Abstract
Purpose
The iodinated cocaine derivative [123I]PE2I is a new selective ligand for in vivo studies of the dopamine transporter (DAT) with SPECT. Recently, a bolus/infusion (B/I) protocol for [123I]PE2I measurements of DAT density was established [Pinborg LH et al. J Nucl Med 2005;46:1119–271]. The aims of this study were, firstly, to evaluate the test–retest variability using the B/I protocol and, secondly, to evaluate the B/I approach in a new group of healthy subjects using two outcome parameters, BP1 (CROI/Cplasma) and BP2 (CROI/CREF).
Methods
Seven healthy subjects were subjected to [123I]PE2I SPECT scanning twice. For both studies, the two outcome parameters BP1 and BP2 were calculated based on two different methods for region of interest (ROI) delineation, namely manual delineation and probability map-based automatic delineation with MRI co-registration.
Results
With manual delineation, striatal test–retest variability (absolute difference between first and second scan as a percentage of the mean) of BP1 and BP2 was 13.9% (range 1.8–35.7%) and 4.1% (range 0.5–9.7%) respectively. The probability map-based automatic delineation resulted in striatal test–retest variability of 17.2% (range 4.3–40.5%) and 5.2% (range 0.1–10.9%) respectively. The B/I approach provided stable brain activity from 120 to 180 min post injection in both high- and low-count regions with a mean % change/hour in striatal BP2 of 10.6.
Conclusion
[123I]PE2I SPECT with the B/I approach yields a highly reproducible measure of striatal dopamine transporter binding. The appropriateness of a B/I protocol with a B/I ratio of 2.7 h (i.e. with a bolus worth 2.7 h of infusion) was confirmed in an independent sample of healthy subjects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The clinical features of idiopathic Parkinson’s disease (IPD)—rigidity, tremor and bradykinesia—may also be present in other neurodegenerative diseases, and clinicopathological correlations in patients diagnosed with IPD show that 10–20% have other diagnoses, e.g. Alzheimer’s disease, vascular parkinsonism (vPD), drug-induced parkinsonism (dPD), essential tremor (ET), progressive supranuclear palsy (PSP) or multiple system atrophy (MSA) [2].
During the past decade, imaging of the dopamine transporter (DAT) has increasingly been used as a biomarker for the integrity of presynaptic dopaminergic nerve cells in patients with movement disorders [2–8]. In clinical practice, DAT imaging with SPECT is an important tool to assist in the clinical diagnosis of IPD and other neurodegenerative diseases. In IPD, DAT imaging has consistently shown: (1) low striatal DAT density [5, 6, 9]; (2) side-to-side asymmetry, with greater losses in DAT density in the striatum contralateral to the clinically most affected side [5]; and (3) earlier onset of decline in DAT density in the putamen as compared with the caudate nucleus [5]. In patients with MSA and PSP, by contrast, the side-to-side asymmetry is less pronounced, and a more homogeneous loss of DAT binding takes place in the caudate nucleus and putamen [4, 9, 10].
PE2I is a cocaine derivative that is structurally related to other DAT tracers for SPECT, such as FP-CIT and β-CIT [11, 12]. PE2I is a highly selective ligand with fast kinetics and a high target to background ratio [1]. In a recent study, we compared in the same subjects different methods for quantification of [123I]PE2I DAT binding [1], both following bolus alone and following bolus plus constant tracer infusion. We concluded that the bolus/infusion (B/I) approach is superior to a bolus alone study since it allows for accurate quantification of [123I]PE2I binding and is easily implemented in a clinical setting [1].
The aims of the present study were to test the reproducibility and reliability of the B/I approach for quantification of [123I]PE2I binding to DAT using BP1 (CROI/Cplasma) and BP2 (CROI/CREF) as outcome parameters and, secondly, to test the feasibility of the previously described B/I protocol in a new sample.
Materials and methods
Subjects
For the reproducibility study, seven healthy subjects (age range 41–71 years, three males and four females) were studied twice, at intervals lasting from 7 to 21 days. The subjects were recruited from a local newspaper advertisement and were asked to complete a questionnaire before entering the study. None of the subjects had any history of neurological or psychiatric disorders, and they all claimed to be alcohol- and drug-free. Physical examinations and routine blood tests were normal in all subjects.
All subjects provided informed written consent. The study was performed in accordance with the ethical standards of the Declaration of Helsinki and was approved by the ethical committee of Copenhagen and Frederiksberg (KF 12–009/04).
