Abstract
Objective
To elucidate the quality of tissue-engineered cartilage after an autologous chondrocyte implantation (ACI) technique with Atelocollagen gel as a scaffold in the knee in the short- to midterm postoperatively, we assessed delayed gadolinium-enhanced magnetic resonance imaging (MRI) of cartilage (dGEMRIC) and T2 mapping and clarified the relationship between T1 and T2 values and clinical results.
Materials and Methods
In this cross-sectional study, T1 and T2 mapping were performed on 11 knees of 8 patients (mean age at ACI, 37.2 years) with a 3.0-T MRI scanner. T1implant and T2implant values were compared with those of the control cartilage region (T1control and T2control). Lysholm scores were also assessed for clinical evaluation. The relationships between the T1 and T2 values and the clinical Lysholm score were also assessed.
Results
There were no significant differences in the T1 values between the T1implant (386.64 ± 101.78 ms) and T1control (375.82 ± 62.89 ms) at the final follow-up. The implants showed significantly longer T2 values compared to the control cartilage (53.83 ± 13.89 vs. 38.21 ± 4.43 ms). The postoperative Lysholm scores were significantly higher than the preoperative scores. A significant correlation was observed between T1implant and clinical outcomes, but not between T2implant and clinical outcomes.
Conclusion
Third-generation ACI implants might have obtained an almost equivalent glycosaminoglycan concentration compared to the normal cartilage, but they had lower collagen density at least 3 years after transplantation. The T1implant value, but not the T2 value, might be a predictor of clinical outcome after ACI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is histologically characterized by a lack of blood vessels and an inability of chondrocytes to migrate through the extracellular matrix. Therefore, articular cartilage has poor regenerative ability and is unable to repair itself. Many attempts have been made to treat articular cartilage injury, but treatment that can completely regenerate hyaline cartilage has not yet been established. Surgical methods to treat cartilage injuries include bone marrow stimulation using microfracture [1–3], autologous osteochondral transplantation (AOT) [4–6], mosaicplasty [7, 8], and autologous chondrocyte implantation (ACI) [9, 10]. Bone marrow stimulation can be performed arthroscopically; however, because the repaired tissue is not hyaline cartilage, but actually fibrocartilage, which deteriorates with time, its durability is short-lived, as has been shown in long-term clinical trials. Also, the indication for bone marrow stimulation can be limited by age, duration from onset, cartilage defect size, body mass index, and preoperative activities [2, 3]. Although AOT can repair the defect with hyaline cartilage tissue, it is limited by the size of the donor site [7].
ACI was developed by Brittberg et al. [9] in 1994 and is a promising method to repair defects; expanded chondrocytes harvested from a non-weight-bearing area are cultured in a monolayer culture system and injected. This cell-based method is representative of first-generation, conventional ACI and has shown excellent clinical results; however, some adverse events related to ACI have been reported [10]. This method has raised concerns about re-expression of the chondrocyte phenotype of the monolayer-cultured fibroblastic cells after transplantation, uneven distribution of cells in the defect, and leakage of the chondrocyte suspension [11]. To resolve these problems, we developed tissue-engineered cartilage, i.e., the third-generation matrix-associated ACI, in which cultured cells are embedded in Atelocollagen gel and cartilage-like tissue is produced ex vivo [11]. Our method preserves the phenotype of the cultured chondrocytes and enables even distribution of cells in the transplanted tissue [12, 13]. It also has a lower risk of chondrocytes leakage from the grafted site [13]. It produced good clinical outcomes in early and mid-term postoperative evaluations [11, 14, 15].
Arthroscopy enables macroscopic examination and measurement of stiffness at the transplantation site [11, 16], and tissue biopsy enables qualitative evaluation of tissue. However, these methods are invasive and difficult to perform repeatedly over time. On the other hand, MRI is useful in evaluations because of its non-invasiveness compared with arthroscopy; however, it is difficult for conventional MRI to evaluate the quality of the cartilage and ACI implants.
