Abstract
The uncommon variant of degenerative hip joint disease, termed rapidly progressive osteoarthritis, and highlighted by severe joint space loss and osteochondral disintegration, is well established. We present a similar unusual subset in the lumbar spine termed destructive discovertebral degenerative disease (DDDD) with radiological features of vertebral malalignment, severe disc resorption, and “bone sand” formation secondary to vertebral fragmentation. Co-existing metabolic bone disease is likely to promote the development of DDDD of the lumbar spine, which presents with back pain and sciatica due to nerve root compression by the ”bone sand” in the epidural space. MRI and CT play a complimentary role in making the diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapidly progressive osteoarthritis is a well-recognized but uncommon destructive subset of osteoarthritis usually involving the hip [1–3] and recently reported in the knee joint [4]. The development of rapidly progressive osteoarthritis is thought to be due to altered articular mechanics, which result in high and unequally distributed biomechanical strains that exceed the physical properties of the supporting osteochondral tissues [4]. To date, this process has not been described in the spine. We present cases that represent a destructive disco-vertebral subset of degenerative disease in the lumbar spine. Patients present with back pain and sciatica and are found to have spinal deformity, advanced discovertebral osteochondrosis, and bony fragments within the spinal canal, resulting in neural compromise. Findings on imaging may be interpreted as infection, tumor, or tumoral calcinosis.
Materials and methods
We interrogated the radiological database at our institution from the year 1995 in order to identify cases with calcific densities within the spinal canal on CT. We used the search terms “calcified” or “bone debris” and “spinal canal” and “lumbar spine”. The patients’ imaging, clinical notes, laboratory results, and available histology in all patients were reviewed by three of the authors. All patients had routine, standard radiographs, CT, and MRI of the lumbar spine. The MRI sequences included T1- and T2-FSE-weighted sequences in the sagittal and axial planes, acquired on a 1.5-T Siemens scanner. CT images were obtained using a 16-slice Siemens scanner. We were advised by the IRB that ethical approval was not required in order to conduct this retrospective review.
Results
A search of the radiological database produced 20 patients. Patients with a diagnosis of infection or Charcot arthropathy were excluded. We identified eight cases that demonstrated severe degenerative change of the lumbar spine with epidural calcific bone debris, designated as “bone sand”, within the spinal canal.
All patients were female, postmenopausal, obese patients who presented with back pain and/or radiculopathy. The average age of the patients at presentation was 64 (range 51–71 years). Four of the patients had long-standing severe rheumatoid arthritis and were on long-term non-steroidal anti-inflammatory drugs and oral corticosteroids. The remaining four patients had no known underlying inflammatory condition but took regular non-steroidal anti-inflammatory drugs for pain. Six of the eight patients also had severe osteoporosis determined by DEXA scans while the other two patients had normal bone density. One patient with osteoporosis had metastatic breast cancer and previous radiotherapy of the thoracic spine. One patient was diabetic while another patient was diagnosed with rapidly progressive osteoarthritis of the hip 1 year prior to presenting with back pain.
There were no concurrent diagnostic markers of infection, co-existing renal failure, connective tissue disorder, or disorder of calcium metabolism in any of the patients. The patients were also neurologically normal with no hallmarks of neuropathy. There was no history of therapeutic steroid injection to the lumbar spine in any of the patients. A review of radiographs of other joints revealed no evidence of calcinosis, which would suggest microcrystalline disorders such as calcium pyrophosphate deposition disease, gout, hemochromatosis, or ochronosis.
In addition to “bone sand” within the spinal canal, the common spinal imaging findings in all the patients at the affected levels were highlighted by vertebral malalignment, insufficiency fractures, and severe discovertebral osteochondrosis with disc pace loss, vacuum phenomenon, and end-plate destruction.
In all patients, there was severe degenerative intervertebral disc disease with complete disc resorption and bone-on-bone contact of the vertebral bodies. There was destruction of the vertebral end plates, resulting in multiple paraspinal and epidural pockets of bone debris. Bone debris could not be identified on conventional radiography. On MRI, bone debris returned low signal on T1- and T2-weighted sequences, which is non-specific. MRI demonstrated compression of nerve roots and/or cauda equina in five patients (Fig. 1). The bone debris was best demonstrated on CT, which showed a calcific density around the vertebrae and within the spinal canal. In three patients, CT also demonstrated gas within the bone debris, which suggested a common origin from the discovertebral space.