Experimental procedures
Procedures were done according to Pinborg et al. [1], except that instead of taking 24 venous blood samples between time 0 and 240 min, only two samples were taken 150 min after injection. Cannulas were inserted into both cubital veins for tracer administration and blood sampling respectively. To block thyroidal uptake of free radioiodine, all subjects received 200 mg potassium perchloride intravenously 30 min before [123I]PE2I injection. In all experiments an average bolus of 73.9 MBq (range 63–93.8 MBq) [123I]PE2I was administered, immediately followed by a constant infusion of [123I]PE2I for 3 h. The B/I protocol was similar in both studies, with a bolus worth 2.7 h (range 2.6–2.8 h) of infusion (the B/I ratio). As already mentioned, two separate venous blood samples were drawn at 150 min. Plasma was sampled from the blood, followed by octanol extraction to derive the lipophilic phase containing [123I]PE2I [13]. One ml of plasma and 2 ml of octanol was shaken for 2 min, and then 1 ml of the octanol phase was pipetted into another counting vial. Finally the octanol fraction was counted in a γ-counter (Cobra II, Packard Instrument Company). The activity in the octanol phase multiplied by 2 thus represents parent [123I]PE2I. Cross-calibration with a phantom containing a well-known concentration of [123I]PE2I was done in order to enable comparison of activity measurements between the SPECT scanner and the γ-counter.
SPECT imaging was performed with a triple-head IRIX camera (Philips Medical, Cleveland, USA) fitted with low-energy, general-purpose, parallel-hole collimators (spatial resolution 8.5 mm at 10 cm). The mean radius of rotation was 13.9 cm. Each camera covered 120° of the circular orbit. Scans were performed in continuous mode with an angular step of 3° and 40 steps. Six SPECT acquisitions each of 10 min duration were obtained between 120 and 180 min post injection. Reconstruction of the images was performed with a MATLAB 6.5 (Mathworks)-based program in 1282 matrices (2.33-mm pixels and identical slice thickness) using standard filtered back projection with a low-pass fourth-order Butterworth filter at 0.3 Nyquist (0.064 mm−1). The images were subsequently corrected with an attenuation algorithm fitted to the individual head area using an attenuation coefficient with an empirical value of 0.10 cm−1.
Magnetic resonance imaging
All subjects underwent two structural magnetic resonance imaging (MRI) scans with a 1.5-T Vision scanner (Siemens, Erlangen, Germany) using the 3D MPRAGE sequence (TI/TD/TE/TR=300/300/4.4/11.4 ms, flip angle 12°) acquired as sagittal plane scans with a spatial resolution of 1.50×1.13×1.02 mm3. There were 130 planes and the in-plane matrix was 230×256. MRI and SPECT scans were acquired on the same days. Co-registration of the MRI to the SPECT images was facilitated by the use of five external fiducials glued to the skin (a single one over the glabella, and one over the temple bone and one behind the ear, bilaterally). The fiducials were made of 5-mm-diameter plastic containers filled with olive oil and 99Tc, thereby being visible on both MRI and SPECT (Fig. 1).
3D view of the semi-automatic co-registration. The left side of the panel shows the alignment of a subject’s MR skull and the fiducial markers (green ROIs), visualised from a threshold SPECT image. In the right panel, the skull is visualised transparently, thus showing the volumes of interest [caudate nucleus (yellow), putamen (orange) and occipital cortex (red)] and the five external fiducials from the threshold SPECT image
Regions of interest
The ROIs were applied to the SPECT images in two different ways: manually or probability map based. With the manual method, ROIs were manually delineated directly on the reconstructed SPECT images guided by the use of Kretschmann and Weinrich’s Neuroanatomical atlas [14]. The direct adaptation of ROI coordinates from the atlas required a reorientation of the reconstructed images to the canthomeatally oriented plane. ROIs were delineated on five consecutive slices (7 mm/slice) in a summed image from all six SPECT frames acquired from 120 to 180 min post injection. In order to mimic the SPECT DAT quantification used in daily clinical practice, a new set of ROIs were drawn for each scan event. With the second method the probability map-based automatic delineation approach developed by Svarer et al. [15] was used: Co-registration of MR and SPECT images was performed using a semi-automatic Matlab (Mathworks Inc., Natick, MA, USA) based method [16]. The corresponding external fiducial markers were manually identified and defined on the MR and SPECT images. A rigid transformation between the images was then estimated automatically by minimising the sum of squared errors between the defined points. This method was chosen as it has been demonstrated that automatic approaches such as the AIR and SPM99 methods [16, 17] are less optimal for alignment of MR and SPECT images with tracer binding confined to selected brain regions [16, 18], as is the case for [123I]PE2I imaging. ROIs were subsequently identified automatically on the MR image and directly transferred to the co-registered SPECT image, as described by Svarer et al. [15]; with this probability map-based delineation approach, the putamen and caudate are identified separately. The probability map-based method includes ROIs covering the volume of the entire region whereas with the manually delineated method, striatal ROIs are calculated on the basis of two-dimensional delineations times the slice distance. The occipital cortex was used as a region representing non-specific binding only.
