Abstract
Purpose
The purpose of the study was the identification of group and individual subject patterns of cerebral glucose metabolism (CMRGlu) in patients with Alzheimer’s disease (AD) and with amnestic mild cognitive impairment (aMCI).
Methods
[18F]fluorodeoxyglucose positron emission tomography (PET) studies and neuropsychological tests were performed in 16 aMCI patients (ten women, age 75 ± 8 years) and in 14 AD patients (ten women, age 75 ± 9 years). Comparisons between patient subgroups and with a control population were performed using Statistical Parametric Mapping.
Results
Clusters of low CMRGlu were observed bilaterally in the posterior cingulate cortex (PCC), in the precuneus, in the inferior parietal lobule and middle temporal gyrus of AD patients. In aMCI patients, reduced CMRGlu was found only in PCC. Areas of low CMRGlu in PCC were wider in AD compared to aMCI and extended to the precuneus, while low CMRGlu was found in the lateral parietal cortex in AD but not in aMCI patients. Individual subject pattern analysis revealed that 86% of AD patients had low CMRGlu in the PCC (including the precuneus in 71%), 71% in the temporal cortex, 64% in the parietal cortex and 35% in the frontal cortex. Among the aMCI patients, 56% had low CMRGlu in the PCC, 44% in the temporal cortex, 18% in the frontal cortex and none in the parietal cortex.
Conclusion
This study demonstrates that both AD and aMCI patients have highly heterogeneous metabolic impairment. This potential of individual metabolic PET imaging in patients with AD and aMCI may allow timely identification of brain damage on individual basis and possibly help planning tailored early interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reductions of cerebral glucose metabolism (CMRGlu) and perfusion have been consistently reported in Alzheimer’s disease (AD). The most commonly described diagnostic hallmarks of AD when studied with neurofunctional imaging techniques are bilateral reductions in CMRGlu and blood flow in the temporal and parietal association cortices, posterior cingulate cortex and, in the advanced stages of the disease, in the frontal association cortex. There is typically a relative sparing of the primary sensori-motor cortex [1–3]. This pattern of cortical dysfunction and sparing has been reported also in the early stages of disease [4, 5]. Therefore, CMRGlu on positron emission tomography (PET) has been extensively used as a biomarker to increase the specificity of the clinical diagnosis of AD and to monitor disease progression [6]. An early and accurate diagnosis is desirable as the benefits of therapy may be diminished when treatment is delayed, missing the opportunity of maintaining the levels of cognitive and functional ability as high as possible [7]. As neuronal degeneration in AD begins several years before cognitive deficits become manifest [8, 9], many studies are now aimed at identifying sensitive and specific methods for the characterisation of patients with preclinical AD, as those with mild cognitive impairment (MCI) who are known to progress to clinical AD in up to 25% of cases per year [10]. Amnestic MCI (aMCI) patients belong to a subgroup of MCI subjects characterised by significant memory deficits but who are normal in other cognitive domains and are at higher risk of developing dementia. Anyhow, not all patients with MCI progress to AD-type pathology, as up to 30% develop other neurologic disorders. Thus, the clinical definition of MCI is not synonymous of pre-clinical AD [11]. Besides affecting individual patients’ management, lack of appropriate classification may significantly influence the outcomes of pharmacological trials in which these patients are enrolled. PET studies of regional CMRGlu in MCI subjects are still limited but may represent a useful approach to identify patients with prodromal AD when a ‘typical’ pattern of temporo-parietal hypo-metabolism can be detected and differential diagnosis from other diseases causing dementia [6, 12]. Most studies have reported that MCI patients that show patterns of CMRGlu reductions similar to those observed in clinical AD patients have a high probability of developing AD in the near future. It has also been shown that longitudinal changes of CMRGlu occur in aMCI patients converting to AD, who also present with a characteristic pattern of metabolic abnormalities in the hippocampal and parahippocampal cortex [13] and in the parietal and posterior cingulate cortical areas before developing dementia [14]. However, it remains to be determined to what extent this typical pattern of hypo-metabolism is detectable in individual patients.
