Abstract
Objective
To determine abdominal adipose tissue parameters on PET/CT in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM) that may serve as predictors of progression of MGUS to MM. We hypothesized that patients with MM had higher abdominal adiposity and higher fat metabolic activity compared to patients with MGUS.
Materials and methods
Our retrospective study was IRB approved and HIPAA compliant. The study group comprised 40 patients (mean age 64 ± 13 years) with MGUS and 32 patients (mean age 62 ± 10 years) with recently diagnosed MM (mean time since diagnosis of MM 3.0 ± 3.9 months) who had not undergone MM treatment. All patients underwent whole body FDG-PET/CT. Total abdominal adipose tissue (TAT), abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) cross sectional areas (CSA) (cm2) and metabolic activity (SUV) were assessed. Groups were compared using ANOVA. ROC curve analysis was performed to determine cutoff values for abdominal adipose tissue parameters to detect MM.
Results
Patients with recently diagnosed MM had higher TAT and SAT CSA (p ≤ 0.03) and higher fat metabolic activity (p < 0.01). VAT metabolic activity showed the highest sensitivity and specificity for identifying patients with MM (area under the curve 0.95 with cutoff value of >0.34, sensitivity 90.6 %, specificity 92.5 %, p < 0.0001).
Conclusions
Patients who were recently diagnosed with MM had higher abdominal fat CSA and higher fat metabolic activity compared to patients with MGUS. These parameters may serve as novel biomarkers of progression of MGUS to MM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a rare but fatal malignancy of plasma cells, accounting for approximately 13 % of hematological malignancies and 2 % of all cancers in the US [1, 2]. Known risk factors for the development of MM include increasing age, male gender, black race, family history of MM, and monoclonal gammopathy of undetermined significance (MGUS) [3]. In fact, studies have shown that MM is consistently preceded by MGUS, a premalignant plasma cell proliferative disorder [4]. The annual risk of progression from MGUS to MM is 1 % [5] and the development of biomarkers to aid in identifying patients at risk for progression from MGUS to MM are of great interest.
Epidemiological studies have suggested that obesity [6–10], especially abdominal adiposity [11], may represent a risk factor for the development of MM. However, these studies used surrogates of obesity; i.e. BMI and waist circumference, to evaluate abdominal adiposity. No studies have quantified distinct abdominal adipose depots and assessed their risk on the progression from MGUS to MM. Increased volume of visceral adipose tissue is associated with elevated cardiometabolic risk and oxidative stress as well as gastrointestinal carcinogenesis [12–14]. Interestingly, the composition and metabolic activity of abdominal adipose tissue has been shown to be associated with cardiometabolic risk [12, 13] and the development of cancers [15]. A biopsy study in patients with colorectal cancer demonstrated a different fatty acid profile in abdominal subcutaneous adipose tissue in cancer patients compared to controls, suggesting that changes in fatty acid metabolism may play a role in carcinogenesis [15]. Volumes of different abdominal fat compartments and their respective metabolic activity can be measured non-invasively using 18-fluorodeoxy-glucose positron emission tomography CT (FDG-PET/CT) [13]. FDG-PET/CT may be performed in patients with MGUS to determine the presence of osteolytic lesions and transformation to MM [16].
The purpose of our study was therefore to determine abdominal body composition parameters on FDG-PET/CT that differentiate patients with MGUS and recently diagnosed MM which may serve as predictors of progression of MGUS to MM. We hypothesized that patients with MM have higher abdominal adiposity and higher fat metabolic activity compared to patients with MGUS.
Materials and methods
Patients
This study was approved by the institutional review board and complied with Health Insurance Portability and Accountability Act (HIPAA) guidelines, with exemption status for individual informed consent. A retrospective search was performed to identify patients with MGUS or recently diagnosed MM (diagnosis <12 months) who had not undergone therapy for MM. All patients underwent FDG-PET/CT at our institution from 1/1/2005 to 12/1/2015. Exclusion criteria were malignancy other than MM/MGUS at the time of FDG-PET/CT and abdominal surgery or prior radiation therapy to the abdomen or pelvis that could confound abdominal adipose tissue measurements.