Derivation of binding parameters
Two different outcome parameters, BP1 and BP2 [1], were calculated according to:
where C 1(∞) denotes the constant tracer concentration in the metabolite-corrected plasma compartment, in this instance the mean of two blood samples drawn at 150 min post injection, and C 2(∞) and C 3(∞) denote the constant radiotracer concentration in the non-specific and specific compartments, respectively. In practice this concentration is computed by SPECT ROIs (counts/ml) from the summed SPECT acquisitions. Emission tomography measures the total concentration of radiotracer in the striatum (C tot). Due to the overall physicochemical constancy of brain tissue, C 2(∞) is assumed to equal the concentration of tracer in an ROI devoid of receptors [C ref(∞)] and C 3(∞) is calculated as C tot(∞)−C ref(∞).
The free fraction of tracer in plasma, f 1, and the free tracer fraction in brain tissue, f 2, were not measured and, accordingly, the binding potential (BP) equal to the ratio of the density of available transporters, B′max, to the affinity constant of the tracer [1], K d, was not directly determined.
Statistical analysis
The stability of the individual time-activity curves and outcome measures was calculated as the slope (linear regression analysis from 120 to 180 min) divided by the average value from 120 to 180 min times 100%.
The variability of measurements was computed as the numerical difference between the two measurements, expressed as a percentage of the mean value of both measurements:
The reliability of the measures was further assessed relative to the between- and within-subject variance by the intraclass correlation coefficient, r 1, calculated as [5]:
where MS B =mean sum of squares between subjects; MS W =mean sum of squares within subjects; n=number of within-subject measurements. MS B and MS W were derived by use of the ANOVA test.
The reliability is a measure of the variability that is due to similarities in individual transporter concentrations. A low variability could be due to a low inter-individual variability of DAT density (B′max). It is not possible to establish a level of significance for reliability and therefore this variable is only useful for comparison of different studies of reproducibility (non-statistically).
Results
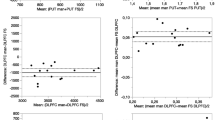
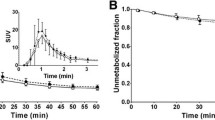
Table 1 shows the individual BP1 and BP2 values and variability measures using manual delineation of ROIs. Figure 2 presents the BP2 inter- and intra-individual variability. The reliability of BP2 was significantly higher (0.95–0.96) than that of BP1 (0.47–0.69). Table 2 shows individual BP1 and BP2 values and variability measures using probability map-based ROI delineation. Both BP1 and BP2 values were significantly lower with the probability map-based ROI delineation than with manual delineation (paired t test: p=0.02, Fig. 3). By contrast, non-specific binding as defined from the occipital region was similar with the two ROI delineation methods. A high variability in free parent compound in the two blood samples drawn at each experiment was found. First experiment: primary blood output, mean 164 Bq/ml; secondary blood output, 168 Bq/ml; individual difference, range 0.1–12.7%, mean 2.3%. Second experiment: primary blood output, mean 142 Bq/ml; secondary blood output, 148 Bq/ml; individual difference, range 0.7–12.2%, mean 4.1%. In subject 7, blood sampling failed owing to technical problems. Figure 4 shows individual striatal BP2 values from manual delineation at different time points from 120 to 180 min at the first and second measurements. Each time point represents the BP2 value normalised with the individual’s mean BP2 value from 120 to 180 min.
Table 3 shows the % change per hour in time-activity curves and BP2 values. The stability measures were calculated from the slope of the regression lines shown in Fig. 4a and b. No correlation was found between the relative difference in stability measures for the first and the second measurement and the relative difference in BP2 values. There was a significant difference in individual BP2 stability between the first and second scans (p≤0.03). Table 4 shows the percent difference in ROI volumes depending on the delineation method; the manual delineation resulted in significantly smaller ROIs (p<0.009). Simple regression analysis showed no correlation between the volumes of individual ROIs as determined by either method and variability in caudate nucleus or putamen.
