Abstract
Purpose
The aim of the study was to investigate the feasibility of shortening the recommended 4-h renoprotective amino acid infusion in patients receiving peptide receptor chemoradionuclide therapy (PRCRT) using radiosensitizing 5-fluorouracil. We evaluated the clearance of radiopeptide from the blood, long-term nephrotoxicity in patients undergoing PRCRT with the conventional 4-h amino acid infusion and renal uptake in patients receiving an abbreviated infusion.
Methods
The whole-blood clearance of 177Lu-DOTA-octreotate (LuTate) was measured in 13 patients receiving PRCRT. A retrospective analysis of short-term and long-term changes in glomerular filtration rate (GFR) in 96 consecutive patients receiving a 4-h infusion was performed. Renal LuTate retention estimated using quantitative SPECT/CT in 22 cycles delivered with a 2.5-h amino acid infusion was compared with that in 72 cycles with the 4-h infusion.
Results
LuTate demonstrated biexponential blood clearance with an initial clearance half-time of 21 min. Approximately 88 % of blood activity was cleared within 2 h. With the 4-h protocol, there was no significant change in GFR (1.2 ml/min mean increase from baseline; 95 % CI −6.9 to 4.4 ml/min) and no grade 3 or 4 nephrotoxicity at the end of induction PRCRT. The long-term decline in GFR after a median follow up of 22 months was 2.2 ml/min per year. There was no significant difference in the renal LuTate retention measured in patients receiving a 2.5-h amino acid infusion compared to those who had a 4-h infusion.
Conclusion
The greatest renal exposure to circulating radiopeptide occurs in the first 1 – 2 h after injection. This, combined with the safety of LuTate PRCRT, allows consideration of an abbreviated amino acid infusion, increasing patient convenience and reducing human resource allocation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of radiolabelled peptides that target somatostatin receptors (SSTR) has been a major advance for both imaging and treatment of neuroendocrine tumours (NETs). Labelling of the peptides with radionuclides emitting beta particles enables specific targeting of the tumours achieving a dose several orders of magnitude higher than that to healthy tissues, which are therefore relatively spared. Peptide receptor radionuclide therapy (PRRT) is therefore an important treatment option for patients with a NET. The principal somatostatin analogue currently used in therapy is 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) [Tyr3]-octreotate (DOTATATE) labelled with 111In, 177Lu (LuTate) or 90Y. PRRT improves quality of life with high rates of objective tumour responses, reduction in clinical symptoms and prolongation of survival [1–5]. To further improve efficacy, our group has combined the use of PRRT with the radiosensitizing agent, 5-fluorouracil (5FU) [6]. The safety profile of such a peptide receptor chemoradionuclide therapy (PRCRT) strategy has been investigated to only a limited extent [6, 7].
In spite of high and specific tumour uptake with PRRT, physiological distribution can result in toxicity, with the kidneys considered to be the critical dose-limiting organ. Renal tubular reabsorption of the radiolabelled peptides forms the principal mode of toxicity. This uptake of the radiopeptide is limited to the proximal tubule [8, 9], is largely irreversible, and is not observed in cortical glomeruli, distal tubules, medulla or pelvis [10]. It is also observed in SSTR subtype-2 (SSTR2) knockout mice, indicating that the mechanism of cortical endocytosis is independent of SSTR2 expression [10]. Therefore the kidneys receive a substantial radiation dose with resultant toxicity ranging from a decrease in creatinine clearance [3, 11], proteinuria and hypertension to development of end-stage renal disease requiring dialysis [12], and thrombotic microangiopathy [13].
There are several means of minimizing the renal radiation effects including altering the structure of the peptide to reduce renal absorption, infusion of competitive inhibitor agents for peptide uptake pathways, dose fractionation and the use of drugs that inhibit renal reabsorption mechanisms or act as radiation protectors [9, 14]. The most widely used strategy is infusing a mixture of the basic amino acids lysine and arginine prior to and following the radiopeptide to inhibit its absorption competitively [15]. The European Neuroendocrine Tumour Society (ENETS) consensus guidelines recommend intravenous administration of 2.5 % lysine, 2.5 % arginine in 1 l of normal saline over 4 h, starting 30 min before administration of the radiopharmaceutical [16]. Another method is infusion of Gelofusine, a gelatin polymer, which inhibits the megalin/cubulin pathway of radiopeptide reabsorption [10].