Body composition by FDG-PET/CT
The FDG-PET/CT studies were performed on an integrated PET/CT scanner (Siemens Biograph 16 or 64, Siemens, Erlangen, Germany or GE Healthcare discovery, Milwaukee, Wisconsin, USA), with a 16 or 64-slice CT and a full-ring HI-REZ LSO PET. Patients fasted 6 h before the exam and blood glucose levels were measured upon arrival and 18 F-FDG was injected only if blood glucose was ≤ 200 mg/ dl. 18 F-FDG was produced using an on-site 230 MeV isochronous cyclotron. The dose injected was based on patient’s BMI (BMI < 30, 15 mCi; 30.1 ≤ BMI ≤44, 20 mCi; BMI >44, 25 mCi). After injection, the patient relaxed in a semi-reclined chair and PET/CT was performed 60 min following the injection of FDG. Attenuation correction CT obtained in mid-expiration phase without intravenous contrast (slice thickness 5 mm; table feed per rotation, 18 mm; time per table rotation, 0.5 s; tube voltage, 120 kVp; tube current, 11 mAs; field of view, 48 cm) and PET images were acquired with the patient’s arms over the head. 3D mode PET images were obtained from the skull base to the mid-thigh, with 6–8 bed positions lasting 3–7 min each. Images were reconstructed to a slice thickness of 2.4 mm. Standard clinical quality assurance measures were performed to assess for reproducibility of scans over time.
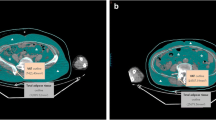
Abdominal fat cross sectional areas (CSA) were assessed on non-enhanced CT using semiautomated methods at the mid-portion of the 4th lumbar vertebra. Fat quantification at his level has been shown to correlate strongly with total abdominal fat volumes [17] and cardiometabolic risk [18, 19]. Analyses were performed using Osirix software version 3.2.1 (www.osirix-viewer.com/index.html). First, automated thresholding methods were applied to identify total abdominal adipose tissue (TAT) cross sectional area (CSA) (cm2) using a threshold set for −50 to −250 Hounsfield units (HU) as described by Borkan et al. [20]. We then manually outlined the subcutaneous and visceral adipose tissue areas, respectively, and the mean CSA (cm2) was determined for each adipose tissue depot (see electronic supplementary material, ESM). This has been shown to be a reliable method for adipose tissue measurements with reported inter-reader correlation coefficients (r) of 0.99 for VAT and SAT [21].
FDG-PET/CT images were analyzed using OsiriX software (www.osirix-viewer.com/index.html). Semiquantitative analysis of FDG uptake was performed at the same level as the adipose tissue CSA measurements and mean standardized uptake values (SUV) were calculated using the following formula: SUV (bw) = Ctis/Dinj/bw, where SUV (bw) is SUV normalized for body weight, Ctis is tissue concentration expressed as megabecquerels per milliliter, Dinj is injected dose expressed in megabecquerels, and bw is body weight expressed as kilograms. Mean SUVs were calculated for VAT, SAT, and TAT CSA (ESM). This method has been used in a prior study to assess fat metabolic activity [13]. In addition, total metabolic activity (= CSA × mean SUV) was calculated. Care was taken to exclude areas of obvious misregistration from peristalsis and breathing.
Statistical analysis
Statistical analysis was performed using JMP software (version 11, SAS Institute, Cary, NC) and MedCalc (version 9.2.1.0; Mariakerke, Belgium). Data are presented as mean ± standard deviation (SD). Groups were compared using analysis of variance (ANOVA). Receiver operator characteristic (ROC) curve analysis of body composition measurements was performed to determine sensitivity, specificity, area under the curve (AUC), and confidence intervals (CI) as well as cutoff values for each parameter to detect MM. p < 0.05 indicated statistical significance and p <0.1 indicated a trend.
Results
Patient characteristics and body composition including abdominal fat CSA and metabolic activity of the MGUS and MM groups are shown in Table 1.
We identified a total of 72 patients (36 men, 36 women; mean age 63 ± 11 years), 40 patients with MGUS (23 men, 17 women; mean age 64 ± 13 years) and 32 patients with recently diagnosed MM who were of similar age (19 men, 13 women; mean age 62 ± 10 years). No patients with smoldering MM or Waldenstrom macroglobulinemia were included. Reason for PET/CT in the MGUS group was evaluation for other plasma cell dyscrasias, such as MM or lymphoma (n = 29), work up of an osseous lesion found on skeletal survey (n = 5), work-up of pulmonary nodules (m = 3), and work-up of other lesions (n = 3). PET/CT in the MM group was performed to evaluate extent of disease and potential complications. In the MGUS group, 35 patients (87.5 %) were white, 3 (7.5 %) were black, and 2 (5 %) identified themselves as other. In the MM group 28 patients (88 %) were white, 1 (3 %) was black, 2 (6 %) were Asian, and 1 (3 %) other. Four patients in the MM group had a family history of MM, while none of the patients in the MGUS group had a family history of MM. Patients with MM were recently diagnosed with mean time from diagnosis of MM to FDG-PET/CT of 3.0 ± 3.9 months. There was no significant difference in age, weight, or BMI between the MGUS and MM groups (p ≥0.2). None of the patients had malignancy other than MM/MGUS at the time of FDG-PET/CT.
