Abstract
In the past two decades, MRI has gained a major role in research and clinical management of patients with inflammatory arthritides, particularly in spondyloarthritis (SpA), rheumatoid arthritis (RA), and osteoarthritis (OA). MRI is regarded as the most sensitive imaging modality for detecting early SpA in young patients with inflammatory back pain and normal radiographs of the sacroiliac joints. The recently published Assessment of SpondyloArthritis International Society classification criteria for axial SpA include for the first time a positive MRI demonstrating sacroiliitis as an imaging criterion indicative of SpA together with at least one clinical feature of SpA. Recent data show that systematic assessment of sacroiliitis displayed on MRI has much greater diagnostic utility than previously reported and highlight the diagnostic relevance of structural lesions. In RA, MRI has predictive value for the development of disease in new onset undifferentiated arthritis, and MR pathology at disease onset is a highly significant predictor of radiographic erosions. Consequently MRI has been credited with an important role in the new ACR/EULAR 2010 classification criteria for RA. In OA, bone marrow edema (BME) and synovitis may serve as biomarkers in interventional trials. Treatment interventions targeting BME and synovitis observed on MRI in inflammatory arthritides may have a disease-modifying effect as these lesions are potentially reversible and have been shown to be associated with structural progression. Research should focus on the prognostic significance of MRI lesions in larger cohorts and whether adding MRI to routine care improves clinical and radiographic outcome in patients with inflammatory arthritides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The introduction of magnetic resonance imaging (MRI) to the field of medicine [1, 2], which lead to the Nobel Prize, has revolutionized pathophysiologic concepts and clinical decision making in many areas of clinical research and daily practice. The past two decades have witnessed advances in knowledge in inflammatory rheumatology disorders by adopting MRI both for research and routine practice. These achievements may well represent just the first step of ongoing progress in the evaluation of inflammatory arthritides by MRI.
Several properties of MRI render this imaging modality particularly attractive for assessing inflammatory arthritides. The capability of visualizing both soft tissues and bone by tomographic imaging with high spatial and contrast resolution allows assessment of all structures involved in the disease process. Moreover, its noninvasive technique and its lack of ionizing radiation makes MRI an ideal imaging tool for monitoring disease outcome and following patients after treatment interventions. Current limitations preventing a more widespread use of MRI are costs and regionally limited availability. However, these expenses have to be weighed against the treatment costs of expensive biologic agents and the indirect costs incurred by delayed diagnosis or by complications resulting from an undiagnosed inflammatory condition.
The goal of this article is to review the contributions of MRI to research and clinical management in axial spondyloarthritis (SpA), rheumatoid arthritis (RA), and osteoarthritis (OA), which are the three most common inflammatory conditions in rheumatology. A focus will be on active lesions represented by bone marrow edema (BME; also termed bone marrow lesion, BML) and synovitis. These MRI signs of disease activity are potentially modifiable by treatment. There is growing evidence that these lesions are associated both with pain and with structural progression in inflammatory arthritides, and interventions targeting BME and synovitis may show a disease-modifying effect. BME and synovitis may also serve as biomarkers in interventional trials. At present, histopathology data from areas of BME displayed by MRI are limited. It is interesting that the uniform appearance of BME in typical subchondral locations displayed by MRI in inflammatory arthritides seems to reflect different histopathological findings. In RA, where the quality of perioperative correlation studies between BME on MRI and histopathology is highest, predominantly active cellular infiltrates with a variety of cytokine signatures have been found [3, 4]. Scarce data in SpA from vertebral facet joints removed during spinal extension surgery and from biopsies of the sacroiliac joint (SIJ) showed predominantly mononuclear cell infiltrates with only partial correlation with BME seen on preoperative MRI [5, 6]. In contrast, bone specimens in OA obtained from patients with advanced disease undergoing joint replacement surgery demonstrated mainly nonspecific findings such as bone marrow fibrosis or bone remodeling, whereas edema in the bone marrow and cellular infiltrates were rare findings [7–10].
MRI has a key role in clinical research in SpA, RA, and OA. Where the technique is available, MRI has also gained an important position in daily routine for early diagnosis of SpA. This is reflected by the inclusion of MRI as an imaging criterion in the recently published Assessment of SpondyloArthritis (ASAS) International Society classification criteria for axial spondyloarthritis [11]. In RA, the present data don’t yet justify a recommendation for routine use of MRI in daily practice; however recent data have confirmed the high predictive value of MRI for structural progression, and the recent recommendations address the use of imaging modalities in the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2010 classification criteria for RA [12]. In particular, the use of MRI is recommended in selected patients in daily routine [13, 14]. MRI in OA is mainly restricted to clinical research, although suspicion of a differential diagnosis may require the occasional use of MRI in routine care of patients [15].
MRI for axial spondyloarthritis
MRI in axial SpA—major component of new classification criteria
The spectrum of SpA comprises the related disorders ankylosing spondylitis (AS), psoriatic arthritis, enteropathic SpA associated with Crohn’s disease and ulcerative colitis, reactive arthritis, juvenile SpA, and undifferentiated SpA. Imaging plays a major role in predominantly axial forms of SpA, which are less accessible to clinical examination than peripheral SpA. While sacroiliitis remains the hallmark for the diagnosis of SpA, disability usually results from inflammation of the spine with progressive axial ossification [16].