In recent years, delayed gadolinium-enhanced MRI for cartilage (dGEMRIC) [17–20] and T2 mapping [20–23] have been introduced as diagnostic imaging methods that are less invasive and can yield more qualitative information about cartilage compared with conventional MRI. The dGEMRIC T1 value reflects the amount of glycosaminoglycan (GAG) in the cartilage tissue [17, 19, 20, 24]. On the other hand, T2 mapping is an imaging method that reflects proton movements in the tissue and is affected by the density of collagen fibers and free water content in the cartilage [22–24]. The cartilage tissue transplanted using the method of Brittberg et al. (i.e. first-generation ACI) has already been validated by dGEMRIC and T2 mapping, and the qualitative characteristics of such cartilage tissue have been clarified [24]. However, to date, there has been no report of third-generation matrix-associated ACI with a scaffold of Atelocollagen gel evaluated by dGEMRIC and T2 mapping at 3.0-T MRI.
The purpose of this cross-sectional study was to elucidate the quality of tissue-engineered cartilage by third-generation matrix-associated ACI using dGEMRIC and T2 mapping in the short- to midterm postoperatively.
Materials and methods
Patients
The subjects were 8 patients (11 knees) who felt pain, a catching sensation, and swelling and were diagnosed with full-thickness cartilage injury on a loadbearing site of the femoral condyle or on the patellar facet (Outerbridge grade IV) of the knee who could undergo follow-up for more than 3 years. The Outerbridge classification is a grading system for chondral and osteochondral injuries of the knee by arthroscopy, (grade 0: normal cartilage; grade I: cartilage with softening and swelling; grade II: a partial-thickness defect with fissures on the surface that do not reach subchondral bone or exceed 1.5 cm in diameter; grade III: fissuring to the level of subchondral bone in an area with a diameter more than 1.5 cm; grade IV, exposed subchondral bone) [25]. Table 1 shows demographic data, including the transplantation site and size, and the number of isolated chondrocytes for all subjects. The average follow-up period after surgery was 5.9 years. Between 1998 and 2005, these patients (3 males and 5 females) underwent a third-generation ACI [mean (± SD) age at ACI, 37.2 ± 12.5 years; age range, 15–52 years]. The mean size of the lesion was 370 mm2 (range, 70–1440 mm2). The causes of osteochondral defects were trauma (8 knees), localized osteoarthritis (2 knees), and osteochondritis dissecans (1 knee). Subjects had undergone ACI transplantation to the medial condyle of the femur (n = 7) and patella (n = 4). The mean time between surgery and evaluation was 5 years and 11 months (range, 3–10 years). The study was approved by the Institutional Committee on Ethics of Shimane University School of Medicine (#362), and informed consent was obtained from all participants included in the study.
Transplantation of tissue-engineered cartilage
The ACI procedure described by Ochi et al. [11] was performed. Briefly, under general or spinal anesthesia, approximately 300 mg of cartilage tissue was harvested arthroscopically from the non-weight-bearing areas of the knee with the articular cartilage injury. The cartilage tissue was subjected to enzymatic digestion, the extracellular matrix was removed, and the chondrocytes were isolated [11]. These chondrocytes were embedded in a three-dimensional culture using Atelocollagen gel (Koken Atelocollagen Implant, Koken Co., Ltd., Tokyo, Japan) for 4 weeks. Transplantation surgery was performed according to the method of Brittberg et al. [9]. The cultured chondrocytes embedded in Atelocollagen were transplanted into a cartilage defect beneath a periosteal patch (Fig. 1).
ACI technique with Atelocollagen gel as a scaffold: the hyaline cartilage cells were gathered from a non-weight-bearing area (1). The cells were embedded in Atelocollagen gel and cultured for 4 weeks (2). The cultured tissue was transplanted to the cartilage lesion and covered with the periosteum (3)
Equipment and imaging
Imaging was performed on a 3.0-T MRI scanner (Signa HDxT 3.0 T, GE Healthcare, Milwaukee, WI, USA) equipped with a knee array coil at a time point more than 3 years after ACI. Imaging was performed in the coronal plane if the transplantation site was the femorotibial joint and in the transverse plane if the transplantation site was the patellofemoral joint. The imaging protocol was based on the method of Burstein et al. [18]. First, proton-density-weighted imaging was performed for morphological assessments. The resulting images were used to determine the slice positions, and T2 mapping was performed. Next, 0.2 mmol/l/kg of Gd-DOTA (gadoterate meglumine) (Magnescope® intravenous injection 38 % Syringe, Guerbet Japan KK, Tokyo, Japan) contrast agent was administered. The subjects performed walking exercise on a treadmill at 3 km/h for 10 min. T1 mapping was performed 120 min after intravenous injection, and the same slice positions were used for the T2 mapping.