Patients with recently diagnosed MM had higher TAT and SAT CSA (p = 0.03 and p = 0.02, respectively) compared to patients with MGUS (Figs. 1 and 2). Similarly, patients with recently diagnosed MM had higher TAT and VAT mean and total metabolic activity (p < 0.02) compared to patients with MGUS.
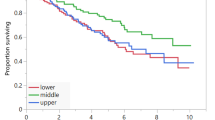
The results of ROC curve analysis are summarized in Table 2. On the basis of ROC curves, VAT metabolic activity (SUV) showed the highest sensitivity and specificity for identifying subjects with MM. The area under the curve (AUC) was 0.95 with a cutoff value of >0.37, sensitivity was 90.0 %, and specificity was 92.5 % (p < 0.0001) (Fig. 3).
Discussion
MGUS is a premalignant plasma cell proliferative disorder, which occurs in 3 % of individuals 50 years of age or older [5]. It is characterized by a serum monoclonal protein at a concentration ≤ 3 g/dL, bone marrow plasmacytosis <10 %, and absence of organ or tissue impairment including bone lesions [22]. MGUS is associated with a life-long risk of progression to MM, which is about 1 % per year [5]. Given the fact that nearly all MM cases are preceded by MGUS, it is of clinical importance to determine predictors of progression from MGUS to MM. Our study showed that patients who were recently diagnosed with MM and who had not undergone MM therapy had higher abdominal fat CSA and higher fat metabolic activity by FDG-PET/CT compared to patients with MGUS, suggesting that these parameters may serve as novel biomarkers of disease progression in patients at risk for MM.
Established risk factors for the development of MM in addition to MGUS include increasing age, male gender, black race, and family history of MM [3]. In our study, only 5 % of all patients had a family history of MM and the majority of patients were white, in concordance with the patient demographics of our hospital. Patients with MGUS often undergo FDG-PET/CT to exclude the presence of myeloma-defining lesions [16, 23]. FDG-PET/CT is also an imaging modality for staging and surveillance of patients with MM [24, 25]. Therefore, it would be valuable to determine novel imaging biomarkers indicating risk of progression from MGUS to MM that could be assessed on FDG-PET/CT performed as part of routine staging or surveillance.
Population studies suggest that obesity is associated with the development of different types of cancer, including MGUS and MM [4, 6, 7, 9, 10, 26, 27]. A recent pooled analysis of MM from 20 prospective cohorts in the National Cancer Institute Cohort Consortium found increased MM mortality for higher BMI and higher waist circumference, indicating that not only overall obesity but particularly abdominal obesity is a risk factor for MM [11]. In our study, patients with MM had increased abdominal adipose tissue CSA compared to patients with MGUS despite similar BMI, suggesting that quantification of abdominal fat may serve as a better biomarker of disease progression from MGUS to MM than BMI. Furthermore, we found increased metabolic activity of abdominal fat in patients with MM compared to patients with MGUS and ROC curve analysis of VAT metabolic activity showed the highest sensitivity, specificity and AUC for identifying subjects with MM, suggesting that VAT metabolic activity may serve as a biomarker to identify patients at risk for developing MM.
Potential mechanisms for the role of abdominal adiposity and increased risk of MM include low levels of adiponectin, an adipokine, which is secreted by adipocytes. Low levels of plasma adiponectin are associated with obesity, insulin resistance, and the metabolic syndrome [18, 28] and low adiponectin concentrations have also been linked to the development of cancers such as pancreatic and breast cancer [29, 30]. A recent prospective study in patients with MM and controls has demonstrated an inverse association between adiponectin levels and subsequent risk of developing MM, suggesting that low levels of adiponectin may play an important role in the mechanisms linking obesity to myelomagenesis [31]. Low adiponectin may lead to the development of MM by stimulating the production of proinflammatory cytokines, such as IL-6 and TNF, while suppressing the production of anti-inflammatory cytokines, such as IL-10 and IL-1RA, thereby promoting transduction pathways associated with survival and proliferation of malignant plasma cells [31–35]. The stimulation of proinflammatory cytokines may account for our observed increased metabolic activity of abdominal fat in MM compared to MGUS. It is also possible that the higher metabolic activity in the abdominal depot reflects a similar process in the bone marrow adipose depot where myeloma begins. For example in mice, high fat feeding results in a rapid but coincident increase in both abdominal adiposity and bone marrow adipose tissue [36]. In a recent study of B6 mice, Lwin et al. demonstrated that diet-induced obesity promoted a myeloma like condition in-vivo, which might be related to changes in the cellular composition of the marrow [37]. Further studies are needed to define whether the metabolic changes associated with obesity in the bone marrow establish a direct relationship between myeloma progression and adipocyte volume.
