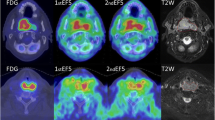
Abstract
Purpose
In order to improve the treatment of squamous cell carcinoma of the head and neck, precise information on the treated tumour’s biology is required and the prognostic importance of different biological parameters needs to be determined. The aim of our study was to determine the predictive value of pretreatment PET/CT imaging using [18F]FDG, a new hypoxia tracer [18F]EF5 and the perfusion tracer [15O]H2O in patients with squamous cell cancer of the head and neck treated with radiochemotherapy.
Methods
The study group comprised 22 patients with confirmed squamous cell carcinoma of the head and neck who underwent a PET/CT scan using the above tracers before any treatment. Patients were later treated with a combination of radiochemotherapy and surgery. Parametric blood flow was calculated from dynamic [15O]H2O PET images using a one-tissue compartment model. [18F]FDG images were analysed by calculating standardized uptake values (SUV) and metabolically active tumour volumes (MATV). [18F]EF5 images were analysed by calculating tumour-to-muscle uptake ratios (T/M ratio). A T/M ratio of 1.5 was considered a significant threshold and used to determine tumour hypoxic subvolumes (HS) and hypoxic fraction area. The findings were finally correlated with the pretreatment clinical findings (overall stage and TNM stage) as well as the outcome following radiochemotherapy in terms of local control and overall patient survival.
Results
Tumour stage and T-classification did not show any significant differences in comparison to the patients’ metabolic and functional characteristics measured on PET. Using the Cox proportional hazards model, a shorter overall survival was associated with MATV (p = 0.008, HR = 1.108), maximum [18F]EF5 T/M ratio (p = 0.0145, HR = 4.084) and tumour HS (p = 0.0047, HR = 1.112). None of the PET parameters showed a significant effect on patient survival in the log-rank test, although [18F]EF5 maximum T/M ratio was the closest (p = 0.109). By contrast, tumour blood flow was not correlated with any of the clinical endpoints. There were no statistically significant correlations among [18F]FDG SUVmax, [18F]EF5 T/M ratio and blood flow.
Conclusion
Our study in a limited number of patients confirmed the importance of MATV in the prognosis of locally advanced squamous cell carcinoma of the head and neck. It is of interest that high uptake of the hypoxia tracer [18F]EF5 showed a stronger correlation with a poor clinical outcome than [18F]FDG uptake. This confirms the importance of hypoxia in treatment outcome and suggests that [18F]EF5 may act as a surrogate marker of radioresistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the European Cancer Observatory [1], 139.500 new cases of head-and-neck squamous cell cancer (HNSCC) were registered Europe-wide (EU 27) in 2012. Despite advances in therapy, 63.500 patients died of the disease in the same year. In the USA an estimated 55.070 new cases will be registered in 2014 while 12,000 patients will die due of the disease [2].
The treatment of head and neck cancer has advanced significantly in recent years with the introduction of new imaging techniques and particularly with the introduction of intensity-modulated radiation therapy. These new techniques have the potential to increase the precision of delivery of (higher) radiation doses to the areas of interest (also within the tumour) resulting in better treatment success and fewer side effects. Because PET with CT can depict tissue function as opposed to anatomy, this imaging modality is rapidly becoming central in therapy planning in malignant neoplasms despite the fact that more validation is needed in this field [3]. In recent years several negative physiological prognostic factors have been identified that influence the outcome of treatment, with low blood flow and hypoxia [4] being among the most important. A high glucose metabolic rate (visualized as high [18F]FDG uptake on PET) has been added to this list. This finding, however, is controversial and has been contradicted by the results of some studies [5].
However, as stressed by Ang in his recent editorial [6], recent findings indicate that the importance of hypoxia in head and neck cancer might have been overestimated and that therefore selection of patients who are most likely to benefit from the new drugs targeting hypoxia is critical. This might not be true only for hypoxia, but also for many other negative prognostic factors, such as human papillomavirus and in particular HPV-16 which is receiving greater attention as studies are repeatedly identifying it as an important independent prognostic factor in the treatment of head and neck cancer [7].
The aim of our current study was therefore to determine the prognostic significance for local recurrence and overall survival of pretreatment [18F]EF5 PET (a new hypoxia tracer), [15O]H2O PET (for blood flow) and [18F]FDG PET imaging in patients with head and neck cancer. The study was designed as a follow-up to our previous study in which we evaluated the feasibility of PET with [18F]EF5 for imaging hypoxia in head and neck cancer [8].