The aims of this study were to identify individual patterns of metabolic impairment in patients with AD and aMCI and to assess the consistency of these patterns in relation to the diagnosis, as well as the relationships between patterns of glucose metabolism in AD and aMCI groups.
Materials and methods
Patients selection
Thirty patients who fulfilled the criteria for AD or aMCI (see below) were recruited at the Center for Research and Treatment of Cognitive Dysfunctions, Institute of Clinical Neurology, Department of Clinical Sciences “Luigi Sacco” Hospital, University of Milan, between 2003 and 2006. They had been referred by general physicians, neurologists or psychiatrists or from other institutions because of cognitive impairment. All patients underwent a diagnostic workup, routinely applied in the outpatient clinic for clinical evaluation of patients with cognitive impairment, which included an interview with the patient and an informant, medical, psychiatric and neurological examinations, structural neuroimaging and routine blood screening.
All patients received the clinical dementia rating (CDR) [15] and underwent a neuropsychological assessment measuring global cognitive functioning (Mini-Mental State Examination [MMSE], and Raven’s Coloured Progressive Matrices 47), long-term verbal and spatial memory (Story Recall, Rey–Osterrieth Complex Figure recall), visuospatial abilities (Rey–Osterrieth Complex Figure copy, Clock Design), executive functions (Weigl’s Sorting Test, Phonemic and Semantic Fluencies, Frontal Assessment Battery) and attention (Number cancellation) [16].
Inclusion criteria
Subjects were selected on the basis of the following criteria: (a) Hachinski ischaemia score [17] less than 4; (b) absence of organic brain pathology or organic illness affecting the brain other than cognitive impairment and absence of structural brain alterations on MRI; (c) no significant history of head injury; (d) absence of major systemic illnesses or medical complications, including vitamin deficiency states, thyroid disorders, recent treatment for neoplastic disease, poorly controlled diabetes mellitus, kidney or liver disorders, serious cardiac diseases or severe inflammatory diseases and sensory disorders (i.e. blindness or deafness); (e) no history of drug or alcohol dependence; (f) no psychoactive medication or treatment that may affect cognitive function or brain metabolism.
Alzheimer’s disease
AD was diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteria [18] for dementia and National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria [19] for probable AD.
Fourteen patients were enrolled: four men and ten women, mean age at examination was 75 ± 9 years. The mean interval between onset of symptoms and the date of examination was 3.4 ± 1.7 years. The mean for years of formal education was 7.7 ± 5.0 years (defined as years at school plus years of apprenticeship or attendance at technical school, college and university). The results of neuropsychological tests are summarised in Table 1.
Amnestic mild cognitive impairment
aMCI was diagnosed according to the diagnostic criteria defined by Petersen et al. [10]. These criteria include memory concerns, usually reported by the patient and preferably corroborated by an informant, objective memory impairment (i.e. a Z score greater than 1.5 SD below the mean, adjusted for age and education, on test measuring long-term memory), normal general cognitive function (MMSE > 24), normal activities of daily living, CDR = 0.5 and not demented [18, 19]. To ensure that all subjects had the amnestic form of MCI, impairment of a cognitive domain other than memory was considered an exclusion criterion. Sixteen individuals were included in the study. The mean age at examination was 75 ± 8 years; ten patients were female and six male. The mean interval between onset of symptoms and the date of examination was 2.1 ± 1.6 years. The mean for years of formal education was 9.1 ± 4.5 years. The results of neuropsychological tests are summarised in Table 1.