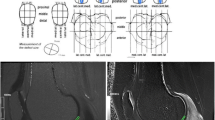
The T1 and T2 values were obtained by measuring a 3-mm2 region of interest (ROI) in the center of the transplantation site of the cultured chondrocytes and the adjacent non-injured cartilage by a senior radiologist who has more than 15 years of experience in the orthopedic field and has specialized in reading orthopedic images. For image analysis, a DT1 map was used for the T1 map, and Cartigram was used for the T2 map (both from GE Healthcare).
T1 and T2 values for pre-transplanted tissue
Atelocollagen gel and cultured, tissue-engineered cartilage were imaged to determine the T1 and T2 values for pretransplanted tissue. Imaging was performed on Atelocollagen gel alone (Fig. 2a) and on tissue-engineered cartilage after 4 weeks of culture (immediately before transplantation); the chondrocytes (n = 1.0 × 106) were derived from another patient (a 76-year-old female) with knee osteoarthritis who had received a total knee arthroplasty (Fig. 2b) for this study. Each sample was immersed in a 0.2 mmol/l solution of Gd-DOTA contrast agent for 24 h and then imaged. The imaging conditions and T1 and T2 measurement method were the same as previously described.
Clinical evaluation
The Lysholm score [26], used for the clinical assessment of knee function, was calculated before surgery and at the same time as MR imaging. The Lysholm score is a clinical outcome measure of knee injuries that contains eight domains: limp, locking, pain, stair-climbing, support, instability, swelling, and squatting; a score of 0 to 100 is calculated, which was divided into four categories: 95 to 100 indicates an excellent result; 84 to 94 indicates a good result; 65 to 83 indicates a fair result; less than 65 indicates a poor result.
Statistical analysis
The Wilcoxon test was performed to compare pre- and postoperative Lysholm scores and to compare T1 and T2 values between the transplanted tissue (implant) and peripheral healthy cartilage (control). Spearman’s rank order correlation was used to evaluate the correlation between clinical outcome (Lysholm score) and T1 and T2. The significance level was set at P ≤ 0.05. Statistical computer software (IBM SPSS 16, IBM Japan, Ltd., Tokyo, Japan) was used to perform all statistical analyses.
Results
Image analysis findings
The mean transplanted tissue T1 value after contrast enhancement (T1implant) was 386.64 ± 101.78 ms, and for the adjacent non-injured cartilage (T1control) it was 375.82 ± 62.89 ms (Fig. 3a and Table 2). There was no statistically significant difference between the T1implant and the T1control (P = 0.424). The mean T2implant value was 53.83 ± 13.89 ms for the transplanted tissue, and for T2control it was 38.21 ± 4.43 ms for the adjacent non-injured cartilage, indicating a significantly higher value for T2implant (P = 0.008) (Fig. 3b and Table 2). By contrast, the mean T1 and T2 values were 2434 ms and 409 ms, respectively, for the center of the Atelocollagen gel without chondrocytes. These values decreased to 1439 ms and 269 ms, respectively, in the implant (chondrocytes embedded in the Atelocollagen gel) cultured for 4 weeks.
Clinical findings
Preoperatively, there were no excellent cases, two good cases, five fair cases, and four poor cases based on the Lysholm score. Postoperatively, there were five excellent, three good, one fair, and one poor case. Clinical scores showed that symptom-free and almost normal function were achieved for all except two cases. One patient (no. 3) showed a low postoperative score for the categories of pain and climbing stairs because of weakness of the femoral quadriceps. The other patient (no. 11) experienced re-injury by trauma 7 years after the ACI transplantation. Overall, the mean Lysholm score was 71.3 ± 13.8 points preoperatively and 86.7 ± 17.1 points postoperatively. There was a significant improvement in the mean Lysholm score after the ACI (P = 0.050).
Relationships among the T1, T2 values and postoperative Lysholm scores
There was a significant correlation between the T1implant and clinical outcomes (r = 0.823, P = 0.002) (Fig. 4a). There was a no significant correlation between the T2implant and clinical outcomes (r = −0.128, P = 0.707) (Fig. 4b).
a Correlation of the T1implant value and the Lysholm score after ACI surgery. There was a significant correlation between the T1implant value and clinical outcomes. b Correlation of the T2impalant value and the Lysholm score after ACI surgery. There was no significant correlation between the T2implant value and clinical outcomes
Discussion
In this study, mean postoperative T1 (dGEMRIC) values for the ACI implant at 3.0 T, at least 3 years after implantation, were not significantly different from those in the adjacent non-injured cartilage, whereas T2 mapping of the implant showed a significantly higher mean value than did the adjacent non-injured cartilage. The T1 and T2 values for both the Atelocollagen gel without chondrocytes and the implant cultured for 4 weeks differed greatly from those of cartilage tissue obtained from the subjects. To the best of our knowledge, this is the first study to reveal the relationship between the dGEMRIC data at 3.0-T MRI and clinical outcomes after third-generation matrix-associated ACI in the short- to midterm postoperatively.
