Abstract
Purpose
The detection of malignant peripheral nerve sheath tumours (MPNSTs) in patients with neurofibromatosis 1 (NF1) remains a clinical challenge. The purpose of this study was to evaluate the use of [18F]2-fluoro-2-deoxy-d-glucose PET/CT (FDG PET/CT with early and delayed imaging) in patients with symptomatic neurofibromas, to revalidate current cut-off values for identification of malignant change within neurofibromas and to examine the relationship between SUV and tumour grade.
Methods
Patients with symptomatic neurofibromas underwent FDG PET/CT imaging at 90 and 240 min. Semiquantitative analysis using maximum standardized uptake value (SUVmax) was performed and correlated with histology.
Result
In 69 patients, 85 lesions were identified for analysis, including 10 atypical neurofibromas and 21 MPNSTs. Sensitivity of FDG PET/CT in diagnosing NF1-associated MPNST was 0.97 (95% CI 0.81–0.99) and the specificity was 0.87 (CI 0.74–0.95). There was a significant difference in SUVmax between early and delayed imaging and in SUVmax between tumours identified as benign and malignant on PET/CT. There was also a significant difference in SUVmax between tumour grades.
Conclusion
FDG PET/CT is a highly sensitive and specific imaging modality for the diagnosis of MPNST in NF1 patients. We recommend performing early (90 min) and delayed imaging at 4 h for accurate lesion characterization and using a cut-off SUVmax of 3.5 on delayed imaging to achieve maximal sensitivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis 1 (NF1) is a common autosomal dominant neurocutaneous disorder with a birth incidence of 1 in 2,500 to 1 in 3,000 [1]. The major diagnostic manifestations include café au lait patches, neurofibromas, skinfold freckling, iris Lisch nodules, optic pathway glioma and distinctive bony dysplasia. The neurofibroma, a benign peripheral nerve sheath tumour, is the most common tumour in NF1 patients and may manifest as focal dermal, cutaneous or subcutaneous growths, intraforaminal spinal nerve root tumours or diffuse plexiform neurofibromas [2]. The complications of NF1 are unpredictable and widespread. Neurological problems originate from central nervous system tumours and malformations and as a consequence of skull and skeletal deformity. Neurological deficit including weakness and sensory impairment results from neurofibromas causing compression of peripheral nerves, nerve roots or the spinal cord. Neurofibromin is the NF1 gene product and acts as a tumour suppressor. Individuals with NF1 have a significantly increased risk of developing malignant peripheral nerve sheath tumours (MPNSTs) with a life-time risk of 8–12% [3, 4].
MPNSTs usually arise from pre-existing benign plexiform neurofibromas, they metastasize widely and frequently have a poor prognosis. Differentiating between benign and malignant tumours has important prognostic and therapeutic implications, but can be difficult, especially in individuals who have multiple benign tumours. The clinical manifestations of malignancy include unremitting pain not otherwise explained, rapid increase in size of a plexiform neurofibroma, change in consistency from soft to hard, and new or unexplained neurological deficit [3]. Whilst cross-sectional imaging is helpful in identifying tumours and delineating relationships with surrounding structures, it cannot be used to reliably identify malignant change. Currently histology remains the gold standard for identifying malignant transformation within a neurofibroma. However, this requires complete excision, which is frequently not technically feasible and if core biopsy is performed the focus of malignant change, particularly within a large heterogeneous tumour, may be missed.
To date a number of studies have addressed the role of FDG PET in the assessment of MPNSTs in NF1 (Cardona et al. also included sporadic MPNSTs) [5–9]. Ferner et al. showed a significant difference in mean standardized uptake value (SUV) between benign and malignant lesions and concluded that the optimum time for measuring SUV in patients with symptomatic neurofibromas is 240 min after injection of [18F]FDG [5]. More recent work from our group has shown that symptomatic neurofibromas with a maximum SUV (SUVmax) of ≥3.5 should be excised and lesions with SUVmax of 2.5–3.5 should be reviewed clinically [9]. In this study tumours identified as benign on PET were clinically followed-up for between 2 and 5 years [9].
Based on this evidence using PET-only scans, the aims of our study were to evaluate the sensitivity of PET/CT, to clarify the value of early and delayed imaging, to revalidate current cut-off values for identification of malignant change within neurofibromas and to examine the relationship between SUV and tumour grade.