Advances in the field of SpA over the past few years include the application of MRI as a major diagnostic tool and the introduction of antitumour necrosis factor alpha (anti-TNFα) therapy [17]. Until recently, diagnosis after clinical assessment relied primarily on radiographs demonstrating sacroiliitis according to the modified New York criteria [18]. While this approach continues to play a major role in clinical practice, it has been shown that several years may elapse before sacroiliitis becomes apparent on radiographs [19], during which time patients may experience significant symptoms and impairment of quality of life comparable to those with established disease [20]. Moreover, patients with early SpA may respond to treatment with anti-TNFα agents, which may be even more effective than in those with established disease [21, 22]. MRI abnormalities may precede radiographic changes by several years [23].
The major relevance of MRI in recognition of early SpA is reflected by the new axial SpA classification criteria developed by the ASAS [11]. For the first time, imaging is included as a major criterion that may be either radiographic (as defined by the modified New York criteria [18]) or MRI evidence of sacroiliitis, which allows classification of patients with preradiographic disease. A companion expert consensus statement by the ASAS/OMERACT working group defined a positive MRI of the SIJ based solely on active inflammatory lesions [24]. It requires subchondral or periarticular BME on STIR sequence or osteitis on contrast-enhanced T1-weighted (T1W) sequence highly suggestive of sacroiliitis; BME needs to be present in at least two lesions on a single SIJ slice or must be observed on two consecutive slices if only one lesion is present.
Apart from the major role of MRI in recognition of clinically suspected, yet radiographically unconfirmed early SpA, MRI may be used to assess patients with established SpA unresponsive to standard treatment or to evaluate response to therapeutic interventions. Moreover, there is growing evidence that inflammatory spinal lesions detected by MRI may be predictive of new syndesmophyte formation.
The spectrum of MRI lesions in sacroiliitis
Several groups have recently reported descriptions of active and structural abnormalities in the SIJ that are usually based on the combined information from both T1W and STIR sequences. Active lesions include subchondral, ligamentous, sacrotuberous, and capsular edema as well as synovitis, capsulitis, joint space inflammation, and ligamentous enthesitis. Structural lesions include fat infiltration, erosion, bone sclerosis, and ankylosis. The histopathology of bone marrow signal abnormalities in SpA is poorly characterized. Histology findings of interstitial, predominantly mononuclear cell infiltrates from CT-guided biopsies of the SIJ [6] and bone tissue from zygoapophyseal joints removed during spinal extension surgery [5] showed a moderate correlation with bone marrow inflammatory lesions on dynamic MRI or on STIR sequences. Moreover, MRI detected only about half of the inflammatory lesions evident on histopathology. Though MRI can reveal diverse lesions, there have been only a few attempts to achieve standardized and validated definitions.
Bone marrow edema
Active lesions can be observed on MRI within a few weeks of presentation of inflammatory back pain [25, 26]. Subchondral edema can be reliably detected on STIR sequences [27]. An online training module for systematic assessment of edema in the SIJ has been developed and validated by demonstrating increased reliability of detection after review of the module [28].
Do contrast-enhanced MRI sequences improve the detection of active SIJ lesions compared to STIR sequence alone? It has been reported that a T1W fat-suppressed sequence after administration of gadolinium contrast (T1WGd) may enhance diagnostic utility for detection of sacroiliitis as compared to the STIR sequence [29]. Although sensitivity for SpA was comparable at 65.7 and 62.9% for the STIR and T1WGd sequences, respectively, 24 and 13% were considered inconclusive, while disease was ruled out in 34 and 47 patients by STIR and T1WGd, respectively. Although the authors concluded that T1WGd had superior diagnostic capacity, this was a retrospective rather than a prospective inception cohort study, and the indication for performing the MRI was left to the discretion of the clinician. Because this may introduce significant bias, further comparative study is necessary in early inception SpA cohorts because contrast enhancement doubles the time required for the patient to lie still in the magnet as well as the costs of the procedure.
A study comparing STIR and gadolinium-enhanced T1W sequences to detect active bone marrow lesions in the SIJ of 40 SpA patients fulfilling the ESSG criteria (27 patients met the modified New York criteria) concluded that both sequences performed nearly equally well [30]. A previous report in 2005 did not show an advantage of contrast-enhanced T1W sequence over STIR sequence alone to detect spinal inflammation in 38 patients with AS [31].
Erosion
Bone erosion has been defined as full-thickness loss of the dark appearance of either iliac or sacral cortical bone of the SIJ with loss of the adjacent marrow signal on T1SE images [32].
It has been suggested that so-called cartilage sequences, such as T2W gradient echo or fat-saturated T1W (T1W FS), may facilitate reliable detection of erosions. These directly demonstrate cartilage as a thin zone of intermediate to high signal intensity with an adjacent low signal intensity cortex and a sharply defined bone margin. Erosions are identified as high or intermediate signal defects in the hypointense cortical bone on these sequences. One study used both T1W and T1FS to identify erosions in the SIJ and showed good reliability for an erosion score based on the extent of involvement of the joint surface, although this study did not directly compare these two sequences as regards reliability of detection or diagnostic utility [32]. A recent report compared T1W FS with spin-echo, three-dimensional-fast low angle shot (3D-FLASH), and three-dimensional-double excitation in the steady-state (3D-DESS) sequences in a retrospective analysis of scans from 30 patients with suspected sacroiliitis and 9 healthy controls [33]. These sequences provide higher resolution, greater contrast, and lower partial volume effects in the assessment of the cartilage. A similar number of patients demonstrated bone and cartilage erosions for each of the sequences, although erosion scores for bone and cartilage based on extent of involvement were significantly higher in 3D-DESS and 3D-FLASH than T1W FS images. The superiority of these sequences over T1W images in the reliable detection of SIJ erosions and their contribution to diagnostic utility requires further study.