This study demonstrated a significant correlation between the T1 values and Lysholm score after ACI at short- to mid-term. Neuman reported that dGEMRIC values for the medial condyle were correlated with the KOOS subgroup QOL after ACL injury at 1.5-T MRI [27]. Furthermore, Årøen showed that dGEMRIC readings in the focal cartilage lesions of the femoral condyle correlated with the KOOS pain score at 1.5-T MRI [28]. These reports were consistent with our results that dGEMRIC might be a predictive factor for mid-term clinical outcome.
In the dGEMRIC, our study showed that there was no significant difference in the T1 value between the transplanted, tissue-engineered cartilage and the adjacent non-injured cartilage for an average 5.9 years after surgery (range, 3–10 years after ACI surgery). Based on this finding, it is speculated that the ACI site might contain similar amounts of GAG as the adjacent area for more than 3 years after third-generation matrix-associated ACI. Watanabe et al. [24] evaluated subjects with a mean age of 22.3 years and on average 1.5 years after first-generation ACI surgery at 1.5-T MRI. The mean T1 value of the transplantation site was significantly lower than that of the unaffected area, and the GAG concentration from biopsy specimens was also significantly lower. On the other hand, Brix reported a significant decrease in the T1 value at the grafted site after second-generation matrix-associated ACI (HyalograftⓇ) compared to the reference site over a follow-up period of 1 year at 3.0-T MRI at midterm [29].
In the T2 mapping, our study showed a significantly higher T2 value for transplanted, tissue-engineered cartilage compared with that of the adjacent non-injured cartilage. This result suggested that the collagen fiber density in the transplantation site might be lower than that of the surrounding cartilage after at least 3 years after third-generation matrix-associated ACI. Salzman reported that the cartilage ultrastructure of first-generation knee ACI was not correlated with clinical or T2 values at 1.5-T MRI [30]. However, he showed a correlation between the Lysholm score and T2 values in the repair tissue after second-generation knee ACI at 1.5-T MRI [31]. Further studies are necessary to determine whether the inconsistency between our results and other works on dGEMRIC and T2 mapping is due to differences in subjects, time from surgery until evaluation, MRI Tesla, or differences between the monolayer culture method and the three-dimensional culture method used in the current study.
There have been no reference T1 and T2 values for transplanted cartilage tissue, especially Atelocollagen gel, because no published values are available from past reports. The T1 and T2 values for the Atelocollagen gel alone differed from those of the cartilage. As chondrocytes embedded in the Atelocollagen gel proliferated, the values of the resulting cartilage tissue approached those of healthy cartilage tissue. These findings suggest that the values might be reduced over time, but after implantation, they may approach the values of healthy cartilage tissue.
Limitations
First, our study had a cross-sectional design and was unable to elucidate the changes in T1 and T2 values over time or the effects of age. Because a contrast agent is used in dGEMRIC, there was a risk of drug allergy. In addition, the study could not be conducted in patients with a renal disorder. The imaging time is longer than that of conventional MRI, and the cost is higher. Second, accurate evaluation is difficult if the cartilage is too thin because of the performance limit of the equipment. Third, the age range was wide, and the follow-up periods varied, although all were more than 3 years. Lastly, biopsy was not performed, so a comparison could not be made between the results of a histological evaluation and the results of imaging.
Conclusion
Our study suggested that the third-generation matrix-associated ACI implants might have obtained an almost equivalent GAG concentration compared to healthy cartilage, but with a lower collagen density, at least 3 years after transplantation. T1 values, but not T2 values, might be predictors of poor clinical outcome after ACI. This study was useful in its provision of a less invasive, quantitative method to evaluate transplanted tissue or other treatments in cartilage repair compared with an invasive biopsy.
References
Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001(391 Suppl):S362–369.
Asik M, Ciftci F, Sen C, Erdil M, Atalar A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24(11):1214–20.
Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–63.