Control subjects
For comparison, seven elderly individuals with well-documented normal cognitive function were selected from our database. They were selected among those undergoing a PET scan for cancer re-staging (NHL in four cases, lung cancer, melanoma and colon in the other patient), thus for reasons unrelated to cognitive deficits. These subjects had no evidence of neurological impairment based on intensive interviews, CDR = 0, MMSE > 28 and normal scores on the same extensive psychometric tests used to assessed cognitive-impaired subjects. Exclusion criteria specific to these subjects were: (a) Hachinski ischemia score [20] greater than or equal to 4; (b) evidence of brain metastases or other organic brain pathology or structural brain alterations on MRI; (c) evidence of depression or abnormal score in the Montgomery Asberg Depression Rating Scale; (d) history of head injury; (e) presence of major systemic illnesses or medical complications, including vitamin deficiency states, thyroid disorders, poorly controlled diabetes mellitus, kidney or liver disorders, serious cardiac diseases or severe inflammatory diseases and sensory disorders (i.e. blindness or deafness); (f) history of drug or alcohol dependence; (g) psychoactive medication or treatment that may affect cognitive function or brain metabolism; (h) abnormal PET scan at visual assessment.
All subjects gave their written informed consent after adequate explanation of the procedure before PET examination.
[18F]FDG PET imaging
PET imaging was performed using an ECAT ACCELL scanner (Siemens Medical Systems, Enlargen, Germany) equipped with lutetium oxyorthosilicate crystals. All patients fasted for at least 4 h before examination to ensure standardised glucose metabolism. An activity of 185–370 MBq of [18F]fluorodeoxyglucose (FDG), depending on the patient’s weight, was injected intravenously under resting conditions with eyes closed and ears unplugged. Attenuation-corrected brain scans were acquired 45 min after [18F]FDG administration. To obtain transaxial images, approximately parallel to the inter-commissural line (anterior commissure–posterior commissure line), subjects were positioned with the canthomeatal line parallel to the detector rings. After data acquisition, corrections for random, dead time and scatter were performed, and images were re-constructed by filtered back-projection with a Hamm filter (cutoff frequency 0.5 cycles per projection element), resulting in 47 slices in a 128 × 128-pixel matrix.
[18F]FDG PET image analysis
All PET images were converted into ANALYZE format and spatially normalised to match a standard stereotactic anatomical space [21] with a voxel size of 2 × 2 × 2 mm, using a 12-parameter affine (linear) and eight-parameter quadratic (nonlinear) three-dimensional transformation, and smoothed with an isotropic Gaussian kernel of 12 mm using Statistical Parametric Mapping (SPM2-Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, UK) programme implemented in Matlab 6.0 (Mathworks, Natick, MA, USA) on a Windows XP personal computer workstation. Global counts were normalised by proportional scaling to remove confounding effects due to global changes. Age was modelled as a nuisance variable.
Group analyses
Groups were compared on a voxel-by-voxel basis using a two-sample t test. Significant differences between groups (i.e. AD vs. controls, aMCI vs. controls and AD vs. aMCI) were examined at p < 0.01 for voxel height, uncorrected, and p < 0.05 for cluster extent. The choice of loose statistical criteria is related to the fact that areas of cortical hypo-metabolism in AD and MCI is expected, as already demonstrated by the literature, and we were looking for differences among AD and MCI.
Single-subject analyses
To assess regional cerebral metabolic abnormalities in single subjects, each PET study was analyzed separately according to a previously described method [22]. The SPM{t} maps of each patient were then converted to binary masks; in this way, new images were created where each pixel could be either zero if the corresponding cerebral region was normal or one if it was abnormal. All the masks were summed together, to generate a map of overlapping regions of metabolic impairment.
Results
Group analyses
AD patients vs. control subjects
AD patients had significant regional CMRGlu reductions in the posterior cingulate cortex, precuneus and parietal and temporal lobes bilaterally (Table 2 and Fig. 1).
aMCI patients vs. control subjects
aMCI patients showed reductions in glucose consumption that were restricted to the posterior cingulate cortex, with a lower level of statistical significance than that observed in AD (Table 3, Fig. 2).
SPM showing areas in which aMCI subjects present lower glucose metabolism than the control subjects. In comparison with Fig. 1, the areas of hypo-metabolism are less widespread and limited to the cuneus and posterior cingulate
aMCI patients vs. AD patients
As compared to aMCI, AD patients showed greater CMRGlu reductions in the temporal, parietal and occipital regions, bilaterally (Table 4, Fig. 3).