There has been limited investigation into the optimal time course for amino acid administration. Jamar et al. concluded that a 10-h infusion reduced renal radiation exposure by –10 – 15 % over a conventional 4-h treatment [17]. Their protocol, however, also involved an increase in the quantity of lysine and arginine delivered from 26.4 to 52.8 g. A second study did not identify any difference in kidney dose for different amino acid protocols including 4 – 5 h before injection, 2 h before injection + 2 – 3 h after injection, 1 h before injection + 2 h after injection [18].
Whilst administration of basic amino acids is widely regarded as a safe practice for preventing adverse renal reactions with PRRT, the infusion of a large volume of hypertonic solution is not well tolerated in all patients. Side effects include nausea, vomiting and biochemical effects such as hyperkalaemia [19, 20]. If administered via a peripheral vein, we have also observed local thrombophlebitic reactions which can result in skin ulceration. A longer duration of infusion may also be a practical impediment to efficient delivery of PRRT, particularly when the treatment is given on an outpatient basis requiring greater allocation of medical and nursing resources.
The aim of this study was to (1) investigate the pharmacokinetics of LuTate blood clearance with a view to optimization of renoprotective measures, (2) evaluate the long-term renal toxicity of LuTate PRCRT in a cohort given a 4-h renoprotective amino acid infusion and (3) estimate any difference in renal LuTate retention when using an abbreviated renoprotective infusion.
Materials and methods
LuTate blood clearance study
In patients receiving a standard 4-h amino acid infusion, serial blood samples were collected from 13 of the patients receiving a therapeutic dosage of LuTate. Blood samples were collected at 0.17, 0.33, 0.5, 1, 2, 4, 24, 48 and 72 h after injection. Activities were measured using a well counter (Biodex Medical, Atomlab 950) and the values were decay-corrected with concentration values scaled to percentages of the patient maximum recorded at T 0. All parts of this study were approved by the Peter MacCallum Ethics Committee. For this prospective part of the study, written informed consent was obtained from all patients.
Long-term renal toxicity measurement
Patients
All patients who underwent PRCRT with LuTate and concomitant 5FU between August 2005 and August 2012 at our institution were identified. All these patients had a documented diagnosis of surgically unresectable NET. In addition, adequate SSTR expression for consideration of PRRT was documented on pretreatment 111In-pentetreotide scintigraphy or 68Ga-DOTATATE PET/CT in all patients by demonstration of intensity of tumoral uptake greater than physiological liver activity. We excluded patients who had 90Y-DOTATATE treatment from analysis. A total of 96 patients were identified for analysis. The demographic characteristics of the patients are presented in Table 1. All the patients had at least two glomerular filtrate rate (GFR) measurements, and 64 had a GFR measurement prior to and at the end of the induction regimen.
Treatment protocol
177Lu was obtained as 177Lu-LuCl3 from IDB-Holland (Petten/Baarle Nassau, The Netherlands). The peptide DOTA-octreotate (provided by the Erasmus Medical Centre, Rotterdam, Netherlands) was labelled with 177Lu through chelation to a DOTA molecule to forming 177Lu-DOTA-octreotate. This labelling procedure was performed in-house and the product was then used as a therapeutic agent. The labelling efficiency was greater than 95 % as determined by thin-layer chromatography and high-performance liquid chromatography. Each patient received 6 – 12 GBq LuTate as part of a serial treatment regimen: an induction course consisting of four cycles separated by 6 – 8 weeks followed by one or two consolidation cycles in selected patients and one maintenance cycle as needed 12 – 18 months after induction. The dose of LuTate was empirically adjusted according to the burden of disease, renal function and body weight. Formal dosimetry was not performed.
Excluding the first cycle, radiosensitizing 5FU was administered at a dose of 200 mg/m2/day starting 4 days prior to radionuclide administration and continuing for up to 3 weeks. The maintenance treatment cycles were individualized for each patient depending on the tumour burden, response to treatment and blood counts. Therefore as the time-points for the maintenance cycles were not uniform in all patients, the time-points used for long-term follow-up GFR measurements were at differing intervals and after varying cumulative administered activities. In this cohort of patients receiving a traditional course or amino acids for renal protection, an infusion of 25 g lysine and 25 g arginine in 1 l of normal saline was given intravenously over 4 h starting at least 30 minutes prior to LuTate administration.
