Abstract
A solitary fibrous tumor (SFT) is documented in several body sites. However, there are few reports on the radiological and corresponding histopathological, including immunohistochemical, features of SFT in the lower extremities. A 58-year-old male presented with a lump in his right thigh of 6 months duration. Plain radiograph revealed a soft tissue lesion in his right thigh, involving the adjacent mid-diaphysis and showing focal cortical thickening and calcification. Magnetic resonance imaging scans displayed two well-defined, T1-isointense and T2 heterogeneously hyperintense lesions, measuring together 15 cm in the intermuscular plane and the juxtacortical location along the mid-diaphyseal region of the right femur. Radiologically, the differential diagnoses considered were undifferentiated pleomorphic sarcoma and synovial sarcoma. Microscopic examination of the core biopsy and the resected tumor revealed a tumor composed of cells with oval to spindle-shaped nuclei in a variably collagenized stroma, including hyalinized blood vessels and focal dystrophic calcification. Mitotic figures were 4/10 high power fields. Immunohistochemically, the tumor cells were positive for CD34, BCL2, and STAT6. Diagnosis of malignant SFT was offered. The tumor displayed NAB2ex4-STAT6ex2 gene fusion on molecular testing. This constitutes a relatively uncommon case report of a large SFT in the thigh, including its radiological and pathological features, confirmed by STAT6 immunostaining. An SFT should be considered in cases of slow-growing, well-defined soft tissue tumors, which are isointense on T1 and heterogeneously hyperintense on T2-weighted sequences, and display calcification and cortical thickening of the adjacent bones. Various differential diagnoses and their treatment-related implications in such cases are discussed herewith.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the recent World Health Organization (WHO) classification of soft tissue and bone tumors, a solitary fibrous tumor (SFT) is defined as a tumor of fibroblastic origin, histopathologically characterized by thin-walled, dilated, and branching (hemangiopericytomatous) blood vessels, a variable amount of stromal, and perivascular hyalinization, and an underlying NAB2-STAT6 gene fusion [1]. Many tumors display hemangiopericytomatous vasculature, such as synovial sarcoma and malignant peripheral nerve sheath tumor. These tumors are differential diagnoses of SFT. Over the years, CD34 has been considered a useful immunohistochemical (IHC) marker for diagnosing SFT. However, its sensitivity and specificity are limited [2, 3]. Lately, signal transducer and activator of transcription (STAT) 6 is a fairly sensitive and specific diagnostic IHC antibody marker for substantiating a diagnosis of SFT [4].
An extrapleural SFT is a ubiquitous tumor and occurs within superficial and deep sites, visceral organs, and rarely in the bones [5, 6]. While extremities constitute 30–40% of extrapleural SFTs, there are very few cases of SFT in the thigh, especially those confirmed by STAT6 immunostaining and NAB2-STAT6 genetic fusion [4, 5, 7,8,9]. Still rare are such cases with detailed radiological features [7, 8].
Herein we present radiological, histopathological, IHC, and molecular features of a large SFT in the thigh. Certain radiological features might be useful in consideration of this tumor in the list of differential diagnoses, which are discussed.
An exact histopathological diagnosis of this tumor requires testing for certain immunohistochemical markers. This is further associated with significant treatment implications.
Case report
A 58-year-old male presented with a lump in his right thigh of 6 months duration, associated with pain for the last 2 months. He disclosed a history of a fall 6 months back.
During his recent clinical examination, a firm, mobile, and palpable lump was identified towards the medial one-third of his right thigh, measuring 6 cm × 6 cm. The lump mobile and was not fixed to the underlying bone. The overlying skin was unremarkable.
He underwent radiological examination, followed by a core biopsy and a complete surgical tumor resection. Subsequently, he underwent external beam radiation therapy (EBRT) with a dose of 50 Gy/25 cycles. He is on follow-up.
Radiological findings
Plain radiograph revealed a soft tissue lesion in his right thigh, involving the cortex of the adjacent mid-diaphysis with focal irregular cortical thickening, along with areas of calcification (Fig. 1).