Images of a 77-year-old female who presented with back pain and nerve root symptoms. a T2W axial MRI at the L4 level showing an extra-dural left paracentral well-defined rounded low signal lesion within the spinal canal compressing the thecal sac. b Axial CT at the same level showing the T2W low signal lesion to be of calcific density containing a small amount of gas. Note the left facet joint subluxation. c Sagittal T2 MRI shows the L4 low signal extradural lesion in continuity with the L3-L4 disc. d Sagittal CT reconstruction shows complete loss of the disc at L3-4 and L4-5 with vacuum phenomenon. There is bone-on-bone contact of the vertebral bodies. Calcific density containing gas is again demonstrated within the spinal canal supporting an L3-L4 discovertebral origin
Insufficiency fractures as a complication of metabolic bone disease was seen in all patients at more than one level of the lumbar spine. This primarily involved the vertebral bodies and in one patient there was involvement of all the lumbar vertebral bodies. Two patients also had fractures of the posterior elements of the vertebrae (Fig. 2).
Images of a 51-year-old lady with rheumatoid arthritis who presented with back and leg pain. a Sagittal T2W MRI showing an oblique insufficiency fracture through the L3 vertebral body. There is also a well-defined low signal extradural lesion posterior to the L3 vertebral body. b Coronal CT shows the fracture through the L3 vertebral body, severe degenerative disc disease and sub-endplate vertebral bone stock loss of the L3, L4, and L5 vertebral bodies. Note the paraspinal calcific debris. c Sagittal CT reconstruction shows the fracture through the L3 vertebral body. “Bone sand” is seen within the spinal canal immediately posterior to the fracture line at the L3-L4 level and the L4-L5 level. d Axial CT reconstruction through the L5 level shows fractures through both pedicles
In all patients, there was radiological evidence of spinal instability that manifested as malalignment of the vertebral bodies in the AP and lateral planes (Fig. 3).Malalignment was defined as > 2 mm vertebral translation in the anteroposterior or lateral planes on standard radiographs. There was also associated advanced degenerative apophyseal disease in each case. One patient had spinal decompression bilaterally at L4/5 and L5/S1 with fusion from L4 to S1. The patient re-presented 10 years later with back pain and sciatica. Imaging showed signs of instability at the level immediately above the fixation and “bond sand” within the spinal canal (Fig. 4).
Images of a 66-year-old female with osteoporosis who presented with back pain and sciatica. a Coronal CT reconstruction showing marked multilevel degenerative discovertebral disease with vacuum phenomenon, end-plate disintegration and scoliosis of the lumbar spine. b Coronal CT reconstruction showing marked facet joint osteoarthritis with sclerosis and vacuum phenomenon. c Axial image through L4-5 showing disc destruction, irregular fragmented bone and vacuum phenomenon within the intervertebral space. Note a fracture of the right L5 lamina also with vacuum phenomenon and bone dust within the adjacent epidural space. d Pelvic radiograph of the same patient showing rapidly progressive osteoarthritis of the left hip 1 year prior to presentation with back pain
Images of a 71-year-old lady who had previous spinal fixation from L4 to S1 presenting with back pain and sciatica. a Coronal CT reconstruction shows solid L4-5 fusion and destructive end-plate change at the adjacent unstable L3-4 segment with complete loss of disc space and intervertebral vacuum phenomenon. b Axial image through the L3 level shows calcific debris or ‘bone sand’ with vacuum phenomenon. c Sagittal CT reconstruction shows forward slip of L3 on L4 and “bone sand” within the spinal canal at this level. d T1W sagittal MRI shows anterolisthesis of L3 and end-plate destruction at the L3-4 level. The calcific debris is intermediate signal. The gas within the debris is seen as low signal
The clinico-radiological findings of the eight patients are summarized in Table 1.