Materials and methods
Patient population
The study was performed after obtaining approval from the Ethics Board of Turku University Hospital and in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from each patient. The study participants comprised 22 patients (17 men and 5 women) with newly diagnosed HNSCC referred to Turku University Central Hospital for radiochemotherapy (RCT) between December 2005 and January 2007. Their average age was 56 ± 12 years (23 – 78 years) and their average body mass was 71 ± 21 kg (50 – 130 kg). The most relevant patient information is presented in Table 1.
Synthesis of [18F]EF5, [15O]H2O and [18F]FDG
The synthesis of all the PET tracers are described in detail in previous papers by our groups [8, 9].
PET/CT device
All data were acquired on a Discovery STE PET/CT scanner (GE Medical Systems, Milwaukee, WI) which combines a helical 64 slice scanner and a bismuth germanate oxide (BGO) block PET tomograph, the latter consisting of 13,440 BGO crystals arranged in 24 rings yielding 47 transverse slices spaced axially at 3.27 mm. The PET imaging field of view is 70 cm in diameter and 15.7 cm in axial length. Attenuation correction was performed using a low-dose ultrafast CT protocol (80 mAs, 140 kV). The performance of the scanners is discussed in more detail by Teras et al. [10].
Data acquisition protocol
Imaging of tumour blood flow with [15O]H2O was followed by PET/CT imaging with [18F]EF5. On a separate day, [18F]FDG PET/CT was performed. Both studies were performed in random order within an average of 7 days (range 1 – 15 days). The imaging protocols were identical to those presented in our previous paper [8]. After the CT acquisition [15O]H2O (1,184 ± 112 MBq) was injected and dynamic emission images immediately acquired over 6 min. Arterial blood was withdrawn using a pump (Alitea, Stockholm, Sweden) at a speed of 6 ml/min to obtain the input function. Blood radioactivity was measured using an online detector (GE Medical Systems, Uppsala, Sweden). After completion of the [15O]H2O blood flow study, patients were removed from the scanner and a slow (approximately 15 s) intravenous bolus of [18F]EF5 (277 ± 70 MBq) was administered. Images (two frames of 300 s each) were acquired at 3 h (171 ± 1 min) after tracer injection.
The FDG study was performed according to the routine oncological FDG protocol used for radiotherapy (RT) planning in our centre. The average injected FDG dose was 346 ± 49 MBq (200 – 389 MBq) and the image was acquired approximately 1 h (58 ± 8 min) after tracer injection.
Image analysis
All images were analysed using noncommercial image analysis software VINCI (Max Planck Institute for Neurological Research, Cologne, Germany; http://www.nf.mpg.de/vinci3/).
Calculation of metabolically active tumour volume, hypoxic subvolume and hypoxic fraction area
FDG images
Metabolically active tumour volume (MATV) was determined using 50 % of the maximum standardized uptake value (SUVmax) as the threshold. Due to very low measured FDG SUVmax (3.71 and 2.97) and the resulting large MATV, the 50 % threshold was substituted by SUV 2.5 as the cut-off value in two patients (patients 12 and 14 in Table 2).
[18F]EF5 images
[18F]EF5 tumour-to-muscle uptake ratios (T/M ratios) were determined by dividing all the voxel values by the average muscle value obtained from a region of interest covering the dorsal neck muscle area contralateral to the tumour in three consecutive planes. The [18F]EF5-avid volume was determined using a T/M ratio threshold of 1.5. The total volume of voxels above this threshold and inside the MATV were considered as the hypoxic subvolume (HS). The ratio between HS and MATV represents hypoxic fraction area (HFA).
Calculation of blood flow and analysis of blood flow images
Blood flow images were calculated using a one-tissue compartment model, which was linearized [11]. Lawson-Hanson nonnegative least squares [12] are used to solve general linear least squares functions. The measured arterial input function for the model was corrected for the measured external dispersion in the tubing by deconvolution with an exponential function. The delay was corrected by fitting the input curve to the measured tissue curve [13, 14]. After calculation of the parametric blood flow image the maximum blood flow value inside the tumour was obtained using the CT and FDG images for spatial orientation.