Fat infiltration
Fat infiltration is evident as bright signal on T1W scans and is often seen in healthy individuals in the SIJ. The fat infiltration observed in SpA patients often occurs adjacent to subchondral bone, may have a distinct border, and is seen in areas of ankylosis and adjacent to other lesions such as edema and sclerosis. It likely indicates prior inflammation though this has not been proven in prospective studies. It is unknown which, if any, of these characteristics contributes to diagnostic utility. A preliminary report from a controlled analysis of 64 individuals concluded that fat infiltration in the SIJ per se has high sensitivity but low specificity for SpA and that its diagnostic utility primarily reflected the presence of associated abnormalities, especially erosions [34].
Diagnostic utility of MRI in preradiographic sacroiliitis
The few studies that were reported over a decade ago demonstrated that MRI had sensitivity and specificity in the range of 54–95% and 83–100%, respectively, but most studies employed gadolinium enhancement with dynamic imaging and lacked age- and sex-matched controls [35–39]. This approach is not used in clinical practice because it is costly, requires prolonged scanning times, and is unreliable. The sum of this data has been incorporated into diagnostic algorithms where the presence of a positive MRI has been assigned a likelihood ratio (LR) of 9 for a diagnosis of SpA [40].
The necessity for standardization of methodology as an approach to knowledge transfer was elaborated in two recent reports by the MORPHO International MRI Group, which aimed at assessing diagnostic utility of MRI by sequences commonly used in clinical practice. MRI scans were assessed from patients with AS meeting modified New York criteria as well as patients with preradiographic SpA [41, 42]. In addition, scans were assessed from age- and sex-matched controls that included healthy individuals as well as those diagnosed with mechanical causes of back pain. The methodological approach was unique in not only implementing standardized definitions of active inflammatory and structural lesions of the SIJ but also a customized online data entry module based on a standardized approach to recording abnormalities in the SIJ (available at www.arthritisimaging.ca). The latter derived from the method developed by the Spondyloarthritis Research Consortium of Canada (SPARCC) for scoring inflammatory lesions in the SIJ [43]. This is based on the division of each SIJ into quadrants and then the assessment of active and structural lesions on a dichotomous basis in all SIJ quadrants of each semicoronal slice. This method has been standardized to assess tilted coronal slices from anterior to posterior, and such methodological details have been incorporated into an online training module that has been validated for reliability and sensitivity to change [44].
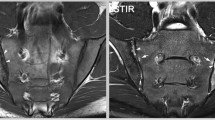
When readers were trained and calibrated to assess SIJ scans and record lesions in this manner, much greater diagnostic utility was evident than reported in earlier studies. In patients without radiographic sacroiliitis, mean sensitivity and specificity of MRI amongst five readers for the clinical diagnosis of SpA made by a rheumatologist was 51 and 97%, respectively (positive LR = 26.0, negative LR = 0.5). Diagnostic utility based solely on detection of BME as defined in the ASAS proposal enhanced sensitivity to 67% but reduced specificity to 88%. This was because a single BME lesion in the SIJ was observed in up to 27% of controls, and BME meeting the ASAS proposal for a positive MRI was recorded concordantly in 23% of mechanical back pain patients and 7% of healthy controls. On the other hand, erosions were recorded concordantly in only 4% of mechanical back pain patients and 2% of healthy controls, and so the inclusion of erosions in addition to BME further enhanced sensitivity to 81% but without changing specificity. Erosions were detected in half of the preradiographic SpA patients, which demonstrates that structural damage of the SIJ starts early in the disease course and contributes substantially to diagnostic utility (Fig. 1).
MRI to confirm a diagnosis of preradiographic spondyloarthritis. A 27-year-old, HLA-B27-negative female patient with inflammatory back pain since 19 months. a Pelvic radiograph. The radiographic modified New York criteria are not met. b MRI of the sacroiliac joints, STIR sequence. Bone marrow edema shows in the sacral portion of both sacroiliac joints and in the right distal ilium (broken arrows). c MRI of the sacroiliac joints, T1SE sequence. A small erosion is seen in the distal iliac part of the left sacroiliac joint (solid arrow). d MRI of the sacroiliac joints 9 months later, T1SE sequence. The erosive process in the distal iliac portion of the left sacroiliac joint is demarcated more clearly (solid arrow). The right iliac joint part shows decreased signal intensity with small bright areas (arrowheads). This may represent bone sclerosis with areas of fat infiltration or metaplastic tissue. The overlaying cortical bone seems preserved
In a second report from the MORPHO group [42], the authors demonstrated that rheumatologists primarily base diagnostic decisions on the finding of BME on the STIR sequence, while experienced radiologists base decisions on information available from both the T1W and STIR sequences, emphasizing the importance of structural lesions. Moreover, diagnostic utility is enhanced when rheumatologist readers are trained to specifically recognize abnormalities on T1W MRI. This is not surprising because abnormalities may often be subtle on one sequence, and the combined information from both sequences may enhance confidence in diagnostic decision making. This observation is also important in pointing to a major unmet need in the continuing education of rheumatologists to achieve an informed dialogue with the radiologist.
MRI for recognition of spinal inflammation
A set of definitions for active and structural lesions in the central, lateral, and posterior compartment of the spine has been reported by the Canada-Denmark MRI International Working Group, and reference images depicting these lesions are available at www.arthritisdoctor.ca [45, 46]. Good to very good interobserver reliability was reported for most of these lesion definitions [47, 48].