GFR measurement
GFR was measured using 51Cr-EDTA plasma clearance. GFR was also estimated using the Modified Diet in Renal Disease (MDRD) formula (eGFR). The eGFR value was measured prior to initiation of treatment and before every further cycle. 51Cr-EDTA GFR was measured before treatment, prior to the last induction cycle and before each maintenance cycle.
Assessment of LuTate renal uptake after abbreviated amino acid infusion
An abbreviated amino acid protocol consisting of a 2.5-h infusion starting 30 min prior to LuTate therapy was administered in 22 treatment cycles in 14 patients. Lysine and arginine were administered at the same rate and concentration, yielding a total dose of renoprotective amino acids of 625 – 750 ml (16 – 19 g of lysine and arginine). Patients with impaired renal function (GFR <60 ml/min) or a small tumour burden were ineligible for the abbreviated amino acid infusion protocol. The comparison group was 72 consecutive treatment cycles in 45 patients receiving the standard 4-h infusion.
Renal LuTate retention was measured with SPECT/CT (Siemens Symbia T6, Munich, Germany) using a quantitative SPECT (Q-SPECT) protocol described previously [21]. Images were acquired at 24 h after injection of LuTate. Kidney volumes were segmented based on anatomical delineation on CT image sets. The resulting volumes of interest were used to record the mean renal standardized uptake value (SUV) on the Q-SPECT dataset.
Statistical analysis
Paired t tests were used to compare GFRs between baseline and after induction in each subgroup. In order to allow for the difference in the GFR time-points and account for all the serial GFR values, a longitudinal linear regression model with patient and time as the random effects was used to estimate the rate of change in GFR. The slope of the curve was obtained to estimate the rate of change of GFR per year. Multivariate analysis was done using the ANCOVA test to determine factors associated with the long-term change in GFR. Factors considered were: age, grade of renal uptake of radionuclide, cumulative dose, mean dose per cycle, diabetes, hypertension and history of previous chemotherapy. Correlations between GFR and eGFR values at baseline and on last follow-up were evaluated using Spearman’s rank correlation coefficient. A two-sided p value of <0.05 was considered significant. For analysis of the abbreviated amino acid infusion, the difference in mean 24-h renal SUV between the abbreviated and 4-h amino acid cohorts was compared using an unpaired t test.
Results
LuTate blood clearance study
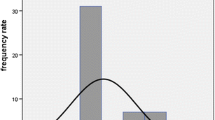
Serial blood samples indicated that LuTate clearance obeyed a biexponential model of clearance from the bloodstream (Fig. 1). Very rapid clearance was observed in the first 1 – 2 h of treatment. The initial clearance half-time was measured at 20.8 min. After 4 h, clearance slowed with measurements indicating a long-term biological half-life of 12.9 h. At 2 h, blood-pool radioisotope concentration was reduced to 12.1 % of its level immediately after injection. At 24, 48 and 72 h, intravenous LuTate concentrations were further reduced to 1.8, 0.9 and 0.8 % of the initial values. Long-term blood-pool LuTate retention could be attributed to a near equilibrium between renal excretion and washout from the tumour volume and other organs.
Intravenous LuTate concentrations obtained through serial blood sampling during radionuclide therapy in 13 patients. Rapid clearance is observed in the first 2 h following administration, a finding attributed to rapid but saturable organ and tumour uptake and redistribution in the extracellular space. The solid line indicates a biphasic double-exponential clearance model. The initial clearance half-time is 21 min
Long-term renal toxicity
In patients who had 51Cr-EDTA GFR measured prior to and at the end of induction therapy (n = 64), the change in mean GFR after administration of a mean activity of 24.6 GBq over 2.8 cycles was 1.2 ml/min (95 % CI −6.9 to 4.4 ml/min) but this was not statistically significant. A subgroup analysis based on grade of baseline GFR is shown in Table 2. Interestingly, patients with impaired renal function at baseline showed a trend to improving renal function, although this was not statistically significant.
