Abstract
Objective
Disuse and/or a non-weight-bearing condition changes muscle composition, with decreased skeletal muscle tissue and increased fat within (intramuscular adipose tissue, IntraMAT) and between (intermuscular adipose tissue, InterMAT) given muscles. Excessive adipose tissue contributes to dysfunctional and metabolically impaired muscle. How these adipose tissues change during orthopedic treatment (e.g., cast immobilization, daily use of crutches) is not well documented. This study aimed to quantify changes in IntraMAT, InterMAT, and thigh and calf muscle tissue during orthopedic treatment.
Materials and methods
We studied 8 patients with fifth metatarsal bone or fibular fractures. The ankle joint involved underwent plaster casting for approximately 4 weeks, with crutches used during that time. Axial T1-weighted MRI at the mid-thigh and a 30% proximal site at the calf were obtained to measure IntraMAT and InterMAT cross-sectional areas (CSAs) and skeletal muscle tissue CSA before treatment and 4 weeks afterward.
Results
Thigh and calf muscle tissue CSAs were significantly decreased from before to after treatment: thigh, 85.8 ± 7.6 to 77.1 ± 7.3 cm2; calf, 53.3 ± 5.5 to 48.9 ± 5.0 cm2 (p < 0.05). None of the IntraMAT or InterMAT changes was statistically significant. There was a relation between the percentage change of thigh IntraMAT CSA and muscle tissue CSA (rs = −0.86, p < 0.01).
Conclusions
The 4 weeks of treatment primarily induced skeletal muscle atrophy with less of an effect on IntraMAT or InterMAT. There is a risk of increasing IntraMAT relatively by decreasing skeletal muscle tissue size during orthopedic treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical orthopedic treatment such as immobilization and/or unloading (non-weight-bearing by cast and crutches) induces muscle atrophy and a decline in muscle function in the lower limb [1, 2]. For instance, cast immobilization (CIM), which stabilizes the ankle and knee angle, is required for a few weeks or months to treat a sprain, fracture, and/or ligament injury, with crutches frequently used for ambulatory activities of daily living (ADLs). Several studies have shown 5–20% decreases in the cross-sectional area (CSA) of knee extensor muscle resulting from muscle disuse and/or unloading for a few weeks [3,4,5,6]. Muscle use and loading of one’s body weight to antigravity muscles is important to maintain the skeletal muscles’ quantity, function, and metabolism [3, 5, 7, 8].
With the decrease in muscle quantity, adipose tissue infiltrates into and/or between muscles. Excessive accumulation of this type of adipose tissue has been frequently confirmed in the presence of disused legs of patients with spinal cord injury and stroke-related hemiparetic patients [9,10,11]. Based to the location of adipose tissue accumulation, these tissues are usually categorized into two types: Adipose tissue that has infiltrated “into” the muscle is known as intramuscular adipose tissue (IntraMAT), and the adipose tissue that accumulates “between” muscles is known as intermuscular adipose tissue (InterMAT) [12,13,14,15]. Gorgey and Dudley [10] reported that the IntraMAT content in the thighs of patients with spinal cord injury was twice as high as that in able-bodied controls. Increased IntraMAT and InterMAT in the thigh could have negative effects on knee extension strength, mobility, and/or physical performance [14, 16]. The infiltration of adipose tissue into muscle is negatively associated with glucose metabolism [16,17,18], eventually inducing type 2 diabetes. Taken together, the information on changes in IntraMAT and InterMAT resulting from orthopedic treatment would be crucial for understanding a deteriorating quality of skeletal muscle metabolic impairment. It is unclear, however, whether the changes in IntraMAT and InterMAT were linked to the change in muscle size during acute longitudinal clinical observation.
The purpose of this study was to assess how clinical CIM affected IntraMAT, InterMAT, and skeletal muscle tissue in the thigh and calf muscles. We hypothesized that IntraMAT and InterMAT might increase along with the decrease in skeletal muscle tissue quantity after 4 weeks of orthopedic treatment in the clinical setting.