Single-subject analyses
AD patients
All AD patients showed at least one cluster of significant hypo-metabolism in cortical regions “typical” of AD. Eighty-six percent of AD patients (12 of 14) had lower CMRGlu in the posterior cingulate cortex and 71% (10 of 14) in the precuneus. The temporal cortex was affected in ten patients (71%) and the parietal cortex in nine (64%). In five patients (36%), a reduction in metabolism was observed in the frontal cortex (in four of these patients, the low frontal CMRGlu was co-existent with temporal and parietal CMRGlu). In only half of the patients (seven subjects), a pattern of parietal and temporal hypo-metabolism was clearly defined (Table 5, Fig. 4a).
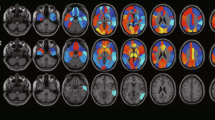
Overlap of metabolic impairment. The color bar indicates the percentage of subjects with overlapping areas of individual metabolic impairment in a 14 AD patients and b 16 aMCI patients. The color scale ranges between 0% (areas where no patient has metabolic impairment) and 100% (areas where all patients show a statistically significant metabolic impairment). It can be seen that almost all AD patients have a significant reduction in [18F]FDG uptake in the posterior cingulate, more than 50% have a reduction in the parietal cortex, about 25% have a reduction in the lateral frontal lobe and very few have a reduction in the mesial frontal lobe. aMCI patients present with a more heterogeneous pattern of metabolic impairment with the posterior cingulate most often impaired
aMCI patients
Two patients had no voxels above the a priori significance threshold. In nine subjects (56%), we found a reduction in metabolism in the posterior cingulate cortex; in seven (44%), reduction was observed in the temporal cortex, mostly asymmetric (in three subjects, the hypo-metabolism was in the right hemisphere, in three subjects in the left hemisphere and in two subjects the hypo-metabolism was bilateral); in three subjects, hypo-metabolism was observed in the frontal cortex (one in the left and one in the right hemisphere and one bilateral); three subjects had impairment in the motor cortex (two bilateral and one left hemisphere), three subjects in the anterior cingulate cortex and one in the precuneus. None of them had deficits of uptake in the parietal areas (Table 5, Fig. 4b).
Case study
This 81-year-old independent woman (FAN in Table 5) had a 3-year history of memory loss. Her past medical history was normal. The neurological examination revealed bilateral palmo-mental reflex and right extensor cutaneous plantar reflex. Baseline investigations, including syphilis serology, folic acid, vitamin B12, thyroid function and electroencephalography (EEG), were within normal limits. MRI revealed diffuse cortical atrophy and small lacunar infarcts in the subcortical white matter. The MMSE and the CDR scored 25 and 0.5, respectively; the neuropsychological assessment revealed impaired verbal and visuospatial memory with sparing of all other cognitive domains. aMCI was diagnosed. A PET scan was performed (Fig. 5). SPM analysis showed a significant reduction in [18F]FDG uptake bilaterally in the frontal cortex (the right side more extended than the left side). A focus of reduced uptake was also observed in the right superior temporal cortex and in the left inferior parietal lobe. Nineteen months later, a progression in memory deficits was reported by her husband. At that time, the autonomy on instrumental activity of daily living was impaired. MMSE and CDR scored 17 and 1, respectively. A second psychometric evaluation showed deficits on tests exploring memory (Story and Rey recalls), semantic fluency and attention. The EEG revealed diffuse slow (θ) activity. On MRI, cortical atrophy had progressed, while the small lacunar infarcts were unchanged. AD diagnosis was established.
Discussion
In this [18F]FDG PET study, we examined the metabolic patterns in AD and aMCI patients as compared to control subjects and to each other, on a group as well as on an individual basis. The single-subject comparisons are particularly relevant to the potential use of FDG PET in a clinical diagnostic setting.