Magnetic resonance image (MRI) revealed two well-defined conglomerates, T1-isointense to mildly hyperintense and T2 heterogeneously hyperintense lesions, as compared to the adjacent skeletal muscle, measuring together 15 cm × 7 cm × 5.5 cm, in the intermuscular plane and the juxtacortical location along the mid-diaphyseal region of the right femur. These were associated with focal cortical thickening. The lesions revealed few T2-hypointense signal intensity areas and an intense post-contrast enhancement with a non-enhancing focus towards the superior aspect. The lesions were seen displacing the vastus medialis muscle with loss of fat planes but without infiltration. The neurovascular bundles appeared unremarkable. The visualized bones showed normal bone marrow intensity due to cortical changes (Figs. 2, 3). Radiologically, the differential diagnoses considered were undifferentiated pleomorphic sarcoma and synovial sarcoma.
Magnetic resonance imaging results (A–B). A Two, conglomerate, well-defined soft tissue lesions (arrows) in the juxtacortical location along the mid-diaphyseal region of the right femur, iso to mildly hyperintense, to the adjacent skeletal muscles on T1-weighted sequence, on coronal view. B Axial view showing a large, well-defined iso to mildly hyperintense, juxtacortical lesion, involving the intermuscular plane in the right thigh
Pathological findings
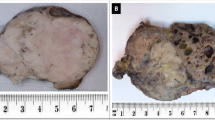
An oriented specimen of tumor resection was received with overlying skin and scar (of the initial biopsy), measuring together 18 cm × 11 cm × 8.5 cm. On cutting, a well-circumscribed tumor was identified, measuring 14 cm × 7.5 cm × 5 cm, which was firm to hard, grey-white in color, with a whorled appearance and focal areas of calcification. The distance of the tumor from all the resection margins was more than 1 cm (Fig. 4). In addition, part of the removed adjacent bone was also received.
Microscopic examination of the biopsy and the resected specimen showed a tumor composed of oval to spindle-shaped nuclei, arranged in a non-descript pattern with stromal and perivascular hyalinization. At places, the tumor cells with an epithelioid cytomorphology were arranged in cords in a densely sclerotic stroma, resembling a sclerosing epithelioid fibrosarcoma. Mitotic figures were up to 4/10 high power fields (HPF). There were geographic areas of hyalinization and focal areas of calcification in some of the sections of the resected tumor. There were no areas of tumor necrosis (Figs. 5, 6).
Microscopic examination of the biopsy (A–B). A Cellular tumor composed of spindle cells with perivascular and stromal hyalinization. Hematoxylin and Eosin, × 200. B Focal areas revealing cells with spindle and rounded nuclei embedded in a dense hyalinized stroma. H and E, × 200. C–D Resection specimen. C. Focal areas resembling sclerosing epithelioid fibrosarcoma (SEF). H and E, × 400. D. Areas of stromal hyalinization and focal dystrophic calcification. H and E, × 100
The tumor was seen superficially abutting the adjacent bone, but not infiltrating into it. All the resection margins were free of tumor (Fig. 4).
By immunohistochemistry, the tumor cells showed patchy positivity for CD34 (an endothelial cell differentiation marker, used to substantiate a diagnosis of SFT), diffuse positivity for BCL2 (to reinforce a diagnosis of SFT), and intense positivity for STAT6 (the most crucial marker for diagnosing SFT), in a significant number of tumor cells, while negativity for markers of epithelial differentiation, such as epithelial membrane antigen (EMA) and pan-cytokeratin (AE1/AE3), as well as S100 protein (a marker of neural/nerve sheath differentiation), smooth muscle actin (SMA), MUC4, and SATB2 (Fig. 7).
A diagnosis of solitary fibrous tumor (SFT) was offered on the biopsy and further confirmed on the resection. According to the risk-stratification system, the case was assigned as high-risk [1]. Furthermore, the tumor was tested for NAB2-STAT6 gene fusion, which constitutes the underlying genetic molecular signature of SFT.
Molecular analysis
Formalin-fixed, paraffin-embedded tissue sections were tested for 8 fusion variants of NAB2-STAT6, using qualitative endpoint reverse-transcriptase (RT)-PCR technique. RNA extraction was performed using Recover All Total nucleic acid extraction kit. Details of primer sequences are enlisted in Table 1.