Materials and methods
Experimental procedure
We targeted patients who came to the Akita Hospital for external injuries (i.e., fracture of the ankle mortise, fifth metatarsal bone, or fibula; Table 1). The subjects’ ankle joints were fixed by plaster cast for approximately 4 weeks. The average CIM duration was 26 ± 1 days. The first magnetic resonance imaging (MRI) scan was acquired at the earliest opportunity following surgery. During CIM, subjects managed their ambulant ADLs on crutches, with the injured leg maintained unloaded (non-weight-bearing). The second MRI scan was acquired when the cast was removed. All patients then started rehabilitation.
Subjects
Eight patients with a fracture of the fifth metatarsal bone or fibula participated in this study (mean age 59.8 ± 5.2 years; height 160.2 ± 3.2 cm; weight 63.5 ± 3.3 kg; body mass index 24.8 ± 1.1 kg/m2; Table 1). Before the experiment, the purpose, procedures, and risks associated with this study were explained to each patient, and written informed consent was obtained from each. All examination protocols were approved by the ethics committees of the Research Center of Health, Physical Fitness, and Sports at Nagoya University and Akita Hospital. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Magnetic resonance imaging
Before MRI scanning, subjects were placed in a supine position for 20 min to avoid fluid shifts. Then, to determine the coronal and sagittal femoral bone axis, the coronal slices were obtained using a locater system scout scan (TR/TE = 185/15 ms, field of view = 400 mm, 128 × 256 matrix) using a 1.5-T whole-body MRI scanner (Excel ART; Toshiba, Tokyo, Japan). T1-weighted spin-echo transaxial images of the injured leg were collected with the following parameters: TR/TE = 625/15 ms, field of view = 250 mm, matrix = 512 × 512, slice thickness = 10 mm, interslice gap = 0 mm. MRI measurements were performed before fixing the fracture using a plaster cast and just after removing the plaster cast.
Analysis of thigh and calf composition
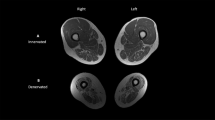
Medical Image Processing, Analysis, and Visualization software (version 4.4.0; National Institutes of Health, Bethesda, MD, USA) was used to analyze images on a personal computer (iMac; Apple, Cupertino, CA, USA). The procedure for MRI data analysis was essentially identical to that described previously [19, 20]. Briefly, we assessed the images of the mid-thigh and 30% of the proximal calf. Mid-thigh was defined according to markers attached at the mid-point between the greater trochanter and the lateral condyle. The calf image was from the marker attached 30% proximally, between the lateral condyle and the fibular lateral malleolus. These anatomical markers were determined by palpation. After obtaining the transaxial images, we identified the thigh’s quadriceps femoris (QF: sum of the rectus femoris, vastus lateralis, vastus intermedius, and vastus medialis muscles), hamstring (HM: sum of the biceps femoris short and long head, semitendinosus, and semimembranosus muscles), and adductor (AD: sum of the sartorius, gracilis, adductor longus, and adductor magnus muscles). We also identified four compartments of the calf: the anterior compartment (including the tibial anterior, extensor digitorum longus, and extensor halluces); lateral compartment (peroneus); deep posterior compartment (including the tibial posterior, flexor hallucis longus, and flexor digitorum longus); superficial posterior compartment (including the soleus and medial and lateral gastrocnemius muscles) (Fig. 1c, f).