Yamashita F, Sakakida K, Suzu F, Takai S. The transplantation of an autogeneic osteochondral fragment for osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1985;201:43–50.
Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318–21.
Bobic V. Autologous osteo-chondral grafts in the management of articular cartilage lesions. Orthopade. 1999;28(1):19–25.
Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125–33.
Robert H. Chondral repair of the knee joint using mosaicplasty. Orthop Traumatol Surg Res. 2011;97(4):418–29.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95.
Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55.
Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg (Br). 2002;84(4):571–8.
Uchio Y, Ochi M, Matsusaki M, Kurioka H, Katsube K. Human chondrocyte proliferation and matrix synthesis cultured in Atelocollagen gel. J Biomed Mater Res. 2000;50(2):138–43.
Katsube K, Ochi M, Uchio Y, Maniwa S, Matsusaki M, Tobita M, et al. Repair of articular cartilage defects with cultured chondrocytes in Atelocollagen gel. Comparison with cultured chondrocytes in suspension. Arch Orthop Trauma Surg. 2000;120(3–4):121–7.
Tohyama H, Yasuda K, Minami A, Majima T, Iwasaki N, Muneta T, et al. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci. 2009;14(5):579–88.
Takazawa K, Adachi N, Deie M, Kamei G, Uchio Y, Iwasa J, et al. Evaluation of magnetic resonance imaging and clinical outcome after tissue-engineered cartilage implantation: prospective 6-year follow-up study. J Orthop Sci. 2012;17(4):413–24.
Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Arthroscopic assessment of human cartilage stiffness of the femoral condyles and the patella with a new tactile sensor. Med Eng Phys. 2002;24(6):431–5.
Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–73.
Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41.
Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49(3):488–92.
Kumahashi N, Tadenuma T, Kuwata S, Fukuba E, Uchio Y. A longitudinal study of the quantitative evaluation of patella cartilage after total knee replacement by delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) and T2 mapping at 3.0 T: preliminary results. Osteoarthr Cartil. 2013;21(1):126–35.
Fragonas E, Mlynárik V, Jellús V, Micali F, Piras A, Toffanin R, et al. Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthr Cartil. 1998;6(1):24–32.
Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthr Cartil. 2001;9(5):393–406.
Domayer SE, Kutscha-Lissberg F, Welsch G, Dorotka R, Nehrer S, Gäbler C, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome—preliminary results. Osteoarthr Cartil. 2008;16(8):903–8.
Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, et al. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239(1):201–8.
Outerbridge RE. The etiology of chondromalacia patallae. J Bone Joint Surg (Br). 1961;43-B:752–7.
Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–4.
Neuman P. Knee cartilage assessment with MRI(dGEMRIC) and subjective knee function in ACL injured copers: a cohort study with a 20 year follow-up. Osteoarthr Cartil. 2014;22(12):84–90.
Årøen A, Brøgger H, Røtterud JH, Sivertsen EA, Engebretsen L, Risberg MA. Evaluation of focal cartilage lesions of the knee using MRI T2 mapping and delayed Gadolinium Enhanced MRI of Cartilage (dGEMRIC). BMC Musculoskelet Disord. 2016;17:73.
Brix OM. Cartilage repair of the knee with Hyalograft C: magnetic resonance imaging assessment of the glycosaminoglycan content at midterm. Inter Orthop. 2013;37:39–43.
Salzmann GM, Paul J, Bauer JS, Woertler K, Sauerschnig M, Landwehr S, et al. T2 assessment and clinical outcome following autologous matrix-assisted chondrocyte and osteochondral autograft transplantation. Osteoarthr Cartil. 2009;17(12):1576–82.
Salzmann GM, Erdle B, Porichis S, Uhl M, Ghanem N, Schmal H, et al. Long-term T2 and qualitative MRI morphology after first-generation knee autologous chondrocyte implantation: cartilage ultrastructure is not correlated to clinical or qualitative MRI outcome. Am J Sports Med. 2014;42:1832–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by the Health Labour Sciences Research Grant (20191301-01).
Rights and permissions
About this article
Cite this article
Tadenuma, T., Uchio, Y., Kumahashi, N. et al. Delayed gadolinium-enhanced MRI of cartilage and T2 mapping for evaluation of reparative cartilage-like tissue after autologous chondrocyte implantation associated with Atelocollagen-based scaffold in the knee. Skeletal Radiol 45, 1357–1363 (2016). https://doi.org/10.1007/s00256-016-2438-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2438-z