Materials and methods
Patient population
Patients were referred from the specialist neurofibromatosis clinic at Guy’s and St Thomas’ Hospitals NHS Trust which has a database of 750 NF1 individuals. Patients were identified from the PET/CT database using the search term ‘neurofibromatosis’ and cross-referenced to the neurofibromatosis database. The search was performed for a 45-month period from August 2004 to April 2008 (the period that PET/CT has been in use at our centre). Patients with classical NF1 and mosaic NF1 were included and the diagnosis was verified by the neurofibromatosis specialist. All patients included in the study were referred for PET/CT either due to pain, increase in the size or change in texture of the neurofibroma, or due to neurological deficit associated with a plexiform neurofibroma. Six patients from a previous study at this institution were included in this study [9]. Patients with positive PET/CT scans underwent biopsy or surgical resection unless the lesion was inaccessible in which case they were followed up clinically with repeat imaging. Neurofibromas were excised from individuals with negative PET/CT scans when clinically indicated. All patients with PET-negative lesions had continued clinical follow-up. Repeat scans in patients with a new lesion of concern were included in the study, but repeat scans for the follow-up of lesions already analysed were not included again.
Ethics
Approval was granted for this study as a multidisciplinary clinical audit of the continuing use of FDG PET/CT in Guy’s and St. Thomas’ NHS Trust for this group of patients.
FDG PET/CT
Scans were performed on a GE Discovery ST (General Electric Medical Systems, Waukesha, WI) BGO four-slice PET/CT scanner. Between January and April 2008 three studies were performed on a GE Discovery VCT 64-slice PET/CT scanner. Following a 6-h fast, patients received an intravenous injection of 350 MBq of [18F]2-fluoro-2-deoxy-d-glucose (FDG) (in children the administered activity was scaled according to body weight calculated from baseline activity of 350 MBq). Half-body scans (cerebellum to mid-thigh) were performed in all patients at 90 min after FDG injection (the ‘early’ scan) with additional local views depending on the site(s) of interest. Delayed local views of the tumour were then acquired at 4 h after FDG injection (the ‘delayed’ scan). The acquisition time for both scans was 5 min per bed position. This imaging time gave adequate count statistics for lesion localization, drawing of the region of interest and SUVmax determination as can be seen from the images in Figs. 1 and 2. Acquired images were reconstructed using OSEM (ordered subset expectation maximization; 30 subsets, one iteration, 5.14 m postfilter, 4.69 mm loop filter).
Axial CT, PET and PET/CT images at 4 h. a Biopsy-proven atypical neurofibroma in the right erector spinae muscle (SUVmax 4.2) shows the focal area of increased [18F]FDG uptake. b Biopsy-proven low-grade MPNST within the posterior musculature of the left thigh (SUVmax 6.2) shows the focal area of increased [18F]FDG. c Biopsy-proven high-grade MPNST occupying the left flank (SUVmax 10.0) shows heterogeneously increased [18F]FDG uptake
Scans were evaluated qualitatively and semiquantitatively using SUVmax. The SUVmax was measured by a single operator hand-drawing a region of interest on the axial PET image using the CT component of the study to ensure the region corresponded to the tumour of interest. The SUVmax was derived as the highest SUV within the tumour and was measured on both the ‘early’ and ‘delayed’ scans at the equivalent image level on each study. If a patient had multiple small neurofibromas that demonstrated low-grade uptake, only the symptomatic lesion of concern was included in the analysis. However, in two patients asymptomatic lesions were identified which demonstrated increased uptake compared to the index lesion and these were included in the analysis. In both patients, the index and incidental lesions demonstrated only low-grade uptake and were therefore classified as benign. All scans were analysed blinded to histological diagnosis. For the purposes of initial analysis, tumours with a SUVmax of ≥3.5 on delayed imaging were classified as malignant on the basis of PET/CT and those with a SUVmax of <3.5 as benign.
Statistical analysis
The SUVmax results for tumours classified as benign or malignant on the basis of PET were compared with two-way repeated measures ANOVA and post-hoc t test analysis using SPSS version 15.0. Sensitivities and specificities were calculated using standard formulae. Following histological correlation, the SUVmax was compared with tumour grade using two-way repeated measures ANOVA and post-hoc t test analysis. ROC curves were drawn for SUVmax values at early and delayed imaging times.
Histology
MPNST were classified histologically as low, intermediate or high grade using the criteria of tumour differentiation, vascular invasion, necrosis and mitotic count [10]. Atypical neurofibroma was defined as a neurofibroma exhibiting nuclear atypia and/or hypercellularity, without the presence of mitosis or necrosis [11, 12].