Representative T1-weighted MRI of the thigh (a–c) and the calf (d−f) before and after cast immobilization. Individual muscles were determined based on the epimysium (c, f). Quadriceps femoris consisted of the rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), and vastus intermedius (VI) muscles. Hamstrings consisted of the biceps femoris short-head and long-head (BFs and BFl), semitendinosus (ST), and semimembranosus (SM) muscles. The adductor group consisted of the sartorius (Sar), gracilis (Gr), adductor longus (AL), and adductor magnus (AM) muscles. The anterior compartment consisted of the tibial anterior (TA), extensor digitorum longus (EDL), and extensor hallucis longus (EHL) muscles. The lateral compartment consisted of the peroneus (PE). The deep posterior calf consisted of the tibial posterior (TP), flexor hallucis longus (FHL) and flexor digitorum longus (FDL) muscles. The superficial posterior consisted of the soleus (SOL) and gastrocnemius medial head and lateral head (MG and LG) muscles. We also traced the femur (Fe), tibia (TI), and fibula (FI). Scale bar = 5 cm
The methodology for determining the thigh and calf composition was identical to that applied by the authors in previous investigations, which used a well-established, non-parametric, non-uniform intensity normalization (N3) algorithm to correct for image heterogeneity due to suboptimal radiofrequency coil uniformity and gradient-driven eddying currents [19,20,21]. This step was essential for subsequent analyses, which assumed homogeneous signal intensity across images. Optimized image correction parameters were determined (end tolerance 0.0001; maximum iterations 100; signal threshold 1; field distance 25 mm; subsampling factor 2; kernel full-width half-maximum 0.15; Wiener filter noise 0.01), and the same parameters were applied to all images from all participants. The investigator then drew six regions of interest (ROIs) of 5 × 5 mm each. Three ROIs were placed on the vastus intermedius for the thigh and the anterior tibia for the calf. The other three ROIs were placed on subcutaneous adipose tissue. The vastus intermedius and tibial anterior comprise 93 and 99% of skeletal muscle respectively, and were chosen so we would obtain a nearly pure skeletal muscle peak on the pixel number–signal intensity histogram. The total number of pixels within the six ROIs and a frequency distribution and histogram of all pixels and signal intensities were produced.
We measured the IntraMAT CSAs, InterMAT CSAs, IntraMAT contents, and skeletal muscle tissue CSAs of the thigh and calf. To ensure minimal investigator bias in the separation of skeletal muscle and adipose tissue in the pixel number–signal intensity histogram, we used the Otsu threshold method, a reliable histogram shape-based thresholding technique used for medical image analyses [22]. To minimize manual tracing-induced errors on thresholding values, the mean of five trials was used, and the values were applied to all muscles of interest for each subject. After carefully tracing the edge of each muscle group, the following parameters were calculated:
-
1.
The number of pixels with a signal intensity lower than the threshold value (skeletal muscle pixel numbers).
-
2.
The number of pixels with a value higher than the threshold (IntraMAT pixel numbers).
-
3.
The number of pixels at the subfascial level and between the muscles with a value higher than the threshold value (InterMAT pixel number.
The IntraMAT CSA and skeletal muscle tissue CSA were calculated using the following equations (FOV = field of view):
Subsequently, the IntraMAT content of each muscle was calculated using the following equation:
The InterMAT CSA was calculated using the following equation:
The images were read in random order, with one investigator (AY) performing all image analyses. Test–retest reliability of IntraMAT content was reported elsewhere [19, 20]. Briefly, we determined the intraclass correlation coefficient (ICC 2.1) for the IntraMAT and InterMAT, which ranged from 0.61 (IntraMAT of the hamstrings) to 0.99 (IntraMAT of the anterior compartment) (p < 0.01).
Statistical analysis
All values are reported as means ± standard error (SE). Wilcoxon signed-rank test was used to compare variables after clinical orthopedic treatment for the IntraMAT, InterMAT, and skeletal muscle tissue CSAs in the thigh and calf. We further compared these variables in each muscle group for the thigh and calf (QF, HM, AD: i.e., each compartment of the calf). The percentage changes per day for the IntraMAT CSA and skeletal muscle tissue CSA in all muscle groups were compared using the Kruskal–Wallis test. We used the Mann–Whitney test to compare the percentage changes per day for the IntraMAT and InterMAT CSAs in the thigh and calf. Spearman’s rank correlation was used to determine the association between the percentage changes per day for the IntraMAT, InterMAT, and skeletal muscle tissue CSAs. The level of significance was set at p < 0.05. All statistical analyses were performed using IBM SPSS statistics software (version 22.0 J; IBM Japan, Tokyo, Japan).