During long-term follow-up, the mean rate of decline of GFR estimated in patients who had more than two GFR measurements (n = 44) was 2.2 ml/min per year (95 % CI, 4.1 to 0.3 ml/min per year; Fig. 2). There was also a good correlation between eGFR estimated using the MDRD formula and GFR estimated using the 51Cr-EDTA method (Spearman’s rho 0.57, p < 0.05), suggesting that the former method can be used as a reasonable surrogate for renal function when 51Cr-EDTA is not available. In the multivariate analysis of the long-term change in GFR, prior chemotherapy was the only factor that showed a statistically significant association (p < 0.05).
Assessment of LuTate renal uptake after abbreviated amino acid infusion
Renal retention of LuTate on Q-SPECT imaging at 24 h was slightly but nonsignificantly greater (5.8 %, p = 0.26) in patients receiving the abbreviated infusion protocol than in those receiving the standard 4-hour infusion protocol. Biological factors that may have affected SUV computation or renal retention including administered LuTate dosage, patient weight and functional renal volume were comparable between the two groups (see Table 3).
Discussion
Renal toxicity is a concern in PRRT as the principal mode of excretion of somatostatin analogues is via the kidneys and this is associated with uptake and retention of a proportion of the filtered peptide in the proximal convoluted tubules. This occurs particularly with 90Y-based PRRT, presumably due to the long beta path length of 90Y that allows beta particles to reach the glomerulus-rich cortical region of the kidney. Otte et al. [11] found stable renal insufficiency in two patients and renal failure requiring dialysis in a further two of 29 patients receiving 90Y-DOTATOC. None of the patients with renal side effects had a renoprotective infusion. They also reported another patient with end-stage renal disease developing after PRRT with 90Y-DOTATOC despite renal protection with Hartmann HEPA 8 % solution [11]. In a large series of 1,109 patients [2] receiving 2,472 cycles of 90Y-DOTATOC and a renoprotective arginine and lysine infusion, 102 patients (9.2 %) experienced severe permanent renal toxicity (grade 4, 67 patients; grade 5, 35 patients). Toxicity was more common in patients with advanced age, low baseline GFR and high renal uptake of tracer. In contrast to these results, Kwekkeboom et al. found that renal toxicity occurred in only 2 of 504 patients treated with LuTate with renoprotective infusion [4]. A preliminary study by van Essen et al. [12] showed no renal toxicity in seven patients treated with concomitant oral capecitabine and LuTate during a short-term follow-up. A preliminary analysis performed in our centre also showed no acute or long-term changes in a smaller group of 27 patients treated with LuTate with infusional 5FU and also receiving a 4-h amino acid infusion [6, 7].
Although amino acid infusion appears to be beneficial in reducing nephrotoxicity, it is not without side effects. Giovacchini et al. [22] found that transient hyperkalaemia frequently occurs with administration of amino acid infusions. Rolleman et al. found that although the radiation dose to the kidneys was lower in patients receiving an infusion containing 75 g lysine, these patients also exhibited a much higher incidence of hyperkalaemia, and vomiting was seen in up to 50 % of patients and muscle weakness in one [20]. These side effects were attributed to both the quantity of amino acid and the hypertonicity of the solution administered. Barone et al. [19] also reported hypophosphataemia. The highest increase in potassium levels has been found with infusion of 75 g lysine alone. We have also noted local cutaneous and phlebitic reactions late during infusion of amino acids into a peripheral vein. Furthermore, a 4-h infusion limits the potential feasibility of administering PRCRT on an outpatient basis in jurisdictions where this is allowable. It also requires significant allocation of staff to supervise treatment, increasing its cost. Therefore, if a reduction in the duration of this infusion were found to be safe, it would have significant clinical advantages.
In this study we analysed the renal toxicity profiles in 96 patients undergoing PRCRT with concomitant 5FU as radiosensitizer. We measured GFR using 51Cr-EDTA plasma clearance as this is a more accurate parameter than serum creatinine or eGFR [23]. We found no significant change in the mean GFR at the end of induction therapy in 65 patients. Whilst mild renal toxicity (grade 1 and 2) was noted in eight patients, no patients developed grade 3 or 4 renal toxicity. Interestingly, patients with baseline impairment of renal function did not have a more marked fall in GFR (Table 2). Rather, there was actually a trend towards improvement in renal function over time. Although not statistically significant and warranting further evaluation, it is postulated that impaired glomerular filtration may decrease presentation of radiopeptide to the proximal tubules, increasing circulation time and increasing bioavailability for tumour uptake. This could augment the therapeutic index and, given that many patients with advanced NET have extensive liver involvement, the response in liver disease may reduce hepatorenal syndrome.