The amplified fusion transcript PCR product was purified using ExoSAP-IT® (USB, Cleveland, OH, USA). The purified PCR product was used as a template for cycle sequencing. The sequencing reaction was performed with the BigDye®Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, USA). The sequencing product was purified using the Optima DTR™ system (Edge Biosystems, Gaithersburg, USA). The purified product was loaded onto ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, USA). The sequencing reads were uploaded in the BLAST search engine of NCBI and showed 100% alignment with the NAB2-STAT6 fusion transcript from the NCBI database.
The tumor showed NAB2ex4-STAT6ex2 fusion transcript (Fig. 8).
A Conventional RT-PCR for NAB2-STAT6 Fusion Transcripts analyzed on 10% PAGE Lane 1. 100 bp molecular weight marker, Lane 2: No band seen for NAB2-STAT6 exon 4-exon 5 fusion PCR, Lane 3: No band seen for NAB2-STAT6 exon 6-exon 18 fusion PCR, Lane 4: 303 bp band seen for NAB2-STAT6 exon 3-exon 3 fusion PCR (exon 4-exon 2 fusion type), Lane 5: No band seen for NAB2-STAT6 exon 6-exon 17 fusion PCR, Lane 6: No band seen for NAB2-STAT6 exon 5-exon 18 fusion PCR, Lane 7: No band seen for NAB2-STAT6 exon 7-exon 2 fusion PCR, Lane 8: No band seen for NAB2-STAT6 exon 5-exon 3 fusion PCR, Lane 9: No band seen for NAB2-STAT6 exon 2-exon 19 fusion PCR, Lane 10: Reagent control. B Sanger sequencing showing NAB2-STAT6 fusion (exon 4-exon 2 fusion type), including forward and reverse primer reads
After the tumor resection and adjuvant EBRT, the patient underwent chest radiographic examination and computed tomogram (CT) scan, which did not reveal any metastatic lesions. He is on a follow-up.
Discussion
Although SFT is a ubiquitous soft tissue tumor, there are few reports on SFT in the thigh [7]. Previously, Anders et al. [7] reported a case of SFT in the thigh, including radiological features, along with a review of 9 such cases. That case was confirmed on histopathologic examination and by CD34-positive immunostaining. Presently STAT6 is considered the most specific diagnostic marker of SFT, especially for tumors occurring at unusual sites [4, 6]. Few cases of SFT have been reported in the lower extremities, confirmed by STAT6 immunostaining and NAB2-STAT6 fusion by molecular testing [4, 9,10,11,12]. Although there are rare reports on histopathological features of SFT occurring in the extremities, there is no such case describing detailed radiological features, further confirmed by STAT6 immunostaining, as well as NAB2-STAT6 gene fusion (Table 2) [5, 9].
The clinico-radiological impression in the present case was of a slow-growing soft tissue sarcoma with secondary ossification. Radiologically, SFTs tend to appear as well-defined, ovoid lesions [8]. MRI typically shows areas of low intensity on T1-weighted images, corresponding to dense collagen and heterogeneously intermediate-to-high signal intensity on T2-weighed images, including similar cases reported in the thigh, as in the present case [8, 13]. Rosaldo-de-Christenson et al. [14] described SFT as an isointense lesion on T1 and variable on T2-weighted images, referring to this as a black and white mixed pattern. In an earlier reported case of SFT in the thigh, the radiological diagnosis considered was a synovial sarcoma and undifferentiated pleomorphic sarcoma, similar to the present case [7]. This has significant treatment-related implications, considering those tumors are high-grade sarcomas and might require adjuvant treatments, especially in large-sized tumors. Although parosteal osteosarcoma with high-grade de-differentiation was another differential diagnosis, because of cortical thickening and calcification, on imaging, it was dismissed given tumor epicenter in the soft tissues and absence of dense ossification within the tumor [15].