Results
The IntraMAT, InterMAT, and skeletal muscle tissue CSAs before and after the orthopedic treatment are shown in Table 2. The IntraMAT and InterMAT CSAs did not change throughout the intervention. Skeletal muscle tissue CSAs in the whole thigh, QF region, superficial posterior compartment of the calf, and whole calf significantly decreased after treatment. IntraMAT content in the anterior compartment of the calf significantly increased after the treatment. The percentage changes per day of the IntraMAT CSA and skeletal muscle tissue CSA were compared among muscle regions, with no significant differences found (Fig. 2).
Figure 3 shows the percentage changes per day of the IntraMAT and InterMAT CSAs in the thigh and calf. There was no significant difference in the percentage changes between the IntraMAT CSA and InterMAT CSA in either the thigh or the calf.
The relation between the percentage changes per day of the IntraMAT, InterMAT, and skeletal muscle tissue CSAs were estimated in the thigh and calf. The percentage change in the IntraMAT CSA in the thigh correlated significantly with that of the skeletal muscle tissue CSA (rs = −0.86, p = 0.007; Fig. 4a). We also found no significant relations for the rest of combinations, i.e., the skeletal muscle tissue CSA and the InterMAT CSA (thigh, rs = 0.36 [Fig. 4b]; calf, rs = 0.41) or the InterMAT CSA and the IntraMAT CSA (thigh, rs = −0.05 [Fig. 4c]; calf, rs = 0.69).
Relations between the changes in the IntraMAT CSA, InterMAT CSA, and skeletal muscle tissue CSA in the thigh. a IntraMAT CSA and skeletal muscle tissue CSA; b InterMAT CSA and skeletal muscle tissue CSA; c IntraMAT CSA and InterMAT CSA. IntraMAT, intramuscular adipose tissue; InterMAT, intermuscular adipose tissue; CSA, cross-sectional area
Discussion
The main findings of this study were as follows:
-
1.
The skeletal muscle tissue CSA significantly decreased after 4 weeks of orthopedic treatment, whereas IntraMAT and InterMAT CSAs did not change significantly.
-
2.
The percentage changes in the IntraMAT and skeletal muscle tissue CSAs among the muscle regions were not different.
-
3.
There was no difference between the percentage changes in the IntraMAT and InterMAT CSAs in the thigh or calf muscles.
-
4.
In the thigh, the percentage change in the IntraMAT CSA was negatively related to that of skeletal muscle tissue CSA.
The 4-week orthopedic treatment resulted in a significantly decreased skeletal muscle tissue CSA in the thigh and calf (Table 2), suggesting that skeletal muscle atrophy might be induced by CIM. We speculated that the decline in force production was due to muscle atrophy. Cook et al. [23] reported that maximum muscle strength and the CSA of the knee extensors decreased by 15.6 and 7.5% respectively, after 30 days of lower-limb unloading, implying that the decline in strength is approximately twofold greater than that of the muscle CSA, a finding similar to that reported in previous studies. Accordingly, it could be speculated that knee extension strength in our subjects might have decreased approximately 20% or more after the treatment, which would have allowed the possibility of risking gait ability dysfunction and metabolic impairment had our patients been older or suffered from diabetes or cardiovascular disease [3, 7, 8, 24]. Several reports proposed the benefit of clinical rehabilitation during orthopedic treatment. For instance, Dirks et al. [25] showed that electrical muscle stimulation prevents muscle atrophy during leg immobilization. Applying this treatment to all types of patients in that clinical situation, however, may be difficult from the viewpoint of labor, cost, and management. It is therefore necessary to introduce a way of preventing muscle atrophy during, not after, orthopedic treatment, focusing on manageability.