One study of WBMRI that included patients with preradiographic SpA and age- and sex-matched controls assessed the diagnostic utility of active spinal inflammatory lesions and showed that the presence of two or more vertebral corner BME lesions had optimal diagnostic utility for SpA (sensitivity 69% and specificity 94%, respectively), especially when located in the thoracic spine [49]. A single vertebral corner BME lesion was observed in up to 26% of healthy controls aged 45 years or less, which is a relevant finding for routine practice to avoid misclassification of healthy individuals as having SpA on the basis of an isolated or doubtful corner inflammatory lesion, particularly if seen in the vicinity of a degenerating disc. Another study focused on the diagnostic utility of vertebral corner BME lesions and of vertebral corner fat lesions separately and concluded that a cut-off of three or more BME lesions and of five or more fat lesions provided optimal diagnostic utility [50, 51]. However, the heterogeneous control group was not age- and sex-matched, the patients had longstanding AS, the influence of spinal location of lesions on diagnostic utility was not assessed and the study design was retrospective. There is a need for more data from systematic studies in a younger population with SpA and age- and sex-matched controls before conclusions on the diagnostic utility of structural spinal lesions can be drawn.
Routine scanning protocols of the spine may not include the lateral segments of the thoracic spine where lesions may be observed at sites such as the costo-vertebral and costo-transverse joints. This is particularly relevant in the presence of even a slight degree of scoliosis, which occurs in about 70% of individuals. When scanning the spine of a patient suspected as having SpA, the number of sagittal slices should be increased to ensure that also the lateral spinal segments are depicted. A systematic analysis in patients with established AS showed that inflammatory lesions were more frequent in lateral versus central segments in the thoracic spine [52]. In one study where the diagnostic utility of active spinal lesions was assessed in early SpA, the presence of active lesions in the lateral segments had very high specificity for SpA but lacked sensitivity [49]. Furthermore a recent study demonstrated inflammatory lesions also in the posterior spinal elements in a majority of AS patients indicating that scrutiny of posterior spinal structures is essential [53].
During the last few years, multichannel and multicoil technology has been introduced into clinical MR scanners. This whole body (WB) MRI method, which provides comprehensive assessment of systemic disorders, allows the scanning of the SIJ, the entire spine, the anterior chest wall, and the shoulder and pelvic girdle within 30 min without repositioning the patient. Scanning the entire spine in one single examination is more convenient for the patient and less time consuming than separate imaging of the upper and lower halves of the spine by conventional MRI. Imaging of the lower extremities is an additional option that may be relevant for some patients with concomitant peripheral enthesitis, but this has to be weighed against an additional examination time of 20 min. WB MRI of the SIJ and spine has been shown to correlate well and demonstrate comparable reliability to conventional MRI for the detection of active inflammatory lesions in patients with established AS [54, 55]. However, it depicts the SIJ in the coronal plane rather than the tilted coronal plane used in conventional MRI so that the images do not focus on the cartilaginous portion of the joint. It therefore requires further assessment of diagnostic utility compared to conventional imaging in early SpA.
MRI to assess patients with established axial SpA unresponsive to standard treatment or to monitor response to therapy
Patients with established axial SpA may have both inflammatory and mechanical sources of back pain, and it may be difficult to distinguish between these on clinical grounds. In this setting, MRI may be useful to confirm active inflammatory features of SpA and as an objective measure of disease activity before initiating long-term treatment with expensive TNFα inhibitors. There is evidence that widespread inflammation in the spine displayed by MRI may be predictive of a favorable clinical response to anti-TNFα treatment [56]. Response to treatment with anti-TNFα agents may be even more pronounced in very early disease that is detected only by MRI than in patients with established radiographic disease [21, 22]. Several scoring systems to assess SIJ and spinal inflammation have been developed for clinical research to objectively monitor response to treatment. As an example, the SPARCC indices were used to assess disease activity in both the SIJ and spine in a recent randomized controlled trial of adalimumab in active AS. They were shown to be reliable and highly discriminatory between treatment groups [27]. Persisting back pain in AS patients despite anti-TNFα treatment may be due to physical deconditioning after longstanding disease or unrelated mechanical causes and not attributable to failure of the biologic agent. An MRI documenting an improvement in axial inflammation may influence decision making towards physiotherapy rather than switching to another anti-TNFα agent.
A combination of MR and high resolution CT with multiplanar reconstruction showed the best sensitivity to detect transspinal fractures in an ankylosed spine [57, 58] (Fig. 2). These serious fractures through intervertebral syndesmophytes, the former disc, and the posterior spinal elements carry a substantial risk of spinal cord injury [59] and are very difficult to locate by standard radiography in most instances.