Results
Of a total of 97 studies initially identified for inclusion in the study, 28 were excluded: in 4 patients the scan was for the assessment of a tumour other than a neurofibroma (three breast cancers and one solitary pulmonary nodule), in 13 patients no delayed imaging was performed as no focal uptake was identified on the initial scan, 5 scans were for the follow-up of a lesion already included in the analysis, in 1 patient the full dataset was irretrievable, and 5 patients had clinical and neurological signs suggestive of a deep-seated lesion, but no focal neurofibroma was visible on PET/CT scan. There were 69 studies in 62 patients (seven patients had two studies) comprising 31 males and 31 females aged 9–86 years (mean age 31.3 years, SD 16.7). The average early imaging time was 108 min (1 h 48 min), median 101 min (1 h 41 min), and the average delayed imaging time was 252 min (4 h 12 min), median 252 minutes. Prior to the scan all patients had serum glucose within the normal range. From the 69 studies 85 lesions were identified as suitable for analysis.
Excision/biopsy was performed on 39 of the lesions including 8 neurofibromas, 10 atypical neurofibromas, and 21 MPNSTs (11 low-grade and 10 high-grade tumours). On the basis of semiquantitative analysis on [18F]FDG PET/CT scans 42 tumours were categorized as benign and 43 as malignant. Histological correlation identified one false-negative scan and six false-positive scans (Table 1). Sensitivity of FDG PET/CT in diagnosing NF1-associated MPNST was 0.97 (95% CI 0.81–0.99) and the specificity was 0.87 (CI 0.74–0.95).
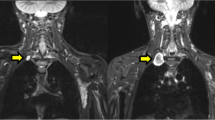
Patients with benign neurofibromas were monitored clinically for 2–41 months (median 19 months, SD 13). Three patients with lesions classified as benign on PET/CT scan underwent excision biopsy due to a high level of clinical suspicion (intractable pain, rapid growth, and/or new neurological symptoms), and of these two had a benign neurofibroma and one an atypical neurofibroma (Table 1). Seven patients with inaccessible lesions classified as malignant on [18F]FDG PET/CT scan did not undergo excision and at the time of this report remained under close clinical review (range 7–46 months; Table 2). The diagnosis was confirmed on histology after excision biopsy in five of six patients with a false-positive [18F]FDG PET/CT scan. However, in one individual an image-guided needle core biopsy was performed at another institution and the site of biopsy did not correlate with the focus of intense FDG uptake on the PET/CT scan (Table 3, patient 6; Fig. 1). At the time of this report, all patients with a false-positive scan remained under clinical follow-up (median 19 months, SD 11.95).
Analysis of PET/CT findings
The mean SUVmax on early imaging (SUVmaxE) was 2.0 (CI 1.8–2.3) for tumours designated as benign on PET/CT scan and 7.0 (CI 5.6– 8.4) for tumours designated as malignant on PET/CT scan. On delayed imaging the mean SUVmax (SUVmaxD) was 1.9 (CI 1.7–2.2) and 8.1 (CI 6.5–9.6) for tumours designated as benign and malignant, respectively, on the basis of PET/CT scan.
Two-way repeated measures ANOVA identified a significant difference in SUVmax between early and delayed imaging ([type irrelevant] F1,83=9.98, p=0.0022) and a significant difference in SUVmax between tumours identified as benign and malignant on PET/CT scan ([time irrelevant] F1,83=56.14, p≪0.0001). There was a significant interaction effect between time and tumour type (F1,83=14.72, p=0.00017). Post-hoc t test analysis revealed a significant difference between SUVmax on early and delayed imaging for tumours classified as malignant on PET/CT scan (p=0.0005) but not for tumours called benign on PET/CT scan (p=0.2).
Analysis with histological correlation
In the tumours with histological correlation the mean SUVmax values on early imaging (SUVmaxE) were 5.1 (CI 4.3–5.9), 7.3 (CI 5.5–9.1) and 12.0 (CI 7.3–16.6) for atypical neurofibromas, and low- and high-grade MPNSTs, respectively. No malignant tumours were identified with SUVmaxD <3.2 and there were six benign tumours with SUVmaxD of >3.5. SUVmaxD values for atypical neurofibromas (Fig. 2a, for example), and low- (Fig. 2b, for example) and high-grade (Fig. 2c, for example) MPNSTs were 5.6 (CI 4.6–6.6), 7.8 (CI 5.9–9.7), and 13.7 (CI8.6–18.8), respectively. Two-way repeated measures ANOVA identified a significant difference in SUVmax between tumour types ([time irrelevant] F2,27=7.91, p=0.002). There was also a significant difference between SUVmax on early and delayed imaging (F1,27=10.58, p=0.003). The interaction effect between tumour type and time was not significant (F1,27=2.27, p=0.1). Post-hoc t test analysis showed the most significant difference in SUVmax between atypical neurofibromas and high-grade MPNSTs on early and delayed imaging (p=0.006 and p=0.004, respectively); there was a significant difference between atypical neurofibromas and low-grade MPNSTs (p=0.035 for early imaging and p=0.047 for delayed imaging) and between low- and high-grade MPNSTs but this was not as strong (p=0.04 for early imaging and p=0.02 for delayed imaging). The overlap in SUVmaxD values for benign, atypical, and low- and high-grade MPNSTs is illustrated in Fig. 3. Unpaired t test also demonstrated a significant difference in mean SUVmaxD values between benign and atypical neurofibromas (p≪0.0001).