Compared with disuse-induced muscle atrophy, the concept of IntraMAT and InterMAT resulting from disuse has received far less attention. It is important to investigate the changes in IntraMAT and InterMAT in the clinical setting because these adipose tissues could have been negatively associated with dysfunction and metabolic changes in skeletal muscle [16, 17, 26]. Interestingly, we found that the IntraMAT and InterMAT CSAs did not change throughout the orthopedic treatment (Table 2). It has been shown that reduced muscle activity induces increased IntraMAT and InterMAT. Manini et al. [27] showed that combined IntraMAT and InterMAT volume increased by 15 and 20% in the thigh and calf, respectively, after 4 weeks of immobilization in healthy young individuals, with 0.5%–0.7% increase per day. Although our results support the reports of an IntraMAT increase with a similar rate (0.6%–1.3% per day), it did not significantly change (Figs. 2, 3). In patients 6 weeks after spinal cord injury, the IntraMAT CSA increased 126% compared with that of healthy control subjects, which corresponded to a 3.0% increase per day [10]. The increased IntraMAT CSA was three times greater in the spinal cord injury patients than in our patients. This discrepancy may be explained by the difference in the models between fracture treatment in the clinical setting and that for spinal cord injury. That is, loading was partly applied in our participants during ADLs, whereas no muscle contraction was expected in patients with a spinal cord injury. Therefore, the change in the IntraMAT CSA would depend on the type of disuse. The duration of our trial could be another key factor in interpreting the results. The average CIM duration was 26 ± 1 days in this study, which is shorter than in previous reports, in which there were statistically significant increases in IntraMAT and InterMAT [10, 27, 28]. We found only a few studies that investigated the time course of the IntraMAT increase accompanied by muscle disuse. For instance, the changes in IntraMAT in patients with a spinal cord injury were investigated on two occasions: in a patient with an injured leg 6 weeks after the injury compared with an able-bodied control leg, and in a patient with an injured leg at 6 weeks versus 18 weeks after the injury [10]. Although the increase at the first time point was 3.0% per day, that at the second time point was 0.3% per day, suggesting that the change in the IntraMAT might not be linear with the duration of disuse. Hence, the time course of changes in these adipose tissues as a result of disuse is still not well understood. Addison et al. [29] reported that the accumulation of IntraMAT was determined by several factors (i.e., age, injury, obesity, inactivity, disease). IntraMAT and InterMAT were affected by the interaction of these factors, which may be a reason why we could not confirm the changes in IntraMAT and InterMAT. We clearly showed that the changes in IntraMAT and InterMAT were negligible in this clinical trial, implying that we should carefully monitor the change in skeletal muscle tissue size rather than the fat infiltration into and between the muscles.
According to disuse and unloading studies, QF region atrophy ranged from 7 to 10% after 20 days of head-down-tilted bed rest or 20 days of unilateral limb unloading, with the degree of muscle atrophy at 0.4% to 0.5% per day [30, 31]. Their degree of atrophic response was similar to that in our study (Fig. 2). Interestingly, our group and others reported that the atrophic response is different among individual muscles in the lower limb [6, 7, 32, 33]. In this study, although the degree of reduction of the QF skeletal muscle tissue was twice as great as other lower-limb muscle regions, there was no significant difference among the muscle groups tested (Fig. 2). This finding may affect the result that the percentage change in the IntraMAT CSA was not different among the muscle groups. It was somewhat contradictory to that of Manini et al. [27], who reported an increase in the sum of the IntraMAT and InterMAT (they called it “IMAT” in their article), which was greater in the calf than in the thigh. The percentage change in the IntraMAT CSA in the anterior compartment of the calf was obviously higher than for other muscle groups, although the difference did not reach statistical significance (Fig. 2). Our participants managed the ambulatory ADLs on crutches during the treatment, with their lower limb muscles avoiding loads as much as possible. We could not completely restrict their ambulation because of a clinical observation trial, which is a limitation of this study. If the loading effect was well controlled throughout the treatment, it could provide more valuable information for estimating how much IntraMAT and skeletal muscle tissue change occurred during orthopedic treatment. It would also help when prescribing the rehabilitation program for reversing muscle atrophy and increasing IntraMAT and InterMAT.