Radiochemotherapy and surgery
External beam RT to the tumour and regional lymph nodes was performed using a linear accelerator. Six patients underwent definitive RT to a median dose of 64.6 ± 6.4 Gy in 29.5 ± 3.9 fractions, and 16 patients underwent preoperative RT to a median dose of 63.8 ± 3.3 Gy in 29.2 ± 3.2 fractions. One patient (patient 20) refused to undergo surgery and received only (definitive) RT to a total dose of 69.3 Gy. All but one patient (patient 21) received concomitant chemotherapy with cisplatin. Of these 21 patients, 20 received a dose of 40 mg per m2 of body surface (BS) per week of RT to a cumulative dose of 387.4 ± 91.9 mg or 199.7 ± 45 mg/m2 of BS. One patient (patient 16) received 100 mg per m2 of BS per 3 weeks twice.
Study end-points
Overall survival and local control were used as the end-points. Local control was defined as sterilization of primary tumour and/or metastases by RCT. Local relapse was determined by subsequent CT imaging. Overall survival was calculated for the period between the first scan and the conclusion of the follow-up period on 30 June 2010. The median follow-up time was 41 ± 15 months.
In our centre the therapy of head-and-neck cancer consists of either definitive RCT or surgery alone or alternatively a combination of preoperative RCT and surgery. The decision is made by a specialist team consisting of an ORL surgeon, an RT specialist and an oncologist.
Statistical analysis
To test the distribution of the data, the Shapiro-Wilk test was used. Due to the nonnormal distribution of the data, Spearman’s correlation coefficients were calculated to determine the correlations among the uptake of FDG and EF5 and blood flow. The differences between the groups were evaluated using the nonparametric Wilcoxon test. For survival analysis the log-rank test and the Cox proportional hazards model were used. P values of less than 0.05 were considered statistically significant. SAS version 9.2 software was used to perform the statistical tests (SAS Institute Inc., Cary, NC).
Results
Pretreatment disease and tumour-specific findings
TNM stage, overall stage and grade in each patient are shown in Table 1.
PET findings
The study protocol was tolerated well by the patients and none of the studies was interrupted due to patient discomfort or complications. In two patients the arterial activity curve was not obtained, in one due to problems with the activity meter and the other due to blood pump malfunction. In these two patients the curve was calculated using a new method introduced in our previous paper [15]. Detailed results of the PET image analysis are presented in Table 2.
The average MATV (derived by applying a threshold of 50 % SUVmax) was 9.26 ± 10.17 cm3 (range 45 – 1.1 cm3) and the average maximum FDG SUV was 12.56 ± 6.92 (range 2.97 – 26.0). The maximum EF5 uptake value was only obtained in the patients with at least one voxel value above T/M ratio 1.5. In 6 of the 22 patients no voxels with values above this threshold were observed. The average maximum T/M ratio in the remaining 16 patients was 2.03 ± 0.53 and the average tumour volume above this threshold (or HS) was 6.04 ± 9.49 cm3 (range 0.10 – 28.85 cm3). This translates into an average HFA of 36.7 ± 35.7 % when taking only the EF5-positive tumours into account or 26.67 ± 34.50 % when taking all tumours into account. The average maximum blood flow in tumours was 102.52 ± 52.2 mL/min/dL (range 31.9 – 207.15 mL/min/dL).
Correlations between PET parameters
The only statistically significant correlations were between MATV and FDG SUVmax (R = 0.524, p = 0.0117) and between MATV and HFA (R = 0.527, p = 0.0123) . MATV was also correlated with EF5 T/M ratio with borderline significance (R = 0.417, p = 0.068). Other correlations were not statistically significant.
Influence of pretreatment tumour characteristics on PET values
None of the PET parameters differed significantly between patients divided according to disease stage (I + II vs. III + IV. Only MATV was significantly different between patients divided according to T stage (T1 + T2 vs.T3 +T4, p = 0.009).
Relationships between pretreatment parameters and clinical end-points
The clinical end-point results are shown in Table 3.
Local control
Pretreatment PET values did not differ significantly between patients divided according to local control (with vs. without local control) at follow-up. Differences in FDG SUVmax, EF5 T/M ratio and blood flow between the groups are shown in Fig. 1.