Transspinal fracture in a patient with longstanding ankylosing spondylitis and fusion of the thoracolumbar segment. A 57-year-old male patient with HLA-B27-positive ankylosing spondylitis. The thoracolumbar spine was completely ankylosed after a disease duration of 32 years. The patient complained about increasing back pain since 3 years without recalling an initiating event. The reason for referring the patient was the question whether TNFα-inhibitor therapy should be started due to persistent flare of ankylosing spondylitis. a Conventional radiography of the thoracolumbar spine. Syndesmophyte bridging of all lumbar levels (broken arrows). Advanced degenerative bony changes on level Th 11 / Th 12 (solid arrow). b Whole body MRI of the spine, STIR sequence. Active inflammation with bone marrow edema in adjacent anterior vertebral corners Th 2 and Th 3 (broken arrow). Polymorph increased signal intensity on level Th 11 / Th 12 (solid arrow). c CT of the thoracolumbar spine. Old transspinal fracture through syndesmophytes, vertebral disc, and posterior elements of the spine (solid arrows) with marked secondary degenerative changes
Emerging role of MRI to predict spinal ossification
Three recent studies concluded that inflammatory spinal lesions detected by MRI are predictive of new syndesmophytes as shown on spinal radiographs 24 months later, although the association with progression differed among studies [60–62]. New syndesmophytes developed significantly more frequently in vertebral corners with inflammation than in those without inflammation seen on baseline MRI. Interestingly, vertebral corner inflammation that resolved upon treatment with anti-TNFα agents was more strongly associated with new syndesmophyte formation [62]. However, vertebral corners that appeared normal on MRI also developed new bone formation. Further research in larger cohorts is needed to determine the prognostic significance of spinal inflammation on MRI and whether progression can be modified by treatment with biologics, especially if therapy starts early in the disease course.
MRI in daily routine for patients suspected to have axial SpA
Patients presenting with clinical features of SpA with equivocal radiographs should be further investigated using T1W and STIR MRI of the SIJ scanned in the tilted coronal orientation as the principal diagnostic tool, especially if they are B27-positive [63]. There are presently no data supporting the routine evaluation of the spine in addition to the SIJ in the absence of symptoms in the spine and/or chest wall. In that setting the radiologist should be alerted to the requirement for an imaging protocol that ensures visualization of lateral segments (available at www.arthritisdoctor.ca). The finding of unequivocal BME and erosions in the SIJ carries a high probability for SpA. In addition, the presence of active spinal inflammatory lesions, especially in the thoracic spine and in the lateral segments, substantially increases the likelihood of SpA.
MRI in rheumatoid arthritis
The spectrum of MRI lesions in RA and their histopathologic correlation
MRI allows assessment of all the structures involved in RA, i.e., synovial membrane, tendons and tendon sheaths, intra- and extraarticular fluid collections, cartilage, bone, and ligaments. MRI has been shown to be more sensitive than clinical examination and radiographs for detection of inflammatory and destructive joint changes in early RA (Fig. 3). MRI and histopathological signs of synovial inflammation are closely correlated [64–66]; in a study of metacarpophalangeal (MCP) joints in patients with early and with established RA, miniarthroscopy confirmed the presence of bone pathology in all joints with MRI bone erosions and histologic and macroscopic synovitis in all joints with MRI synovitis [67]. MRI BME represents inflammatory infiltrates in the bone marrow, i.e., osteitis, as demonstrated by comparison with histological samples obtained at surgery in RA patients [3, 4]. Whereas erosions reflect bone damage that has already occurred, BME appears to represent the link between joint inflammation and bone destruction.
MRI findings in patients with early rheumatoid arthritis. a–c Coronal STIR sequence of the wrist and metacarpophalangeal joints of three different patients with early rheumatoid arthritis. a shows no bone marrow edema, b bone marrow edema (high signal intensity) in many wrist bones, but not in the metacarpophalangeal joints, whereas c shows bone marrow edema in various wrist bones as well as in the second metacarpophalangeal joint (arrow). d Coronal T1SE sequence and e axial T1SE sequence of the same patient as illustrated in c. These images show bone erosion, confirmed in two planes, in the second metacarpal head (arrow). Courtesy Susanne J. Pedersen, MD, Copenhagen
A high level of agreement for detection of bone erosions in wrist and MCP joints in RA patients (concordance at 77–90% of sites) between MRI and computed tomography (CT), the gold standard reference for detection of bony destruction, documents that MRI erosions represent true bone damage [68–71].
The majority of MRI studies in RA investigated knee, wrist, or finger joints. Reports on other peripheral joints are few and not essentially different. Although only one formal comparison with follow-up of other joints exists, MRI of unilateral MCP and wrist joints are most commonly recommended for MRI follow-up of RA patients, whereas MRI of other joints is only to be obtained if specifically clinically indicated.
Compared with radiography, MRI offers clear advantages but also has disadvantages due to increased cost and lower availability. However, MRI costs represent only a fraction of the total expense incurred in the management of RA patients when the costs of biological RA treatment or the indirect costs of sick leave/early retirement are considered. Dedicated extremity MRI units are increasingly used and offer improved patient comfort at lower cost than conventional MRI units [72–80]. The better of the dedicated low-field MRI units provide similar information on erosions and synovitis as conventional high-field MRI devices [75, 76]. However, performance characteristics of different machines differ widely [81], emphasizing the need for careful testing of the individual machines.
Optimal MRI assessment of synovitis requires use of intravenous gadolinium contrast media, while assessment of BME and erosion does not [82]. Thus, for assessment of synovitis, BME, and erosions, a pre- and post-contrast T1-weighted sequence in two planes plus a T2-weighted fat-saturated or short tau inversion recovery (STIR) sequence is recommended [83] (Fig. 4).