Although we showed that the percentage changes in the IntraMAT CSA per day was approximately five times higher than that of the InterMAT CSA in the thigh, a statistically significant difference was not confirmed (Fig. 3). This result was presumed to be a large variation among individuals and a limited number of participants. IntraMAT is defined as adipose tissue infiltration into muscles or muscle groups, and InterMAT is defined as adipose tissue of the subfascia between muscles [12,13,14, 28]. It is well known that these adipose tissue types are related to the amount of visceral fat and insulin resistance [17, 18, 26]. However, the individual characteristics of these two adipose tissues have not been clarified. We further investigated the relations between the percentage changes in the IntraMAT, InterMAT, and skeletal muscle tissue CSAs in the thigh and calf (Fig. 4). As expected, although the change in the skeletal muscle tissue CSA was related to that of the IntraMAT CSA (rs = −0.86, p < 0.01), there was no significant relation to the change in the InterMAT CSA (rs = 0.36, p = 0.39) in the thigh (Fig. 4a, b). These findings indicate that IntraMAT has greater adaptive plasticity than InterMAT when there is a change in the size of the muscle, suggesting that IntraMAT could quickly fill the space left by skeletal muscle tissue that has atrophied or disappeared. However, characteristic InterMAT was less plastic than IntraMAT. In addition, we found that there was no significant correlation between the changes in the IntraMAT CSA and InterMAT CSA in the thigh and calf (thigh, rs = −0.05; calf, rs = 0.69; p > 0.05; Fig. 4c). Therefore, the adaptation to disuse is different in the IntraMAT and InterMAT CSAs, even though both are categorized as adipose tissue. Further research is needed to investigate the physiological characteristics and mechanisms of these adipose tissues.
There were some limitations to this study. First, the IntraMAT and InterMAT obtained by MRI could be affected by several factors, including daily physical activity, nutrition, and aging, among others. For instance, a previous study confirmed that energy restriction significantly decreased the IntraMAT content in middle-aged patients with type 2 diabetes, suggesting that daily food intake might be seriously related to IntraMAT and/or InterMAT [34]. We could not explore all the parameters related to lifestyle, e.g., physical activity, food intake, time to put the feet on the ground, because the participants spent their daily lives in their houses. Furthermore, the response of these adipose tissues and skeletal muscle to the orthopedic treatment would be different depending on age (the age range was 46–84 years in this study). These factors may induce the dispersion of the results within a limited number of participants (n = 8); however, this is one of the limitations of clinical experiments. We further need to investigate using a wider range of participants. Second, we did not have a control arm/group in the study. Previous studies that examined the effects of unloading or bed rest reported on before and after the thigh CSA, which consisted of IntraMAT, InterMAT, and skeletal muscle results versus those of a control. They found that the thigh CSA did not change [6, 35]. Data from a control group emphasized the effects of the clinical treatment intervention, although we observed changes in skeletal muscle tissue that were obviously decreased by physical activity, as described in previous studies. Third, we tracked only the changes in the skeletal muscle, IntraMAT, and InterMAT CSAs using pixel-by-pixel analysis on MRI scans. It would have been better to measure contractile and fat tissue via molecular levels in muscle biopsy specimens or by magnetic resonance spectroscopy. These methods, however, are invasive and costly. In addition, MRI analysis is not well established with regard to its consistency with the results of other IntraMAT and InterMAT measuring methodologies, e.g., T2 mapping, chemical-shift-based water–fat separation techniques (i.e., Dixon). Further investigation is thus needed.
In conclusion, we observed changes in thigh and calf muscle composition (i.e., IntraMAT, InterMAT, skeletal muscle tissue) during clinical orthopedic treatment (i.e., CIM with cast and crutches). Although muscle atrophy in both thigh and calf was confirmed after the treatment, the IntraMAT and InterMAT did not change. Furthermore, the percentage changes in IntraMAT and skeletal muscle tissue were not notable in thigh and calf muscle groups. These results suggest that the disuse-induced relative increase in adipose tissue that had infiltrated into the muscle (i.e., IntraMAT) was due to the decreasing skeletal muscle tissue, not an increase in adipose tissue, during the orthopedic treatment. Hence, regaining skeletal muscle tissue would be an essential treatment goal for the patients with an orthopedic injury of the lower limbs.