Consistent with previous findings, we found that AD patients show reduced glucose metabolism in the posterior cingulate, precuneus and parieto-temporal association areas. However, on an individual basis, the pattern of parieto-temporal hypo-metabolism was evident only in half of the patients with a clinical diagnosis of AD. The brain region most commonly hypo-metabolic in AD patients was the posterior cingulate cortex, followed by the precuneus, the temporal cortex, the parietal cortex and finally the frontal cortex. These results suggest that the term ‘typical AD pattern’ defines the overall population findings, while, in individual subjects, the patterns of reduced tracer uptake may be quite variable. This heterogeneity was previously reported in AD where metabolic profiles ranging from bilateral temporal, bilateral parietal or bilateral parieto-temporal hypo-metabolism or highly asymmetric temporo-parietal hypo-metabolism or isolated posterior cingulate hypo-metabolism were observed by various investigators [23]. Our data indicate that [18F]FDG PET examinations of individual patients should focus particularly on the presence of hypo-metabolism in the posterior cingulate cortex and precuneus, rather than on temporal and parietal association cortices alone.
aMCI patients also showed impaired glucose metabolism in the posterior cingulate cortex, consistent with previous findings [14]. Metabolic reductions in the posterior cingulate cortex were found in most (56%) of our aMCI patients. On the other hand, metabolic dysfunctions in the associative temporal cortical areas were observed in only seven aMCI patients (44%), while none reached statistical significance for lateral parietal cortex hypo-metabolism. The posterior cingulate cortex has often been reported to be involved in the early stages of AD [24–26]; therefore, our finding in aMCI is consistent with the hypothesis that aMCI is a pre-AD state. The involvement of the parieto-temporal association cortex is typical of more advanced disease [14]. In our study, bilateral metabolic abnormalities in the temporal–parietal and posterior cingulate cortex were found in AD patients, while, in aMCI patients, the extent of hypo-metabolism was more limited and did not involve the parietal association cortex.
This study also demonstrates that besides the metabolic impairment in the posterior cingulate, some aMCI patients had areas of metabolic impairment in the frontal lobe without a clearly defined ‘metabolic pattern,’ as shown by individual voxel-based analysis. Differently from AD patients, in whom the reduction in CMRGlu in frontal areas is usually detectable when also the parietal deficits are evident, suggesting an advanced stage of the disease, in aMCI subjects, we found that two of them (FAN and MA) had a reduction in FDG uptake in the frontal lobe (one asymmetric and one bilateral, despite mild signs of diffuse cortical atrophy on the MRI of both patients), without abnormalities of FDG uptake neither in the posterior cingulate cortex nor in the temporal cortex, which suggests that early fronto-temporal dementia (FTD) rather than early AD may be present [27]. Another patient (VL) had a reduction in uptake in the posterior cingulate cortex and in the frontal lobe. This may be due to dual pathology (AD and FTD), an atypical appearance of FTD or to a frontal lobe variant of AD. Further clinical follow-up and, ultimately, autopsy verification will be needed to resolve these possibilities.
We observed not only the lack of symmetrical hypo-metabolism, except in two cases, but also a broad heterogeneity of focal alterations and their combinations. In our study, the principal hallmarks associated with the clinical diagnosis of aMCI versus AD were the general lack of parietal hypo-metabolism and significant heterogeneity of focal hypo-metabolism in areas that become involved at later AD stages.
Among the aMCI patients, in two subjects, we could not identify any cluster of metabolic impairment, suggesting that neuropsychological assessment and neurofunctional imaging are not strictly correlated.
Since reductions in posterior cingulate metabolism are considered a major risk factor for developing AD among aMCI patients [24, 28], follow-up examinations of our patients are necessary to assess whether the patients with posterior cingulate cortex abnormalities will go on developing dementia, while those with more heterogeneous abnormalities will remain stable or develop other dementias. Similarly, follow-up will be necessary to determine whether the aMCI subjects without posterior cingulated CMRGlu reduction have non-progressive courses or whether an alternative neurological explanation for memory complaint is identified.