Differences in FDG SUVmax, EF5 T/M ratio and blood flow between patients with stages I – III and stage IV, between patients with and without local control at follow-up, and between patients with T stage 1 or 2 and T stage 3 or 4. Despite visible trends, none of the pretreatment or posttreatment clinical findings had any significant effect on the most relevant PET values
Overall survival
Overall survival did not differ significantly between patients divided according to the median value of each PET parameter. The difference was closest to significance between patients divided according to the median EF5 T/M ratio (log-rank test, p = 0.109; Fig. 2).
Analysed using the Cox proportional hazards model, only MATV (p = 0.008; HR = 1.11), EF5 T/M ratio (p = 0.015; HR = 4.08) and HS (p = 0.005; HR = 1.12) were significantly correlated with overall survival. None of the other PET values showed statistical significance.
Discussion
We present here the results of a study of the prognostic value of pretreatment PET imaging of hypoxia ([18F]EF5), metabolic activity ([18F]FDG) and blood flow ([15O]H2O) in HNSCC. The results obtained in 22 patients indicate that MATV is an important prognostic factor. This value is similar to the anatomic tumour size, which was also confirmed in our study by the significant influence of T classification on MATV. This is in line with previous findings [16]. We also explored the correlations between individual PET parameters as well as pretreatment tumour characteristics and PET parameters. The most relevant results, however, were obtained in the analysis of correlation between PET parameters and clinical end-points, in our case overall survival. The log-rank test showed only a borderline significant effect of pretreatment [18F]EF5 uptake on overall survival. However, the Cox proportional hazards model showed a significant correlation between [18F]EF5 maximum pretreatment uptake and overall survival of patients.
The Kaplan-Meier analysis (among others) showed no significant difference between patients with EF5 T/M ratio <1.5 and patients with EF5 T/M ratio >1.5. On the one hand this can be explained by the image-derived cut-off values that are always to some extent arbitrary in nature, and which rarely translate into clinically significant differences; this also tends to be true for FDG cut-off values. On the other hand this might be explained by the relatively small number of patients. In choosing an image-based threshold for significant hypoxia, we were aware of the highly arbitrary nature of the suggested EF5 T/M ratio threshold of 1.5 for relevant hypoxia. This and the reasons for choosing this value have been discussed in greater detail in our previous paper [8]. Regardless of these shortcomings, we believe that some value is needed to obtain comparable results and future (hopefully larger) trials will show whether this value is indeed justified or not.
While the importance of hypoxia in the progression and treatment outcome of head and neck cancer has been well established in large studies [4], the noninvasive PET imaging of tumour hypoxia is not yet routinely used clinically. Only recently have positive correlations between the uptake of hypoxia tracers other than [18F]FMISO and survival of patients been reported [17]. This is no doubt because the relatively new field of PET hypoxia research has produced a number of viable alternatives to [18F]FMISO only in recent years. The development of reliable hypoxia probes has proven to be more demanding than initially estimated and in particular the development of hypoxia PET tracers is a very long and costly process that has only recently entered the clinical stage [18].
The induction of anaerobic glycolysis and therefore the increases in [18F]FDG uptake under hypoxic conditions have been demonstrated previously [19, 20], although contradictory results have also been reported, most recently by Busk et al. [21]. We tried to explore this in this study by investigating the correlations between the three parameters [18F]FDG SUV, hypoxia and blood flow. As observed in previous studies by our and other groups, the correlations between blood flow of tumours and outcome and between blood flow and parameters such as [18F]FDG SUV and hypoxia are far from simple. While it is true that tumours with low blood flow cannot grow as successfully as those with an ample supply of oxygen and nutrients, it is also true that hypoxic tumours (which tend to have a lower blood flow) have been proven to develop successful strategies for surviving in this hostile environment finally resulting in more aggressive phenotypes. In addition the relationship between blood flow and hypoxia seems to be very complex. While it is true that well-perfused tumours tend not to be hypoxic, it is the dynamic nature of hypoxia that poses challenges to measuring and understanding this relationship. Whereas chronic hypoxia is by definition quite stable over shorter periods of time, the distribution and the amount of acute hypoxia, on the other hand, does change with time. This poses a challenge to interpreting the results obtained at different time points. We tried to avoid this by obtaining [15O]H2O and [18F]EF5 images on the same day. Although the time difference was only around 4 hours, the question remains as to whether the mismatch between the two parameters was due to this phenomena or some other biological mechanism. In addition the use of [15O]H2O for imaging blood flow in malignant tumours poses additional questions. The overproduction of vascular growth factors in malignant tumours results in chaotic and dysfunctional vascular structures. These vessels have among other characteristics a variable lumen diameter. This means that there might be a mismatch between the areas, of the tumour reached by the very small [15O]H2O molecule that cannot be reached by red blood cells that deliver oxygen to the user cells. This method would therefore overestimate the blood flow of poorly perfused areas. Conversely, the similarly small molecule [18F]EF5 could reach those areas unreachable by red blood cells and therefore correctly image hypoxic areas. This would introduce another potential bias in the interpretation and correlation of perfusion and hypoxic images.