Biopsy of the bone debris was taken in one patient. Two patients had posterior decompression and the bone debris was surgically removed in one patient. The term “bone sand” was used in the operation notes to denote the physical similarities of the bone debris to wet grains of sand on handling. Histology showed a large amount of amorphous eosinophilic debris with numerous fragments of bone and occasional fragments of cartilage. The precise nature of the debris was uncertain but it stained with Von Kossa, indicating the likely presence of calcific deposits (Fig. 5). CT imaging actually confirmed the presence of calcification.
Discussion
Degenerative joint disease consists of both regressive and progressive remodeling processes. It can be defined as the expression of the joint’s inability to cope with the mechanical stress placed upon it [5]. Osteophyte formation, which usually predominates in non-pressured segments of the joint, and subchondral bone overgrowth in pressured segments are examples of progressive remodeling [6]. Destructive or regressive subsets of osteoarthritis are uncommon but well known in the hip [1–3] and recently shown to involve the knee joint as well [4]. Rapidly destructive osteoarthritis is thought to be due to altered articular mechanics, which result in high and unequally distributed biomechanical strains which exceed the physical properties of the osteochondral tissues [4]. Coexisting metabolic disease is not uncommon promoting osteochondral failure leading to rapid joint destruction, bone stock loss and bone debris [7–9]. There are a few cases describing rapidly destructive arthritis in the hip in patients with coexisting rheumatoid arthritis [1]. To date, this process has not been described in the lumbar spine. We cannot describe our cases of destructive discovertebral degenerative disease (DDDD) of the spine as ‘rapid’ because we do not have imaging of the spine prior to clinical presentation that shows a rapid temporally related progressive process. Nevertheless, our study points to a similar rare destructive subset of degenerative disease affecting the lumbar spine. It is of interest that one patient with DDDD had a diagnosis of rapidly progressive osteoarthritis of the left hip a year previously treated by hip replacement.
In all eight cases of DDDD of the spine there was severe scoliosis or malalignment in the anteroposterior or lateral directions. The imaging features suggest that malalignment is a key feature that promotes abnormal motion and unequally distributed discovertebral biomechanical strains. Severe facet joint disease is a common denominator leading to malalignment. Apophyseal joint synovitis and superficial erosions, which can cause abnormal AP and lateral movement of the corresponding vertebral bodies have been reported in patients with rheumatoid arthritis [10–12]. In our cases, the four patients with rheumatoid arthritis had advanced degenerative facet joint arthropathy, which may have been promoted or accelerated by previous synovitis and erosions of the apophyseal joints. The four patients without rheumatoid arthritis also had severe degenerative facet joint arthropathy. One patient had spinal instability due to advanced degenerative apophyseal disease, which developed as a result of internal spinal fixation at the levels below. Long-term use of non-steroidal anti-inflammatory drugs may also play a role in this process. Repeated mechanical insult to a compromised joint may be rendered less painful by NSAIDs leading to bone destruction as seen in cases that were previously labeled as “analgesic hips” [13]. The absence of a clinical neuropathic disorder is important as a neuropathic state (Charcot spine) needs to be considered in the differential diagnosis of DDDD of the spine.
Severe loss of the intervertebral disc space is the second hallmark of this process. Abnormal motion between the vertebral bodies contributes to abnormal stresses across the discovertebral junctions, which accelerate the degenerative process of the intervertebral disc. Alterations at the discovertebral junction in the thoracic and lumbar spine may also be a result of rheumatoid arthritis. A number of articles were published showing radiologic characteristics similar to those seen in our cases with marked deformity and disc height loss in patients with rheumatoid arthritis [10–12, 14]. Primary rheumatoid involvement of the discovertebral unit has been proposed as a cause for discovertebral changes in rheumatoid arthritis [15]. Histological evidence for this hypothesis is very weak, and it is more likely to be mechanical secondary to instability from apophyseal synovial and soft tissue ligament involvement. Regardless of the mechanism of disc space loss, however, once the protective effect of the disc is overcome, friction between the exposed vertebral end plates leads to degradation and subchondral failure shown by irregularity, erosion, and fragmentation of the end plates with associated bone stock loss.