The main limitation of our study is the retrospective and cross-sectional study design which limits our ability to ascertain causality. However, this was a pilot study to evaluate potential biomarkers of transformation from MGUS to MM. Prospective longitudinal studies are necessary to confirm these findings. Another limitation of assessing abdominal fat metabolic activity is potential misregistration due to peristalsis and breathing. Care was taken to exclude areas of obvious misregistration and we only included patients who did not have intra-abdominal neoplasms, prior abdominal surgery or other intra-abdominal pathology that could lead to abnormal metabolic activity. A limitation was the use of different imaging protocols and equipment over time. However, as both groups were imaged over the same time period, we do not think that those changes would introduce systemic bias. We also performed standard clinical quality assurance measures to assess for reproducibility of scans over time. Strengths of our study are the availability of FDG-PET/CT and detailed measures of abdominal adiposity and metabolic activity in a large cohort of patients with MGUS and patients with recently diagnosed MM.
In conclusion, our study showed that patients who were recently diagnosed with MM had higher abdominal fat CSA and higher fat metabolic activity by FDG-PET/CT compared to patients with MGUS, suggesting that these parameters may serve as novel biomarkers of disease progression in patients at risk for MM. Larger longitudinal studies are necessary to confirm our findings.
References
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6:225–47.
Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7.
Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9.
Alexander DD, Mink PJ, Adami HO, et al. Multiple myeloma: a review of the epidemiologic literature. Int J Cancer. 2007;120 Suppl 12:40–61.
Blair CK, Cerhan JR, Folsom AR, Ross JA. Anthropometric characteristics and risk of multiple myeloma. Epidemiology. 2005;16:691–4.
Brown LM, Gridley G, Pottern LM, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12:117–25.
Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259–68.
Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni Jr JF. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43.
Teras LR, Kitahara CM, Birmann BM, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014;166:667–76.
Christen T, Sheikine Y, Rocha VZ, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3:843–51.
Oliveira AL, Azevedo DC, Bredella MA, Stanley TL, Torriani M. Visceral and subcutaneous adipose tissue FDG uptake by PET/CT in metabolically healthy obese subjects. Obesity (Silver Spring). 2015;23:286–9.
Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2012;330:1–10.
Cottet V, Vaysse C, Scherrer ML, et al. Fatty acid composition of adipose tissue and colorectal cancer: a case-control study. Am J Clin Nutr. 2015;101:192–201.
Dammacco F, Rubini G, Ferrari C, Vacca A, Racanelli V. (1)(8)F-FDG PET/CT: a review of diagnostic and prognostic features in multiple myeloma and related disorders. Clin Exp Med. 2015;15:1–18.
Ryo M, Kishida K, Nakamura T, Yoshizumi T, Funahashi T, Shimomura I. Clinical significance of visceral adiposity assessed by computed tomography: a Japanese perspective. World J Radiol. 2014;6:409–16.
Bredella MA, Torriani M, Ghomi RH, et al. Adiponectin is inversely associated with intramyocellular and intrahepatic lipids in obese premenopausal women. Obesity (Silver Spring). 2011;19:911–6.
Hyun YJ, Kim OY, Jang Y, et al. Evaluation of metabolic syndrome risk in Korean premenopausal women: not waist circumference but visceral fat. Circ J. 2008;72:1308–15.
Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–7.
Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond). 2007;31:500–6.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;121:749–757
Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457–63.
Caldarella C, Treglia G, Isgro MA, Treglia I, Giordano A. The role of fluorine-18-fluorodeoxyglucose positron emission tomography in evaluating the response to treatment in patients with multiple myeloma. Int J Mol Imaging. 2012;2012:175803.
Lu YY, Chen JH, Lin WY, et al. FDG PET or PET/CT for detecting intramedullary and extramedullary lesions in multiple Myeloma: a systematic review and meta-analysis. Clin Nucl Med. 2012;37:833–7.
Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85.
Bao Y, Giovannucci EL, Kraft P, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2012;105:95–103.
Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–704.
Hofmann JN, Liao LM, Pollak MN, et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood. 2012;120:4418–20.
Fowler JA, Lwin ST, Drake MT, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82.
Klein B, Tarte K, Jourdan M, et al. Survival and proliferation factors of normal and malignant plasma cells. Int J Hematol. 2003;78:106–13.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5.
Doucette CR, Horowitz MC, Berry R, et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J Cell Physiol. 2015;230:2032–7.
Lwin ST, Olechnowicz SW, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29:507–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study. The study was approved by the local Institutional Review Board (IRB) and HIPAA compliant.
IRB approval
The study was IRB approved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3353 kb)
Rights and permissions
About this article
Cite this article
Veld, J., O’Donnell, E.K., Reagan, M.R. et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skeletal Radiol 45, 1277–1283 (2016). https://doi.org/10.1007/s00256-016-2425-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2425-4