ROC analysis was performed for early and late imaging. This showed that a cut-off SUVmaxD of 3.1 achieved maximal sensitivity (1.0) with a specificity of 0.766 (Fig. 4). To achieve maximal sensitivity on early imaging a cut-off SUVmaxE of 2.35 would be required, resulting in a specificity of 0.6 (Fig. 5).
Discussion
Based on our previous experience with PET we have advocated the use of early and delayed imaging in the diagnosis of MPNSTs. We recommended that symptomatic neurofibromas with SUVmax of 3.5 and above should be excised and lesions with SUVmax of 2.5–3.5 should be reviewed clinically and those with an SUV <2.5 considered as benign [9].
We found a significant difference between the SUVmax of benign and malignant tumours on PET/CT scan, which is in accordance with the findings of previous PET studies [5, 8, 9]. However, in this study we also demonstrated the different behaviour of benign neurofibromas and MPNSTs. The SUVmax of the malignant tumours increased significantly with time but the SUVmax of the benign tumours did not. These changes follow the time–activity uptake curves that we previously identified in soft-tissue sarcomas [13]. Albeit an expected finding, this change in SUV emphasizes the importance of both early and delayed imaging in differentiating between benign and malignant lesions. Early imaging also helps to identify and localize the area(s) of interest for the delayed study, and reveals nontarget lesions which may influence decisions regarding patient management [8, 9].
Interestingly we also demonstrated a correlation between mean SUVmax and tumour grade. While atypical neurofibromas, and low- and high-grade MPNSTs all behaved in the same way with SUVmax increasing over time, the actual SUVmax increased with increasing tumour grade. The difference in SUVmax was most marked between benign and atypical neurofibromas and atypical neurofibromas and high-grade MPNSTs. To our knowledge this is the first study to demonstrate such a relationship and suggests that FDG PET/CT can be used to grade the malignancy of the tumour. However, there was overlap between tumour grades and therefore it is not yet possible to predict tumour grade accurately on an individual basis. Ducatman et al. did not find a significant correlation between survival and tumour grade, but recently Brenner et al. reported a small retrospective study of 16 patients in which tumour SUVs predicted long-term survival [7, 14]. A larger prospective study with a 5-year follow-up should resolve the question as to whether SUV correlates with survival.
It is of note that there was a significant difference in mean SUVmax between atypical neurofibromas and benign neurofibromas. This supports our previous hypothesis that atypical neurofibromas are at the lower end of the malignant spectrum [9]. The patient with a false-negative PET/CT scan (SUVmax 3.2) had an atypical neurofibroma and this might have reflected the differing views of pathologists on the definition of atypical neurofibroma. A consensus on the histological diagnosis of atypical neurofibroma and whether it represents a benign or malignant lesion will be extremely helpful for future studies [11, 12]
ROC curve analysis of SUV on delayed imaging identified a cut-off value of SUVmax of 3.1 to achieve maximal sensitivity and specificity. In our study this would have identified all malignant tumours (including the false-negative scan with an SUVmax of 3.2). However, the number of false-positive scans would have increased from 6 to 11, and therefore we continue to recommend a cut-off of 3.5 for malignancy. We suggest that tumours with an SUVmaxD value in the range 3.0–3.5 (this includes tumours whose SUVmax increases from early to late imaging to within this range) should be reviewed clinically and further patient management discussed at specialist multidisciplinary meetings. The lower limit of this range is slightly higher than previously suggested and is probably related to the use of CT attenuation correction that results in an increased SUV compared to rod-based attenuation [15]. The overlap range is of interest in that it is possible that a combination of tracers will be necessary in this select area to try and further separate patients requiring biopsy or excision from those who can be observed.