Metabolic bone disease, confirmed by DEXA, was found in six of our eight patients. This seems to suggest a third important biomarker of DDDD. Weakening of the subchondral bone leads to fragmentation of the end plate and vertebral body fractures. Insufficiency fractures were seen in all eight cases and provide evidence of underlying bone disease. In our patients, poor bone quality was a result of postmenopausal status, steroid treatment, and/or rheumatoid arthritis. Bone involvement in rheumatoid arthritis was first described by Barwell in 1865 [16]. An increased incidence of generalized osteoporosis and increased risk of vertebral fracture have also been reported [17]. The underlying etiology of bone mineral density loss in rheumatoid arthritis is multifactorial. Severity of osteoporosis is related to duration of disease, postmenopausal state, impaired mobility, vitamin D deficiency, and calcium malabsorption [13]. High-dose corticosteroid treatment is also related to bone mass loss [13], however, the effect of low-dose corticosteroid treatment remains controversial. Nevertheless, a past history of steroid use particularly in high doses is a risk factor for stress fractures regardless of the patient’s bone mineral density [18].
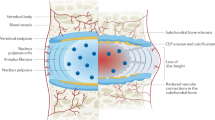
It appears that the destructive nature of the discovertebral disease seen in all the patients is due to three prognostic biomarkers: malalignment, intervertebral degenerative disc resorption, and end-plate failure with metabolic insufficiency fractures. The mechanism is similar to that seen in rapidly progressive osteoarthritis in the hips and knee (Fig. 3). If the rate of fracturing and fragmentation of the bone is faster than the physiological resorptive mechanisms, these fragments form “bone sand” accumulations in the paraspinal tissues and epidural space resulting in symptoms of neural compromise. It is therefore not surprising that patients with this form of destructive arthritis present with symptoms of nerve root compression.
“Bone sand” is one of the main hallmarks of DDDD of the spine. The imaging appearances of the epidural “bone sand” mass need to be differentiated from a sequestrated disc herniation. MRI is suggestive when there is an epidural mass associated with marked disc resorption and evidence of vertebral bone stock loss, but CT is more specific in making the diagnosis. The other principal imaging differential diagnoses encompass infection, neuropathic arthropathy of the spine (Charcot spine) and tumoral calcinosis of the spine. Infection and tumor can be excluded on the basis of clinical, biochemical, imaging, and histological evidence. In one of our patients with previous metastatic breast cancer, the presence of bone sand and intervertebral vacuum phenomenon on CT was particularly helpful in differentiating degenerative disc disease from metastatic disease (Fig. 6).
Images of a 59-year-old lady with known metastatic breast cancer and previous radiotherapy to the thoracic spine, who presented with back pain. a T1 W sagittal MRI shows destructive end-plate change at L5-S1. There is intermediate signal soft tissue anteriorly at the L5-S1 level. b Sagittal CT reconstruction demonstrates end-plate sclerosis, intervertebral vacuum phenomenon at L5-S1 and destruction of the anterior S1 vertebral. c Sagittal CT reconstruction shows intraosseous disc herniation at L4 and L5 in keeping with bone softening. “Bone sand” is seen within the spinal canal at the L5-S1 level. d Axial CT reconstruction shows bone debris or “bone sand” within the spinal canal at the L5-S1 level
The literature also includes intraspinal tumoral calcinosis as a potential differential diagnosis. Based on biopsy material, Durant et al. described 21 cases of so-called tumoral calcinosis of the spine associated with imaging features of either discovertebral or facet joint disease [19]. Of the four cases with discovertebral disease, three of the patients described had underlying rheumatoid arthritis. MRI images for two of these patients were published, which showed evidence of discovertebral disease similar to our cases. These patients also had lesions within the spinal canal on MRI studies that were shown to be calcific masses within the spinal canal. The masses had a gritty, chalky texture on macroscopic examination as in our cases. Histology of these masses showed deposits of calcium hydroxyapatite surrounded by histiocytic and foreign-body giant cell reaction but some cases showed fragments of bone mixed with the calcium [19]. In light of our study, it is likely that these three patients were examples of DDDD of the spine with “bone sand” within the spinal canal, based on review of the images and histology description, rather than tumoral calcinosis. Of note, in Durant’s series, these three cases had been misdiagnosed as infection with abscess formation on MRI with gadolinium enhancement prior to surgery by virtue of the discovertebral destruction and the presence of a non-enhancing epidural lesion. However, as we have shown CT is useful in the diagnosis of DDDD as it easily shows vacuum phenomenon and bone sand, which should easily differentiate it from infection.