We also analysed the long-term renal effects of PRCRT with a median follow-up period of 22 months in the entire cohort of 96 patients. The mean rate of decline in GFR was 2.2 ml/min per year. This change in GFR appears to be only marginally more than the average age-related decrease in creatinine clearance of 0.75 ml/min per year found by Lindeman et al. [24] and is of minor clinical significance. Our results are consistent with the results of Bodei et al. [25] who showed that after a median follow-up period of 30 months, none of the patients treated with LuTate PRRT without chemotherapy showed renal toxicity. This further suggests that the addition of radiosensitizing chemotherapy does not increase the renal toxicity compared with stand-alone PRRT. Therefore, there seems to be an acceptable therapeutic index with this therapy to allow consideration of a less-aggressive renoprotective amino acid infusion protocol.
Imaging and blood sample measurements indicate that the pharmacokinetics of somatostatin analogues in radionuclide therapy obey a two-phase model. Initial organ uptake mostly occurs in the first 1 – 2 h after injection of therapeutic agent. This phase is marked by rapid clearance from the bloodstream with a clearance half-time of approximately 20 min. Clearance is primarily attributed to absorption by tumour, liver and spleen with simultaneous renal excretion. Following tissue uptake, washout occurs at a much slower rate. Therefore the initial 2 – 3 h of the uptake phase, representing approximately six to nine of the rapid clearance half-times, is the ideal time for the renoprotective amino acid infusion. These results are consistent with measurements made by Jamar et al. [17]. We have previously shown an inverse relationship between both tumour burden and patient size and renal retention of radiopeptide [26]. Accordingly, patients with a small disease volume or small body habitus may still need a longer infusion time, and this should also be considered in patients with preexisting renal impairment.
Given the rapid clearance of LuTate from the blood, we propose that a shorter duration of amino acid infusion would provide equivalent renoprotection. Patients who received the shorter infusion did not show a higher renal concentration of LuTate than patients receiving the conventional 4-h renoprotective amino acid infusion (Table 3). The major limitation of this analysis was the lack of formal dosimetric data for the kidneys. Both prospective and retrospective dosimetry calculations have their own limitations. With respect to prospective dosimetry, the lack of amino acid infusion at the time of pretherapy diagnostic somatostatin analogue imaging (111In octreotide or 68Ga-octreotate) significantly limits the ability to assess the likely renal dose. Therefore we have adopted a pragmatic dosing regimen that assigns an administered activity in the range 6 – 12 GBq primarily based on tumour burden but also influenced by renal function and body habitus as well as preexisting myelosuppression, e.g. from previous chemotherapy. Other aspects considered in determining the dose also include logistic aspects such as the dose delivered by the supplier and the number of patients being treated on a given day. However, quantitative SUV data from the SPECT/CT imaging has been used as a surrogate for renal dose [21]. We are continuing follow-up of patients who received the abbreviated amino acid infusion to ensure that long-term renal function is not compromised.
Conclusion
Serial blood data indicate that the greatest renal exposure to circulating radiopeptide occurs in the first 1 – 2 h after injection. Since PRCRT with LuTate and concomitant 5FU when combined with a renoprotective amino acid infusion has minimal acute or long-term renal toxicity, we propose that it would be safe on the basis of these data to shorten the duration of amino acid infusion to a period from 30 min before to 2 h after LuTate infusion in selected patients. This would make the treatment more convenient for the patient, efficient and less resource intensive for health professional staff, while simultaneously limiting potential direct side effects.
References
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35. doi:10.1007/s00259-011-1902-1.
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–23. doi:10.1200/JCO.2010.33.7873.
Kwekkeboom DJ, Bakker WH, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA(0),Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2003;30:417–22. doi:10.1007/s00259-002-1050-8.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi:10.1200/JCO.2007.15.2553.
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–62. doi:10.1200/JCO.2005.08.066.
Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. 177Lu-octreotate, alone or with radiosensitising chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity 111In-octreotide. Eur J Nucl Med Mol Imaging. 2010;37:1869–75. doi:10.1007/s00259-010-1483-4.
Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–11. doi:10.1007/s00259-010-1631-x.