MRI to detect (teno-)synovitis in a patient with early rheumatoid arthritis. Axial T1SE sequence of the second to fifth metacarpophalangeal joints in a patient with early rheumatoid arthritis before (a) and after (b) intravenous contrast agent injection. Severe synovitis is seen in all metacarpophalangeal joints (horizontal arrows). Flexor tendon tenosynovitis is also noted, particularly at the second and third finger (vertical arrows). Courtesy Susanne J. Pedersen, MD, Copenhagen
Monitoring disease activity and structural damage
To be valuable for monitoring joint inflammation and destruction, a measure must be reproducible and sensitive to change [84]. MRI allows quantitative measurement of the early contrast augmentation (“enhancement”) rate after intravenous injection of gadolinium, which reflects the degree of synovitis, the measurement of synovial volume, as well as less detailed (qualitative: presence/absence; semiquantitative: scoring) evaluation of synovitis and bone erosions. In observational studies and randomized clinical trials, semiquantitative scoring has been the most frequently used approach. The OMERACT (Outcome Measures in Rheumatology) RA MRI scoring system (RAMRIS) involves semiquantitative assessment of synovitis, bone erosions, and BME in RA finger and wrist joints [83]. This method was developed and validated through iterative multicenter studies under OMERACT and EULAR banners [83, 85–88]. Consensus MRI definitions of important joint pathologies and a “core set” of basic MRI sequences were also suggested [83].
The OMERACT erosion scores are closely correlated with erosion volumes estimated by MRI and CT [69, 70]. Using the RAMRIS, very good intrareader reliability, good interreader reliability, and a high level of sensitivity to change have been reported, demonstrating that the OMERACT RAMRIS system, after proper training and calibration of readers, is suitable for monitoring joint inflammation and destruction in RA [89, 90]. A EULAR-OMERACT RA MRI reference image atlas has been developed, providing an easy-to-use tool for standardized RAMRIS scoring of MR images for RA activity and damage by comparison with reference images [91]. Scoring systems for tenosynovitis [92] and joint space narrowing [93] may be used in addition to the OMERACT scoring system.
The OMERACT synovitis score is sensitive to change over weeks as well as months, and MRI, as assessed according to the OMERACT system, is increasingly used in trials of biologic agents [71, 94–100]. Haavardsholm et al. reported that a combined score of synovitis, tenosynovitis, and BME was more sensitive than conventional biomarkers and clinical measures as well as the individual MRI parameters [101].
Quantitative methods of synovitis (synovial membrane volumes and post-contrast enhancement rates), which in knee joints have been shown to be closely related with histopathological synovitis [64–66] and to be sensitive to change [65, 102], have only been used sparsely in clinical trials, probably due to them being laborious (particularly the measurement of synovial volume) and/or having limited reproducibility with multicenter use (particularly early enhancement rates by dynamic MRI). However, recent software improvements, providing more automated methods, potentially increase assessment speed and reproducibility [103–107]. This encourages the reappraisal of the usefulness of such quantitative methods.
Several studies have demonstrated that MRI is more sensitive than radiography for monitoring erosive progression in individual joint regions [108–111]. RAMRIS scoring of unilateral wrist and MCP joints is more sensitive to change than Sharp/van der Heijde radiography scoring of bilateral hands, wrist joints, and forefeet [112].
The superior sensitivity to change and discriminatory ability of MRI compared to radiography has been demonstrated in randomized controlled clinical trials (RCTs) [94, 113, 114]. Quinn et al. demonstrated a significantly lower erosion progression rate by MRI, but not by the Sharp/van der Heijde radiography method, in 12 early RA patients treated with methotrexate plus infliximab compared to 12 patients receiving methotrexate alone [94]. This was the first study to verify that the use of MRI in RCTs allows shorter observation periods and/or fewer patients for discriminating between different therapies concerning reduction of structural joint damage. This has been confirmed by subsequent studies [113, 115]. A recent large study of 318 methotrexate-naïve patients demonstrated that inhibition of erosive progression by biological therapy compared to placebo can be demonstrated by MRI using half the patients and half the follow-up time of radiography [116]. These latter data reinforce the superior sensitivity of MRI as compared to radiography assessment for measuring change owing to erosive damage.
Diagnostic utility of MRI in undifferentiated peripheral arthritis
A number of relatively small studies (≤50 patients per study) provided ambiguous results regarding the differential diagnostic value of MRI [117–121]. Recently, data from two large follow-up studies of undifferentiated arthritis have allowed a more thorough investigation of the utility of MRI to diagnose RA [122, 123]. Tamai et al. investigated 129 patients with nonclassifiable arthritis despite routine examination, including C-reactive protein and biochemical tests, and developed a prediction model containing anticyclic citrullinated peptide (anti-CCP) and/or IgM rheumatoid factors (RF), MRI-proven symmetric synovitis, and MRI-proven BME and/or bone erosion. Of patients positive for more than two of these variables, 71.3% developed RA within 1 year (specificity 75.9% and sensitivity 68.0%, respectively). Presence of BME had a positive predictive value of 86.1% for subsequent development of RA [122].
In a study of 116 patients with early undifferentiated arthritis, Duer-Jensen et al. documented that MRI BME in metatarsophalangeal (MTP) and wrist joints is an independent predictor of future RA in patients with early undifferentiated arthritis. A prediction model, including clinical hand arthritis, morning stiffness, positive RF, and MRI BME score in MTP and wrist joints correctly identified the development of RA or non-RA in 82% of patients [123].
Two systematic literature reviews have been published recently [124, 125], but too early to include the study by Duer-Jensen et al. [123]. The former study states that widespread use of MRI for diagnosing RA is scientifically not justified, because the sensitivity and specificity of MRI findings for RA differ substantially among studies, depending upon the criteria applied. However, the authors emphasize that the diagnostic performance of MRI was better when lower quality studies or studies in RA patients with longer disease duration were excluded [124]. A systematic literature review by Machado et al. concludes that BME and combined synovitis and erosion pattern seem useful in predicting development of RA from undifferentiated peripheral arthritis. The difference in conclusions seems partly explained by whether poor-quality studies are included [124] or not included [125] in the systematic literature review.