In summary, we present cases of destructive discovertebral degenerative disease of the lumbar spine that has not been previously described. We propose three diagnostic biomarkers: malalignment, intervertebral degenerative disc loss, and end-plate failure due to metabolic bone disease. Similar findings were seen in patients with and without underlying rheumatoid arthritis. The unusual common feature seen was the presence of “bone sand” within the spinal canal, which resulted in nerve root compression. It is important to recognize the features of discovertebral degeneration and differentiate these findings from infection or tumor.
References
Yoshino K, Momohara S, Ikari K, Tomatsu T. Acute destruction of the hip joints and rapid resorption of the femoral head in patients with rheumatoid arthritis. Mod Rheum. 2006;16:395–400.
Batra S, Batra M, McMurtie A, Sinha AK. Rapidly destructive osteoarthritis of the hip: a case series. J Orthop Surg Res. 2008;3:3.
Boutry N, Paul C, Xavier L, Fredoux D, Migaud H, Cotton A. Rapidly destructive osteoarthritis of the hip: MRI imaging findings. AJR Sept. 2002;179(3):657–63.
Walker E, Davis D, Mosher T. Rapidly progressive osteoarthritis: biomechanical considerations. Magnetic Resonance Imaging Clinics of North America. 2011;19(2):283–94.
Kellgren H. Osteoarthrosis in patients and populations. Br Med J. 1961;2(5243):1–6.
Aoki J, Yamamoto I, Kitamura N, Sone T, Itoh H, Torizuka K, Takasu K. End plate of the discovertebral joint: degenerative change in the elderly adult. Radiology. 1987;164(2):411–3.
Davies M, Cassar-Pullicino VN, Darby AJ. Subchondral insufficiency fractures of the femoral head. Eur Radiol. 2004;14(2):201–7.
Yamamoto T, Bullough PG. The role of subchondral insufficiency fracture in rapid destruction of the hip joint: a preliminary report. Arthritis Rheum. 2000;43(11):2423–7.
Tokuya S, Kusumi T, Yamamoto T, Sakurada S, Toh S. Subchondral insufficiency fracture of the humeral head and glenoid resulting in rapidly destructive arthrosis: a case report. J Shoulder Elbow Surg. 2004;13(1):86–9.
Heywood AWB, Meyers OL. Rheumatoid arthritis of the thoracic and lumbar spine. J Bone Joint Surgery. 1983;68(B(3)):363–8. May.
Shichikawa K, Matsui K, Oze K, Ota H. Rheumatoid spondylitis. Int Orthop. 1978;2:53–60.
Sims-Williams H, Jayson MIV, Baddley H. Rheumatoid involvement of the lumbar spine. Ann Rheum Dis. 1977;36:524–31.
Doherty M, Holt M, MacMillan P, Watt I, Dieppe P. A reappraisal of ‘analgesic hip’. Ann Rheum Dis. 1986;45:272–6.
Kawaguchi Y, Matsuno H, Kanamori M, Kimura T, et al. Radiological findings in the lumbar spine in patients with rheumatoid arthritis and a review of the pathologic mechanisms. J Spinal Disord Tech. 2003;16(1):38–43.
Resnick D. Diagnosis of Bone and Joint Disorders. Vol 4. 4th ed. Philadelphia: WB Saunders Co Ltd; 2002. p. 926.
Barwell R. Diseases of the joints. London Hardwicke; 1865.
Woolf AD. Osteoporosis in rheumatoid arthritis—the clinical viewpoint. Br J Rheum. 1991;30(2):82–3.
Durant DM, Riley LH, Burger PC, McCarthy EF. Tumoral calcinosis of the spine. Spine. 2001;26(15):1673–9.
Kay LJ, Holland TM, Platt PN. Ann Rheum Dis. 2004;63:1690–2.
Acknowledgments
Histology slide (Fig. 5) kindly provided by Dr. A. Darby.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Charran, A.K., Tony, G., Lalam, R. et al. Destructive discovertebral degenerative disease of the lumbar spine. Skeletal Radiol 41, 1213–1221 (2012). https://doi.org/10.1007/s00256-012-1446-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-012-1446-x