As demonstrated in our previous work with [18F]EF5 [8], there is an obvious similarity between early [18F]EF5 images and perfusion images that could potentially be used in future to obtain a semiquantitative or qualitative images of blood flow using only a single tracer. This, however, does require additional work which was beyond the scope of this study. These are complex problems that need to receive more attention in the future if we wish to obtain more knowledge on the roles of hypoxia and blood flow and their involvement in disease progression.
We believe that the results of the current study are interesting and important, particularly since neither of the other two PET parameters (FDG SUV and blood flow) showed similar correlations with survival. This, however, is in line with the lack of correlations among the PET parameters in our study, and once again emphasizes the need for multitracer imaging in pretreatment tumour evaluation and treatment planning. We are also aware that the number of patients investigated in this study was very low, and this might have affected the results. In particular, FDG SUVs have been shown to be positively correlated with treatment outcome in a study with a larger number of patients [22]. On the other hand, we did observe a significant correlation between MATV and survival, which is in line with the results of studies showing that MATV is a stronger predictor of poor survival than SUV [23, 24]. Due to high costs and complex imaging protocols, this is common to many PET studies, which, nevertheless, does not decrease the importance of the problem.
In two patients an alternative method for determining MATV was used, as explained in the Methods section. We are aware of the problems related to the use of a relative SUVmax threshold (50 %) and fixed SUV thresholds (2.5) for determining the MATV. While it would no doubt have been preferable to use one single method for determining MATV in all patients, it is clear that by doing so we would have introduced an unacceptable degree of error in some patients in whom this generally reliable method would have failed. We therefore believe that using a different method in some patients actually reduced the bias that would have been introduced by using a single method when this method would clearly have failed. While Hatt et al. found large differences in MATV depending on the method used, they found that the choice of method did not have any significant impact on the predictive value of MATV [25]. We are aware that is a subject of intense research and discussion and in our opinion is beyond the scope of this paper.
Regarding the methodology of the study, we have to note that even though we are aware that MRI and FDG PET/CT have been proven to be more sensitive for monitoring local control in patients with head and neck cancer, these modalities were not routinely used in our study in the follow-up of all patients. Particularly since some of the patients were followed in regional hospital after treatment in the Turku University Hospital. For this reason and because we wanted to avoid any potential bias introduced by using a more sensitive method in some patients we decided to use contrast-enhanced CT as the sole method for determining local control of the disease. The treatment that the patients received was the standard treatment in our centre at the time of study. While it is true that there were some differences between treatment protocols that individual patients received, these were considered standard and optimal treatments for each individual patient’s stage. Therefore, while did not take these differences into consideration when analysing the results, we do not believe that these differences had any significant influence on the results obtained. This, of course, is subject to speculation. Finally, the results obtained with [18F]EF5 in this study seem to be in line with recently published results obtained with [18F]FAZA [16]. This is true for the uptake values as well as correlations with survival, and for this reason the question as to the most suitable PET hypoxia tracer remains unresolved until a direct head-to-head comparison is performed.
Conclusion
Despite the small number of patients who participated in our study, hypoxia as represented by [18F]EF5 uptake clearly show had the highest level of correlation with overall survival, and seems to be predictive of radioresistance. As such [18F]EF5 remains a strong potential candidate for pretreatment patient stratification and treatment planning. However, the definite clarification of this question would require a larger, and possibly multicentre study. Also [18F]FDG SUV showed a trend that might have been significant had the number of patients been higher. On the other hand, the lack of correlation between the uptakes of the three evaluated tracers confirms the need for multitracer imaging to obtain all the significant information on tumour biology.