Reproducibility of ROI volumes was significantly higher for the probability map-based method (1%) than for the manual delineation method (8%) (p<<0.001).
Discussion
In this study we have shown that [123I]PE2I SPECT measurements of DAT with the B/I approach are highly reproducible, particularly with BP2 as the outcome parameter. Manual ROI delineation on the functional image was associated with significantly lower reproducibility of volume determination and also resulted in significantly smaller volumes as compared with automatic delineations based on MR probability maps. Nevertheless, the latter approach resulted in a slightly higher variability of BP2 as compared with manual delineation. Finally, in an independent sample of healthy controls we confirmed that a B/I protocol of 2.7 h resulted in stable plasma and brain tissue levels of parent compound.
The reproducibility of [123I]PE2I SPECT measurements of DAT with the B/I approach will depend upon both methodological and biological variability. In our study it was not possible to assess the relative contribution of each of these factors, but methodological variability is determined by variations in, for example, SPECT acquisition, movement artefacts, the reconstruction process and delineation of ROIs. Whereas the former variability potentially can be minimised, we attempted to assess the contribution from variations in ROI delineations by comparing two different methods. In most clinical settings, manual ROI delineations are done directly on the SPECT images, not least because the corresponding structural images are often not available. With this approach, an ROI set is defined from an anatomical atlas and delineated on the individual SPECT scans. Interestingly, although the manual approach was both less precise and less reproducible in terms of volume assessment, it provided a functional reproducibility measure that was better than the probability map-based one. For both delineation methods, BP2 test–retest variability was considerably lower than has been reported in similar studies using other DAT ligands (FP-CIT: range 5–16%; β-CIT: range 7–16%) [19–22]. With the probability map-based approach, test–retest variability was slightly higher and BP2 values were significantly lower than with the manual approach. The latter is probably primarily a partial volume effect related to a larger ROI volume using the probability map- based approach compared with the manual “hot spot” delineation approach, where ROIs are delineated on a few slices only (no attempts were made to manually move the MR-defined volumes to the “hot spots” on the SPECT image). Although the volumes were highly reproducible, the outcome measures showed a much larger variability with the probability map-based approach that was unrelated to the volume of the ROI. This was most likely caused by difficulties in the MR/SPECT co-registration. It is likely that in patients with striatal DAT pathology, the probability map-based approach is superior to manual delineation, simply because the striatal tracer concentration is closer to the non-specific tracer concentration, thereby hampering correct adjustment of ROIs. In this study only two venous blood samples were drawn. If a suitable reference region does not exist, we recommend drawing at least six venous blood samples [1] for reproducible metabolite correction of the input curve.
Analysis of test–retest steady state conditions
At constant tracer levels, quantification of DAT density is both experimentally and analytically simple. Outcome parameters are calculated as simple tissue to venous blood ratios (BP1) or, if a suitable reference region exists, as simple tissue to reference tissue ratios (BP2).
In this study, where the stability of the striatal and occipital time-activity curves was evaluated in a separate group of healthy subjects, we found that in the first and second examinations the percent change in plasma activity varied somewhat over time. The time-activity curves (Fig. 4) and the fact that the stability measures were lower in the high count striatal ROIs than in the low count occipital cortex ROI imply that the variability was primarily due to random noise. This is also supported by the fact that, although the stability was significantly different from the first to the second scan, no difference was found in the reproducibility. In our recent study, we were able to demonstrate both experimentally and through computer simulations that a twofold variation in the terminal plasma clearance rate only translates into a 10% difference in BP1 and BP2 calculated from 120 to 180 min after tracer administration and that outcome parameters were stable in all subjects in the same time period [1]. Accordingly, in spite of variations in plasma levels, the outcome parameters for dopamine transporter binding were highly reproducible.
Conclusion
In this study we have demonstrated that [123I]PE2I SPECT with the bolus/infusion approach yields highly reproducible and reliable values for striatal dopamine transporter binding. Both the manual delineation and the probability map-based method yielded highly reproducible BP2 quantification in healthy subjects. Further, the generalisability of the applied bolus/infusion protocol was confirmed in an independent group of healthy volunteers, and this approach allows the study of three or more subjects within a normal working day.
[123I]PE2I is a highly selective ligand for the dopamine transporter site that provides highly reproducible measures of striatal binding and allows for imaging within 2 h after injection. Future studies must assess the reproducibility of dopamine transporter binding with PE2I SPECT in patients with basal ganglia pathology.
