imaging. 2012;39(2):271–82.
Memorial Sloan-Kettering Cancer Center. Prostate Cancer Nomograms: PSA Doubling Time. New York, NY: Memorial Sloan-Kettering Cancer Center, 2014. http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx.
Vassiliev D, Krasikova R, Kutznetsova O, Federova O, Nader M. Simple HPLC method for the detection of N,N-dimethylaminoethanol in the preparation of [N-methyl-11C] choline. Eur J Nucl Med Mol Imaging. 2003;30(2 Suppl):342P.
Schmid DT, John H, Zweifel R, Cservenyak T, Westera G, Goerres GW, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology. 2005;235(2):623–8.
Oprea-Lager DE, Vincent AD, van Moorselaar RJA, Gerritsen WR, van den Eertwegh AJM, Eriksson J, et al. Dual-phase PET-CT to differentiate [18F] Fluoromethylcholine uptake in reactive and malignant lymph nodes in patients with prostate cancer. PLoS One. 2012;7(10):e48430.
Mertens J, Dobbeleir A, Ham H, D’Asseler Y, Goethals I, Van de Wiele C. Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2012;39(9):1441–8.
Brogsitter C, Zöphel K, Kotzerke J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur J Nucl Med Mol Imaging. 2013;40 Suppl 1:S18–27.
FDA approves 11C-choline for PET in prostate cancer. J Nucl Med. 2012;53(12):11N.
Brenot-Rossi I. Focus: Prostate cancer and PET-choline. Prog Urol. 2014;24(1):3–8.
Ceci F, Herrmann K, Castellucci P, Graziani T, Bluemel C, Schiavina R, et al. Impact of (11)C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging. 2014;41(12):2222–31.
Giovacchini G, Picchio M, Garcia-Parra R, Briganti A, Abdollah F, Gianolli L, et al. 11C-choline PET/CT predicts prostate cancer-specific survival in patients with biochemical failure during androgen-deprivation therapy. J Nucl Med. 2014;55(2):233–41.
Giovacchini G, Incerti E, Mapelli P, Kirienko M, Briganti A, Gandaglia G, et al. [11C]Choline PET/CT predicts survival in hormone-naive prostate cancer patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2015;42(6):877–84.
Breeuwsma AJ, Rybalov M, Leliveld AM, Pruim J, de Jong IJ. Correlation of [11C] choline PET-CT with time to treatment and disease-specific survival in men with recurrent prostate cancer after radical prostatectomy. Q J Nucl Med Mol Imaging. 2012;56(5):440–446.
Visvikis D, Hatt M, Tixier F, Cheze Le Rest C. The age of reason for FDG PET image-derived indices. Eur J Nucl Med Mol Imaging. 2012;39(11):1670–2.
Krak NC, Boellaard R, Hoekstra OS, Twisk JWR, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32(3):294–301.
Mertens J, De Bruyne S, Van Damme N, Smeets P, Ceelen W, Troisi R, et al. Standardized added metabolic activity (SAM) in 18F-FDG PET assessment of treatment response in colorectal liver metastases. Eur J Nucl Med Mol Imaging. 2013;40(8):1214–22.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–71.
Fleming JS, Tossici-Bolt L, Guy M, Kemp P. Comment on Mertens et al.: Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2013;40(5):788–9.
Mahmood U. 2014 SNMMI highlights lecture: oncology. J Nucl Med. 2014;55(11):9N–24N.
Compliance with ethical standards
ᅟ
Funding
This work was supported by grants from the French National Agency for Research called “Investissements d’Avenir”, Labex IRON no. ANR-11-LABX-0018-01 and Equipex ArronaxPlus no. ANR-11-EQPX-0004.
Conflicts of interest
None.
Informed consent
For this retrospective study, we obtained informed consent from all patients to allow the use of their clinical data for research purposes under a protocol approved in our institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colombié, M., Campion, L., Bailly, C. et al. Prognostic value of metabolic parameters and clinical impact of 18F-fluorocholine PET/CT in biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42, 1784–1793 (2015). https://doi.org/10.1007/s00259-015-3123-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3123-5