This study, which to our knowledge is the largest study of PET/CT imaging in MPNSTs, supports these findings: adopting a cut-off SUVmaxD value of ≥3.5 resulted in a sensitivity of 0.97 and a specificity of 0.87. Only one atypical neurofibroma with SUVmaxE of 1.6 and SUVmaxD of 3.2 was misdiagnosed as a benign lesion. With this strategy it is inevitable that there will be false-positive results because of the well-recognized overlap of SUV in benign and malignant tumours. It is clearly justifiable to biopsy lesions that may turn out to be benign, rather than misdiagnose a malignant tumour as benign given the potential clinical consequences. Utilizing PET/CT to identify the area of maximal uptake within the tumour will also help to reduce the risk of false-negative biopsies due to inadequate sampling. If it is felt to be clinically helpful the PET/CT data can also be registered with MRI as has been demonstrated by Somer et al. [16]. In the future, the hope is that new tracers will become available with increased specificity for MPNST identification in these equivocal tumours. It is possible that a proliferation marker such as FLT or alternatively a radiolabelled l-amino acid, such as 11C-methionine, may fulfil this role.
A limitation of our study was the relatively short follow-up time in a number of the lesions identified on PET/CT scans as benign that had not been biopsied and remained under clinical review. However, based on previous work from this institution in which 56 individuals who were deemed to have benign neurofibromas were followed for over 2 years (31 for over 5 years) and no patient developed clinical evidence of malignant disease, we feel that this is a reasonable assumption to make.
In conclusion, we demonstrated that FDG PET/CT is a highly sensitive and specific imaging modality for the diagnosis of MPNST in NF1 patients. We recommend performing early and delayed imaging at 4 h for accurate lesion characterization and using a cut-off SUVmaxD of 3.5 to achieve maximal sensitivity whilst maintaining specificity in the diagnosis of MPNSTs. Patients with NF1-associated MPNSTs require management by specialist multidisciplinary teams conversant with complex NF1 and malignancy.
References
Compston DAS, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet 1989;26(11):704–11.
Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol 2007;6(4):340–51.
Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res 2002;62:1573–7.
Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002;39(5):311–4.
Ferner RE, Lucas JD, O’Doherty MJ, Hughes RAC, Smith MA, Cronin BF, et al. Evaluation of 18fluorodeoxyglucose positron emission tomography (18FDGPET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatr 2000;68:353–7.
Cardona S, Schwarzbach M, Hinz U, Dimitrakopoulou-Strauss A, Attigah N, Mechtersheimer G, et al. Evaluation of F18-deoxyglucose positron emission tomography (FDG-PET) to assess the nature of neurogenic tumours. Eur J Surg Oncol 2003;29:536–41.
Brenner W, Friedrich RE, Gawad KA, Hagel C, von Deimling A, de Witt M, et al. Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging 2006;33:428–32.
Bredella MA, Torriani M, Hornicek F, Ouellette HA, Palmer WE, Williams Z, et al. Value of PET in the assessment of patients with neurofibromatosis type 1. AJR Am J Roentgenol 2007;189:928–35.
Ferner RE, Golding JF, Smith M, Calonje E, Jan W, Sanjayanathan V, et al. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol 2008;19(2):390–4.
Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histological grading system. Int J Cancer 1984;33(1):37–42.
Valeyrie-Allanore L, Ortonne N, Lantieri L, Ferkal S, Wechsler J, Bagot M, et al. Histopathologically dysplastic neurofibromas in neurofibromatosis 1: diagnostic criteria, prevalence and clinical significance. Br J Dermatol 2008;58:1008–12.
Lin BT-Y, Weiss LM, Medeiros LJ, Jeffrey L. Neurofibroma and cellular neurofibroma with atypia: a report of 14 tumors. Am J Surg Pathol 1997;21:1443–9.
Lodge MA, Lucas JD, Marsden PK, Cronin BF, O’Doherty MJ, Smith MA. A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med Mol Imaging 1999;26:22–30.
Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumours. A clinicopathologic study of 120 cases. Cancer 1986;58:2006–21.
Visvikis D, Costa DC, Croasdale I, Lonn AHR, Bomanji J, Gacinovic S, et al. CT-based attenuation correction in the calculation of semi-quantitative indices of [18F]FDG uptake in PET. Eur J Nucl Med Mol Imaging 2003;30:344–53.
Somer EJ, Benatar NA, O’Doherty MJ, Smith MA, Marsden PK. Use of the CT component of PET-CT to improve PET-MR registration: demonstration in soft tissue sarcoma. Phys Med Biol 2007;52:6991–7006.
No grants received.
The authors have had full control of all primary data and agree to allow the EJNM to review the data if requested.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warbey, V.S., Ferner, R.E., Dunn, J.T. et al. [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur J Nucl Med Mol Imaging 36, 751–757 (2009). https://doi.org/10.1007/s00259-008-1038-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-1038-0