References
Shaffer MA, Okereke E, Esterhai JL Jr, Elliott MA, Walker GA, Yim SH, et al. Effects of immobilization on plantar-flexion torque, fatigue resistance, and functional ability following an ankle fracture. Phys Ther. 2000;80(8):769–80.
Stevens JE, Walter GA, Okereke E, Scarborough MT, Esterhai JL, George SZ, et al. Muscle adaptations with immobilization and rehabilitation after ankle fracture. Med Sci Sports Exerc. 2004;36(10):1695–701.
Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991;70(4):1882–5.
Hather BM, Adams GR, Tesch PA, Dudley GA. Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol. 1992;72(4):1493–8.
Schulze K, Gallagher P, Trappe S. Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc. 2002;34(2):303–13.
Akima H, Hotta N, Sato K, Ishida K, Koike T, Katayama K. Cycle ergometer exercise to counteract muscle atrophy during unilateral lower limb suspension. Aviat Space Environ Med. 2009;80(7):652–6.
Heath GW, Gavin JR 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol. 1983;55(2):512–7.
Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135(7):1824S–8S.
Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83(12):1703–7.
Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–9.
Ramsay JW, Barrance PJ, Buchanan TS, Higginson JS. Paretic muscle atrophy and non-contractile tissue content in individual muscles of the post-stroke lower extremity. J Biomech. 2011;44(16):2741–6.
Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol. 2001;90(6):2157–65.
Ruan XY, Gallagher D, Harris T, Albu J, Heymsfield S, Kuznia P, et al. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J Appl Physiol. 2007;102(2):748–54.
Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637.
Hausman GJ, Basu U, Du M, Fernyhough-Culver M, Dodson MV. Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocytes. 2014;3(4):242–55.
Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88(11):1336–44.
Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–85.
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury—a cross-sectional study. Spinal Cord. 2004;42(12):711–6.
Akima H, Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, et al. Skeletal muscle size is a major predictor of intramuscular fat content regardless of age. Eur J Appl Physiol. 2015;115(8):1627–35.
Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, Sakakibara H, et al. Three-dimensional comparison of intramuscular fat content between young and old adults. BMC Med Imaging. 2017;17(1):12.
Sled JG, Zijdenbos AP, Evans ACA. Nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97.
Sezgin M, Sankur B. Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging. 2004;13(1):146–65.
Cook SB, Brown KA, Deruisseau K, Kanaley JA, Ploutz-Snyder LL. Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J Appl Physiol. 2010;109(2):341–9.
Prado-Medeiros CL, Silva MP, Lessi GC, Alves MZ, Tannus A, Lindquist AR, et al. Muscle atrophy and functional deficits of knee extensors and flexors in people with chronic stroke. Phys Ther. 2012;92(3):429–39.
Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. 2014;210(3):628–41.
Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–5.
Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85(2):377–84.
Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Atrophy IFM. Intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25(9):865–72.
Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570.
Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, et al. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand. 2001;172(4):269–78.
Akima H, Ushiyama J, Kubo J, Fukuoka H, Kanehisa H, Fukunaga T. Effect of unloading on muscle volume with and without resistance training. Acta Astronaut. 2007;60(8–9):728–36.
Vandenborne K, Elliott MA, Walter GA, Abdus S, Okereke E, Shaffer M, et al. Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve. 1998;21(8):1006–12.
Grosset J-F, Onambele-Pearson G. Effect of foot and ankle immobilization on leg and thigh muscles’ volume and morphology: a case study using magnetic resonance imaging. Anat Rec. 2008;291(12):1673–83.
Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–72.
De Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583(3):1079–91.
Acknowledgements
The authors gratefully thank the volunteers for their participation and the staff of the Department of Radiology in Akita Hospital.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Yoshiko, A., Yamauchi, K., Kato, T. et al. Effects of post-fracture non-weight-bearing immobilization on muscle atrophy, intramuscular and intermuscular adipose tissues in the thigh and calf. Skeletal Radiol 47, 1541–1549 (2018). https://doi.org/10.1007/s00256-018-2985-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-2985-6