A new role for MRI has recently been added. Due to the limited sensitivity of the ACR 1987 classification criteria for RA in early disease, new criteria have been developed and recently reported, the ACR/EULAR 2010 criteria [12]. Classification as definite RA is based on the presence of definite clinical synovitis (swelling at clinical examination) in one or more joints, absence of an alternative diagnosis that explains the synovitis, and achievement of a total score ≥6 (range 0–10) from the individual scores in four domains: number/site of involved joints (range 0–5), serologic abnormality (range 0–3), elevated acute-phase response (range 0–1), and symptom duration (range 0–1) [12]. MRI and ultrasound may be used to detect joint inflammation and to count joints in the “joint involvement” domain of the new RA classification criteria [13, 14].
In summary, two large studies document an independent predictive value of MRI for the development of RA, implying a diagnostic utility when used in combination with clinical parameters. Furthermore, MRI and ultrasound have an important role in the recent ACR/EULAR 2010 classification criteria for RA. Thus, MRI could be used in routine clinical practice for confirming an early diagnosis of RA made on clinical grounds.
Prognostication
Various studies have reported that MRI pathology (synovitis, bone erosions or, most often, BME) of the wrist and MCP joints at disease onset in early RA predicts radiographic erosions [108, 126–134]. Some looked at the short-term predictive value of MRI after 1 year [108, 126, 128, 130, 131], whereas other studies had 2 [128, 132], 3 [134], 5 [133], 6 [127], or 10 years [129] of follow-up. All studies found MRI to be a highly significant predictor of radiographic erosions.
With the exception of two early RA cohorts [131, 132], all MRI studies in RA did not include anti-CCP or take into account other potential prognostic markers such as smoking or shared epitope carriage. Haavardsholm et al. [131] reported BME and male gender (but not anti-CCP) to be independent predictors of radiographic progression after 1 year in a single-center cohort of 84 patients treated according to standard clinical practice. A study by Hetland et al. was the first clinical trial with a standardized treatment protocol that investigated the predictive value of a variety of potential prognostic markers including both imaging modalities MRI and conventional radiography, immunologic (anti-CCP, IgM RF, and IgA RF), environmental (smoking, educational level), genetic (shared epitope), and disease activity markers [132]. The main finding was that in this comprehensive model, MRI BME at presentation was the strongest independent predictor of radiographic progression 2 years later in early RA patients as determined in both multivariate linear and logistic regression analyses. Three-year [135] and 5-year [133] follow-up studies in the two respective cohorts have documented that MRI BME is also a predictor of long-term radiographic progression. A recent systematic literature review, not incorporating the latest data, reports that when studies of all qualities are included in the analysis, the sensitivity and specificity of MRI findings for predicting erosive progression are so variable that the authors do not recommend clinical use of MRI for this purpose. However, they also note that BME was predictive of erosions if only the highest quality data were included in the analysis [124].
A relation between baseline MRI findings and long-term functional disability has only been documented in one study [136]. Data from the same cohort revealed that extensive MRI BME and erosions at the wrist in early rheumatoid arthritis predicted tendon dysfunction and impaired hand function [137]. Furthermore, a high baseline MRI tendinopathy score was predictive of tendon rupture at 6 years (odds ratio 1.52) [138]. Further studies are required to determine the clinical relevance of these findings.
Another issue of high clinical importance is whether MRI is useful in patients in clinical remission to predict the disease course. Residual synovitis on MRI is frequent in patients in clinical remission [139, 140]. The impact of subclinical imaging findings has been studied by Brown and coworkers [141]. Seventeen controls and 102 RA patients on conventional treatment judged to be in remission by their treating rheumatologists underwent clinical, laboratory, functional, and quality of life assessments during a 12-month period. Radiographs of hands and feet, and MRI and ultrasound of unilateral hand and wrist were performed at baseline and after 12 months. Despite clinical remission, 19% of patients experienced progression in radiographic joint damage during the study period. Baseline ultrasound synovial hypertrophy, ultrasound power Doppler signal and MRI synovitis scores in individual joints were significantly associated with progressive radiographic damage. The study encourages further exploration of MRI and ultrasound for predicting the disease course and for evaluating disease status, including defining what constitutes true disease remission.
MRI in osteoarthritis
MRI has become the imaging modality of choice to assess active and structural joint lesions in OA. The main focus of interest for MRI in OA is clinical research whereas routine use of MRI for the evaluation of patients with OA in daily practice is not justified by currently available data [15]. In clinical practice, MRI for OA may be ordered to exclude relevant conditions in the differential diagnosis such as avascular osteonecrosis or perhaps to assess the extent of structural lesions prior to joint replacement surgery. An emerging indication for daily routine is preradiographic OA where early subtle joint pathology can be detected by MRI long before structural changes are seen on radiography. Research using MRI in OA has focused on the knee joint because this large joint allows good discrimination of anatomical structures, has a relatively thick articular cartilage compared to other joints, and because the prevalence of knee OA in the population is high [142].
Semiquantitative multi-feature whole-joint assessment systems in knee OA
Whole joint MRI evaluation methods reflect the concept of knee OA as a multifactorial disorder of the whole synovial joint organ resulting in changes in structure and function. MRI captures many features of soft tissue, cartilage, and bone that are relevant to clinical manifestations, functional impairment, or pathophysiology of OA. These articular features include bone marrow lesions (BML), joint effusion, synovitis, and bursitis that represent active lesions as well as structural features such as articular cartilage degeneration, subchondral bone attrition and cysts, osteophytes, meniscal, cruciate and collateral ligament integrity, and intraarticular loose bodies.