Although the findings of this study are consistent with other studies on AD and MCI patients, a major difference needs to be discussed. We choose to study neurologically normal subjects by selecting them among those referred for a PET scan for reasons not related to cognitive functions. Obviously, those are ‘patients’ and not ‘globally’ normal individuals; nevertheless, they were normal on extensive neuropsychological evaluation, which excluded cognitive dysfunction and depression. By doing this, we did not submit normal subjects to the radiation exposure that would derive from a PET investigation. Most of our control subjects were exposed to recent chemotherapy for cancer: whether and how this, as well as having cancer, might influence brain uptake of [18F]FDG is not known in details. Secondary depression is common in oncology patients, and this may influence brain uptake of the tracer even when patients do not present with psychiatric symptoms [29]. This can represent a limitation of this study, and along with the low number of control subject we used, may explain the limited extension of the hypo-metabolic regions both in AD and MCI patients and reduce the accuracy of our findings.
In conclusion, this study demonstrates that individual AD and aMCI patients show highly heterogeneous distributions of metabolic impairment. Nevertheless, the consistency of hypo-metabolism in the posterior cingulated cortex, together with absence of abnormality outside of the areas ‘typically’ involved in AD, may be sufficient to identify individual subjects who will have a progressive course of cognitive decline. This may be of great clinical relevance as it would allow timely identification of AD and pre-AD brain damage and help guide early treatment interventions. At present, other measures such as neuropsychological evaluation, biochemical markers and genotyping have failed to provide reliable differentiation of aMCI patients with respect to individual prognosis [25].
References
Mielke R, Kessler J, Szelies B, Herholz K, Wienhard K, Heiss WD. Normal and pathological aging: findings of positron-emission-tomography. J Neural Transm 1998;105:821–37.
Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. Radiology 2003;226:315–36.
Coleman RE. Positron emission tomography diagnosis of Alzheimer’s disease. Neuroimaging Clin N Am 2005;15:837–46.
Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med 2000;41:1155–62.
Johnson KA, Albert MS. Perfusion abnormalities in prodromal AD. Neurobiol Aging 2000;21:289–92.
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–46.
Farlow MR, Cummings JL. Effective pharmacologic management of Alzheimer’s disease. Am J Med 2007;120:388–97.
Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995;16:271–8.
Smith AD. Imaging the progression of Alzheimer pathology through the brain. Proc Natl Acad Sci USA 2002;99:4135–7.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8.
Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology 2007;69:1622–34.
Matsuda H. The role of neuroimaging in mild cognitive impairment. Neuropathology 2007;27:570–7.
Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology 2005;64:1860–7.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 2003;30:1104–13.
Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9(Suppl 1):173–6.
Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995.
Moroney JT, Bagiella E, Desmond DW, Hachinski VC, Mölsä PK, Gustafson L, et al. Meta-analysis of the Hachinski Ischemic Score in pathological verified dementias. Neurol 1997;49:1096–105.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44.
Moroney JT, Bagiella E, Desmond DW, Hachinski VC, Mölsä PK, Gustafson L, et al. Meta-analysis of the Hachinski Ischemic Score in pathological verified dementias. Neurology 1997;49:1096–105.
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988.
Signorini M, Paulesu E, Friston K, Perani D, Colleluori A, Lucignani G, et al. Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and nonquantitative [18F]FDG PET: a clinical validation of Statistical Parametric Mapping. Neuroimage 1999;9:63–80.
Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007;69:871–7.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997;42:85–94.
Small GW, Leiter F. Neuroimaging for diagnosis of dementia. J Clin Psychiatry 1998;59(Suppl 11):4–7.
Ishii K. Clinical application of positron emission tomography for diagnosis of dementia. Ann Nucl Med 2002;16:515–25.
Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Förstl H, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging 2007;28:42–50.
Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol 2001;49:202–13.
Kumano H, Ida I, Oshima A, Takahashi K, Yuuki N, Amanuma M, et al. Brain metabolic changes associated with predispotion to onset of major depressive disorder and adjustment disorder in cancer patients—a preliminary PET study. J Psychiatr Res 2007;41:591–9.
Acknowledgements
The authors wish to thank Prof. K.A. Frey from University of Michigan Ann Arbor for his helpful comments and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Sole, A., Clerici, F., Chiti, A. et al. Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: an FDG PET study. Eur J Nucl Med Mol Imaging 35, 1357–1366 (2008). https://doi.org/10.1007/s00259-008-0773-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0773-6