A definite diagnosis, in this case, was rendered on histopathological examination of the biopsy with immunohistochemical staining. The closest differential diagnosis was a synovial sarcoma. Patchy immunostaining for CD34 made the possibility of synovial sarcoma, less likely and led to consideration of an SFT. Moreover, negative staining for EMA and AE1/AE3 and positive staining for STAT6 helped rule out a synovial sarcoma. Fibromatosis was ruled out because of the lack of SMA and β-catenin and positive CD34 immunostaining. The other morphological differential diagnosis of an SFT includes a dermatofibrosarcoma protuberans (DFSP), given CD34 positivity seen in both the tumors. It is noteworthy that variable CD34 immunostaining is also observed in several cutaneous and soft tissue tumors, including vascular tumors, neurofibromas, and the recently described NTRK-rearranged tumors, which constitute other differential diagnoses of an SFT [16, 17]. Given focal areas of stromal sclerosis and epithelioid cells in the present case, a possibility of a sclerosing epithelioid sarcoma was also considered, which was ruled out because of CD34 and STAT6 immunoexpression and the lack of MUC4 immunostaining [18]. Negative staining for SATB2 helped in ruling out a tumor with osteoid, including extraskeletal osteosarcoma [19]. This immunoprofile also ruled out an adult fibrosarcoma, which was another differential diagnosis. In this way, STAT6 was useful in substantiating a diagnosis of SFT in the present case and differentiating it from its various mimics [1, 4, 5].
In 2013, Robinson et al. [20] identified recurrent NAB2-STAT6 gene fusions in SFT by integrative sequencing. Subsequently, various investigators validated a high specificity of this gene fusion, along with a significant amount of sensitivity in cases of SFT, occurring across various body sites [5, 6, 10, 12]. While studying variants of NAB2-STAT6, across various SFTs, Barthelmeβ et al. [21] observed NAB2ex4-STAT6ex2/3 as the most common type, associated with classic pleuropulmonary SFTs occurring in older patients, associated with relatively benign behavior. On the other hand, they found NAB2ex6-STAT6ex16/17 fusion occurring in cases of SFTs in younger patients with deep-seated tumors and a relatively aggressive clinical behavior. The present case revealed NAB2ex4-STAT6ex2 fusion, which constitutes the most frequent out of the other variants. In another study, Huang et al. [22] found no significant relationship between types of variants and clinical behavior. Instead, they found increased mitoses associated with clinical aggressiveness.
Therapeutically, complete surgical excision, especially in cases of SFT occurring in the extremities, remains the treatment mainstay [4, 7,8,9]. Given the tumor was amenable to complete surgical resection; this was the preferred treatment modality in the present case. Subsequently, adjuvant EBRT was offered because of malignant features. There have been rare instances of malignant transformation and subsequent recurrences and metastasis in a classical SFT, after complete tumor resection [8]. Lately, a risk-stratification scheme has been proposed to predict the risk of metastasis in cases of SFT, based on the age of the patient, tumor size, mitotic count, and tumor necrosis [1, 23]. Accordingly, the present case was assigned high-risk. The postoperative chest radiograph and CT scan of the patient did not reveal any specific abnormality. He has been recommended a follow-up. Among various tumors which constitute differential diagnoses of SFT, extraskeletal osteosarcoma and a synovial sarcoma would be treated with adjuvant chemotherapy, especially for large-sized tumors, such as the present case.
In conclusion, this constitutes a relatively uncommon report of a large SFT of the thigh, including detailed radiological features, corresponding histopathological and immunohistochemical features, and molecular profile. SFT should be included in the list of differential diagnoses of a slow-growing, large-sized soft tissue tumor, showing calcification, despite cortical thickening of the adjacent bone on radiological imaging. A definite diagnosis can be ascertained on histopathological examination. Immunohistochemical stains, such as CD34 and STAT6, are necessary, the latter constituting as its most specific immunohistochemical marker. Surgical resection constitutes the treatment mainstay, irrespective of tumor size. Demonstration of the underlying gene fusion is the diagnostic gold standard for SFT, especially in tumors occurring at unusual sites. A correct diagnosis, including its separation from its differential diagnosis, has treatment-related implications.