Lambert B, Cybulla M, Weiner SM, Van De Wiele C, Ham H, Dierckx RA, et al. Renal toxicity after radionuclide therapy. Radiat Res. 2004;161:607–11.
Vegt E, de Jong M, Wetzels JF, Masereeuw R, Melis M, Oyen WJ, et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51:1049–58. doi:10.2967/jnumed.110.075101.
Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M. Localisation and mechanism of renal retention of radiolabelled somatostatin analogues. Eur J Nucl Med Mol Imaging. 2005;32:1136–43. doi:10.1007/s00259-005-1793-0.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26:1439–47.
van Essen M, Krenning EP, Kam BL, de Herder WW, van Aken MO, Kwekkeboom DJ. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:743–8. doi:10.1007/s00259-007-0688-7.
Cybulla M, Weiner SM, Otte A. End-stage renal disease after treatment with 90Y-DOTATOC. Eur J Nucl Med. 2001;28:1552–4. doi:10.1007/s002590100599.
Rolleman EJ, Melis M, Valkema R, Boerman OC, Krenning EP, de Jong M. Kidney protection during peptide receptor radionuclide therapy with somatostatin analogues. Eur J Nucl Med Mol Imaging. 2010;37:1018–31. doi:10.1007/s00259-009-1282-y.
Hammond PJ, Wade AF, Gwilliam ME, Peters AM, Myers MJ, Gilbey SG, et al. Amino acid infusion blocks renal tubular uptake of an indium-labelled somatostatin analogue. Br J Cancer. 1993;67:1437–9.
Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudla B, de Herder WW, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220–6. doi:10.1159/000225951.
Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, et al. 86Y-DOTA0)-D-Phe1-Tyr3-octreotide (SMT487) – a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging. 2003;30:510–8. doi:10.1007/s00259-003-1117-1.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, et al. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging. 2003;30:207–16. doi:10.1007/s00259-002-1023-y.
Barone R, Pauwels S, De Camps J, Krenning EP, Kvols LK, Smith MC, et al. Metabolic effects of amino acid solutions infused for renal protection during therapy with radiolabelled somatostatin analogues. Nephrol Dial Transplant. 2004;19:2275–81. doi:10.1093/ndt/gfh362.
Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30:9–15. doi:10.1007/s00259-002-0982-3.
Beauregard JM, Hofman MS, Pereira JM, Eu P, Hicks RJ. Quantitative 177Lu SPECT (QSPECT) imaging using a commercially available SPECT/CT system. Cancer Imaging. 2011;11:56–66. doi:10.1102/1470-7330.2011.0012.
Giovacchini G, Nicolas G, Freidank H, Mindt TL, Forrer F. Effect of amino acid infusion on potassium serum levels in neuroendocrine tumour patients treated with targeted radiopeptide therapy. Eur J Nucl Med Mol Imaging. 2011;38:1675–82. doi:10.1007/s00259-011-1826-9.
Hartlev LB, Boeje CR, Bluhme H, Palshof T, Rehling M. Monitoring renal function during chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1478–82. doi:10.1007/s00259-012-2158-0.
Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–56. doi:10.1007/s00259-008-0778-1.
Beauregard JM, Hofman MS, Kong G, Hicks RJ. The tumour sink effect on the biodistribution of 68Ga-DOTA-octreotate: implications for peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2012;39:50–6. doi:10.1007/s00259-011-1937-3.
Acknowledgments
Professor Hicks and Dr. Jackson were supported by a Translational Research Grant from the Victorian Cancer Agency. Dr. K Raghava Kashyap was supported by Endeavour Awards, a venture of Austraining International in partnership with the Department of Education, Employment and Workplace Relations, Government of Australia. We acknowledge the support and inspiration provided to our facility over the past 15 years from the Department of Nuclear Medicine at the Erasmus Medical Centre under the leadership of Professor Eric Krenning.
Conflicts of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Kashyap, P. Jackson and M. S. Hofman contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kashyap, R., Jackson, P., Hofman, M.S. et al. Rapid blood clearance and lack of long-term renal toxicity of 177Lu-DOTATATE enables shortening of renoprotective amino acid infusion. Eur J Nucl Med Mol Imaging 40, 1853–1860 (2013). https://doi.org/10.1007/s00259-013-2504-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2504-x