References
Pinborg LH, Ziebell M, Frokjaer VG, de Nijs R, Svarer C, Haugbol S, et al. Quantification of 123I-PE2I binding to dopamine transporter with SPECT after bolus and bolus/infusion. J Nucl Med 2005;46(7):1119–1127
Allard PO, Rinne J, Marcusson JO. Dopamine uptake sites in Parkinson’s disease and in dementia of the Alzheimer type. Brain Res 1994;637(1–2):262–266
Bao SY, Wu JC, Luo WF, Fang P, Liu ZL, Tang J. Imaging of dopamine transporters with technetium-99m TRODAT-1 and single photon emission computed tomography. J Neuroimaging 2000;10(4):200–203
Brucke T, Asenbaum S, Pirker W, Djamshidian S, Wenger S, Wober C, et al. Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I] beta-CIT and SPECT. Correlation with clinical findings and comparison with multiple system atrophy and progressive supranuclear palsy. J Neural Transm Suppl 1997;50:9–24
Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci USA 1993;90(24):11965–11969
Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol 2000;47(4):493–503
Marshall V, Grosset DG. Role of dopamine transporter imaging in the diagnosis of atypical tremor disorders. Mov Disord 2003;18(Suppl 7):S22–S27
Prunier C, Bezard E, Montharu J, Mantzarides M, Besnard JC, Baulieu JL, et al. Presymptomatic diagnosis of experimental parkinsonism with 123I-PE2I SPECT. Neuroimage 2003;19(3):810–816
Knudsen GM, Karlsborg M, Thomsen G, Krabbe K, Regeur L, Nygaard T, et al. Imaging of dopamine transporters and D2 receptors in patients with Parkinson’s disease and multiple system atrophy. Eur J Nucl Med Mol Imaging 2004;31(12):1631–1638
Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [123I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord 2001;16(6):1023–1032
Carroll FI, Rahman MA, Philip A, Lewin AH, Boja JW, Kuhar MJ. Synthesis and receptor binding of cocaine analogs. NIDA Res Monogr 1991;105:147–153
Emond P, Garreau L, Chalon S, Boazi M, Caillet M, Bricard J, et al. Synthesis and ligand binding of nortropane derivatives: N-substituted 2beta-carbomethoxy-3beta-(4′-iodophenyl)nortropane and N-(3-iodoprop-(2E)-enyl)-2beta-carbomethoxy-3beta-(3′,4′-disubstituted phenyl)nortropane. New high-affinity and selective compounds for the dopamine transporter. J Med Chem 1997;40(9):1366–1372
Videbaek C, Knudsen GM, Bergstrom KA, Hiltunen J, Pinborg LH, Kuikka JT, et al. Octanol extraction yields similar results as HPLC for quantification of [123I]PE2I metabolism. Eur J Nucl Med 1999;26:1139
Kretschmann H-J, Weinrich W. Neuroanatomy and cranial computed tomography. Stuttgart: Thieme; 1986
Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage 2005;24(4):969–979
Ashburner J, Friston K. Multimodal image coregistration and partitioning—a unified framework. Neuroimage 1997;6(3):209–217
Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992;16(4):620–633
Pietrzyk U, Herholz K, Fink G, Jacobs A, Mielke R, Slansky I, et al. An interactive technique for three-dimensional image registration: validation for PET, SPECT, MRI and CT brain studies. J Nucl Med 1994;35(12):2011–2018
Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med 1998;39(11):1879–1884
Morrish PK. How valid is dopamine transporter imaging as a surrogate marker in research trials in Parkinson’s disease? Mov Disord 2003;18(Suppl 7):S63–S70
Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffmann M, et al. Progression of dopaminergic degeneration in Parkinson’s disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord 2002;17(1):45–53
Seibyl JP, Marek K, Sheff K, Baldwin RM, Zoghbi S, Zea-Ponce Y, et al. Test/retest reproducibility of iodine-123-betaCIT SPECT brain measurement of dopamine transporters in Parkinson’s patients. J Nucl Med 1997;38(9):1453–1459
Acknowledgements
The authors thank Karin Stahr for expert technical assistance. This work was supported by the Danish Medical Research Council, the Toyota Foundation, the Elsass Foundation, the University of Copenhagen and H:S Hovedstadens Sygehusfællesskab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziebell, M., Thomsen, G., Knudsen, G.M. et al. Reproducibility of [123I]PE2I binding to dopamine transporters with SPECT. Eur J Nucl Med Mol Imaging 34, 101–109 (2007). https://doi.org/10.1007/s00259-006-0161-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0161-z