Change history
16 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00256-021-03929-y
References
Demicco EG, Fritchie KJ, Han A. Solitary fibrous tumor. In: World Health Organization (WHO) classification of tumours editorial board, eds. World Health Organization classification of tumours. 5th edition. Soft tissue and bone tumours. Lyon: IARC Press; 2020: 104–8.
Miettinen MM, Fetsh JF, Antonescu CR, Folpe AL, Wakely PE. Fibroblastic/ myofibroblastic neoplasms with variable biologic potential, In: Miettinen MM, Fetsh JF, Antonescu CR, Folpe AL, Wakely PE. eds. Tumors of soft tissue. AFIP Atlas of tumor pathology. 4th series, fascicle 20.Maryland: ARP press; 2014: 165–221.
Westra WH, Gerald WL, Rosai J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994;18:992–8.
Doyle LA, Vivero M, Fletcher CDM, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–5.
Rekhi B, Shetty O, Tripathi P, et al. Molecular characterization of a series of solitary fibrous tumors, including immunohistochemical expression of STAT6 and NATB2-STAT6 fusion transcripts, using Reverse Transcriptase(RT)-Polymerase chain reaction(PCR) technique: an Indian experience. Pathol Res Pract. 2017;213:1404–11.
Rekhi B, Bapat P, Tripathi P, Shetty O, Puri A. A rare case of a solitary fibrous tumour of bone showing NAB2-STAT6 exon 3-exon 19 fusion. Histopathology. 2018;73:708–11.
Anders JO, Aurich M, Lang T, Wagner A. Solitary fibrous tumor in the thigh: review of the literature. J Cancer Res Clin Oncol. 2006;132:69–75.
Yoshimura Y, Sano K, Isobe K, Aoki K, Kito M, Kato H. A recurrent solitary fibrous tumor of the thigh with malignant transformation: A case report. Int J Surg Case Rep. 2016;21:111–4.
Vogels RJ, Vlenterie M, Versleijen-Jonkers YM, et al. Solitary fibrous tumor - clinicopathologic, immunohistochemical and molecular analysis of 28 cases. Diagn Pathol. 2014;9:224.
Tai HC, Chuang IC, Chen TC, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol. 2015;28:1324–35.
Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552–9.
Chuang IC, Liao KC, Huang HY, et al. NAB2-STAT6 gene fusion and STAT6 immunoexpression in extrathoracic solitary fibrous tumors: the association between fusion variants and locations. Pathol Int. 2016;66:288–96.
Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol. 2011;196:487–95.
Rosado-de-Christenson ML, Abbott GF, McAdams HP, Franks TJ, Galvin JR. From the archives of the AFIP: localized fibrous tumor of the pleura. Radiographics. 2003;23:759–83.
Jelinek JS, Murphey MD, Kransdorf MJ, Shmookler BM, Malawer MM, Hur RC. Parosteal osteosarcoma: value of MR imaging and CT in the prediction of histologic grade. Radiology. 1996;201:837–42.
Tardío JC. CD34-reactive tumors of the skin. An updated review of an ever-growing list of lesions. J Cutan Pathol. 2009;36:89–102.
Suurmeijer AJH, Dickson BC, Swanson D, Zhang L, Sung YS, Cotzia P, et al. A novel group of spindle cell tumors defined by S100 and CD34 co-expression shows recurrent fusions involving RAF1, BRAF, and NTRK1/2 genes. Genes Chromosomes Cancer. 2018;57:611–21.
Doyle LA, Wang WL, Dal Cin P, et al. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444–51.
Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63:36–49.
Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–5.
Barthelmeß S, Geddert H, Boltze C, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184:1209–18.
Huang SC, Li CF, Kao YC, et al. The clinicopathological significance of NAB2-STAT6 gene fusions in 52 cases of intrathoracic solitary fibrous tumors. Cancer Med. 2016;5:159–68.
Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30:1433–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent
The patient consent was obtained.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rekhi, B., Bapat, P., Chakrabarty, N. et al. A case of a large solitary fibrous tumor in the thigh, displaying NAB2ex4-STAT6ex2 gene fusion. Skeletal Radiol 50, 2299–2307 (2021). https://doi.org/10.1007/s00256-021-03829-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03829-1