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Troost EG, Schinagl DA, Bussink J, Oyen WJ, Kaanders JH. Clinical evidence on PET-CT for radiation therapy planning in head and neck tumours. Radiother Oncol. 2010;96:328–34.
Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–9.
Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, Hawes SE, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res. 2007;13:3255–63.
Ang KK. More lessons learned from the suffocation of hypoxia. J Clin Oncol. 2010;28:2941–3.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Komar G, Seppanen M, Eskola O, Lindholm P, Gronroos TJ, Forsback S, et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J Nucl Med. 2008;49:1944–51.
Eskola O, Grönroos TJ, Forsback S, Tuomela J, Komar G, Bergman J, et al. Tracer level electrophilic synthesis and pharmacokinetics of the hypoxia tracer [(18)F]EF5. Mol Imaging Biol. 2012;14:205–12.
Teras M, Tolvanen T, Johansson JJ, Williams JJ, Knuuti J. Performance of the new generation of whole-body PET/CT scanners: Discovery STE and Discovery VCT. Eur J Nucl Med Mol Imaging. 2007;34:1683–92.
Blomqvist G. On the construction of functional maps in positron emission tomography. J Cereb Blood Flow Metab. 1984;4:629–32.
Lawson CL, Hanson RJ. Solving least squares problems. Philadelphia, PA: Society for Industrial Mathematics; 1987
Meyer E. Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by the H215O autoradiographic method and dynamic PET. J Nucl Med. 1989;30:1069–78.
Van den Hoff J, Burchert W, Muller-Schauenburg W, Meyer GJ, Hundeshagen H. Accurate local blood flow measurements with dynamic PET: fast determination of input function delay and dispersion by multilinear minimization. J Nucl Med. 1993;34:1770–7.
Komar G, Oikonen V, Sipilä H, Seppänen M, Minn H. Noninvasive parametric blood flow imaging of head and neck tumours using [15O]H2O and PET/CT. Nucl Med Commun. 2012;33:1169–78.
Haerle SK, Huber GF, Hany TF, Ahmad N, Schmid DT. Is there a correlation between 18F-FDG-PET standardized uptake value, T-classification, histological grading and the anatomic subsites in newly diagnosed squamous cell carcinoma of the head and neck? Eur Arch Otorhinolaryngol. 2010;267:1635–40.
Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20.
Carlin S, Humm JL. PET of hypoxia: current and future perspectives. J Nucl Med. 2012;53:1171–4.
Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625–32.
Minn H, Clavo AC, Wahl RL. Influence of hypoxia on tracer accumulation in squamous-cell carcinoma: in vitro evaluation for PET imaging. Nucl Med Biol. 1996;23:941–6.
Busk M, Horsman MR, Jakobsen S, Bussink J, van der Kogel A, Overgaard J. Cellular uptake of PET tracers of glucose metabolism and hypoxia and their linkage. Eur J Nucl Med Mol Imaging. 2008;35:2294–303.
Suzuki H, Kato K, Fujimoto Y, Itoh Y, Hiramatsu M, Maruo T, et al. (18)F-FDG-PET/CT predicts survival in hypopharyngeal squamous cell carcinoma. Ann Nucl Med. 2013;27:297–302.
Schinagl DA, Span PN, Oyen WJ, Kaanders JH. Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur J Nucl Med Mol Imaging. 2010;38:1449–58.
Romesser PB, Qureshi MM, Shah BA, Chatburn LT, Jalisi S, Devaiah AK, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–34.
Hatt M, Visvikis D, Albarghach NM, Tixier F, Pradier O, Cheze-le Rest C. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38:1191–202
Acknowledgments
We would like to thank the staff at the Turku PET Centre, the Department of Oncology and Radiotherapy, and the Department of Otorhinolaryngology for help in various phases of this project. We would also like to thank Irina Lisinen, MSC, for her help with the statistical analysis. The study was financially supported by the European Union’s FP6 Commission BioCare (Molecular Imaging for Biologically Optimized Cancer Therapy), under contract number 505785, by the Finnish Cancer Organizations and by the Turku University Foundation.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komar, G., Lehtiö, K., Seppänen, M. et al. Prognostic value of tumour blood flow, [18F]EF5 and [18F]FDG PET/CT imaging in patients with head and neck cancer treated with radiochemotherapy. Eur J Nucl Med Mol Imaging 41, 2042–2050 (2014). https://doi.org/10.1007/s00259-014-2818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2818-3