Several semiquantitative whole joint scoring systems have been proposed: the Whole Organ Magnetic Resonance Imaging Score (WORMS) [143], the Knee Osteoarthritis Scoring System (KOSS) [144], the Boston-Leeds Osteoarthritis Knee Score (BLOKS) [145], and the system proposed by Biswal and colleagues [146]. The scoring modules apply different subregional divisions for the femur and the tibial plateau. None of them uses contrast-enhanced MRI. To date no single whole-joint scoring method has gained widespread acceptance as a standard reference for clinical research. A comparison between these scoring systems regarding construct validity is limited by the absence of histopathological data while assessment of discriminatory capacity is limited by the absence of therapeutic modalities that have major treatment effects.
In a cross-sectional study of 115 knees with radiographic OA at high risk of cartilage loss, the WORMS and BLOKS scoring systems performed similarly for prevalence and severity of cartilage loss, bone marrow lesions, and meniscal damage [147]. A comparison of 1.0 Tesla extremity MRI versus large-bore 1.5 Tesla MRI using the WORMS system showed moderate (weighted kappa for effusion and synovitis 0.53 and 0.54, respectively) to substantial agreement (0.75 and 0.71 for cartilage and BME, respectively) between the two techniques [148].
Quantitative measurement methods have also been developed to assess cartilage integrity in knee OA. These systems are less observer-dependent and may better detect small changes in the cartilage structure over large areas compared to semiquantitative methods. However, quantitative measurement requires specialized software and time-consuming segmentation between different articular structures by trained operators [142].
Bone marrow lesions and synovitis
MRI signs of disease activity such as BML and synovitis/joint effusion are particularly interesting as they are potentially modifiable by treatment [149]. Interventions targeting BML and synovitis may not only result in symptomatic improvement but may have a potential for disease modification as active MRI lesions are associated with both knee pain and progression of structural damage. It is possible that osteochondral fracture and subsequent osteonecrosis may develop in some cases as a consequence of weakening of inflamed bone rather than the inflammation developing as a response to the injury or necrosis.
Bone marrow lesions
BML appear on T2-weighted sequences as ill-defined areas of increased signal intensity (Figs. 5 and 6). The pathophysiology of BML is poorly understood. Histology of BML in knee and hip joint tissue removed at total joint replacement surgery in patients with advanced OA showed predominantly nonspecific findings such as bone marrow fibrosis and necrosis or trabecular microfractures, but only sparse interstitial edema [7–10]. These histopathology findings in OA suggest that BML seen on MRI reflects metaplastic fibrovascular tissue in the bone marrow together with trabecular remodeling. However, most BML fluctuate in size over time, which may be observed after only a few months suggesting that BML reflect more active histopathological changes than structural remodeling [150–155]. In one of these reports, fluctuations in BML scores over 3 months were positively correlated with changes in urinary C-terminal crosslinking telopeptide of type II collagen, a biomarker reflecting cartilage turnover [150].
Discrepancy between severity of hyaline cartilage damage and presence of bone marrow lesions. Bilateral knee MRI (right knee a and b, left knee c and d) with short tau inversion recovery (STIR) and proton density (PD) images. A 60-year-old woman with bilateral knee pain and instability. Right knee symptoms have been present for longer, and on the left side, symptoms have deteriorated recently. Bilateral knee osteoarthritis is severe. On the right side (a and b), this is associated with remodeling of the articular surfaces and subchondral sclerosis. No edema or cyst formation is present. On the left, extensive bone marrow lesion (c) is present with a focal area of diminished signal seen adjacent to the tibial plateau (arrow in d). This presumably represents a sclerotic response to more focal stress/hyaline cartilage damage
Unpredictability of presence and distribution of bone marrow lesions in osteoarthritis. Right knee MRI in two patients (patient 1 a and b; patient 2 c and d) with STIR and PD images. Two 60-year-old women with right knee pain and instability with recent onset of symptoms with near-normal weight-bearing radiographs 8 weeks previously (preserved joint space with tiny osteophytes only). Both patients had tears of the root attachment of the posterior horn of the medial meniscus (not shown) and some medial extrusion of the body of the meniscus. Cartilage loss is a little worse in the first patient, and an extensive bone marrow lesion in the femoral condyle is associated with a focal area of low signal in the subchondral bone without loss of integrity of the articular contour (arrow in b). This likely represents occult osteochondral injury without any substantial area of necrosis. The second patient has an extensive bone marrow lesion in the tibial plateau (c). Although the association of an overweight middle-aged female with meniscal root or radial tear and rapid deterioration of osteoarthritis is well established, the exact distribution of bone marrow signal change, speed of progression, and the presence of co-existent osteonecrosis are unpredictable
A cross-sectional study of patients with knee OA found a strong association between BML and pain [156]. Amongst patients with pain, 77.5% showed BML on MRI, while these lesions were detected in only 30% of patients without pain. In 2007, the same working group reported the results of a 15 month follow-up study [157]. An increase in BML volume was observed in 49.1% of patients with painful knee OA as opposed to only 26.8% of OA patients without knee pain. Among patients with no BML at baseline, the development of new BML was more common in painful knees than in control knees (32.4 versus 10.8%). A recently published self-matched case-control study in patients with knee OA showed that changes in BML extent and synovitis were associated with fluctuations in knee pain; improvement of BML, but not of synovitis or effusion, was associated with a decreased risk of knee pain [158]. However, a recent systematic literature review evaluating the association between active and structural MR findings and pain in patients with knee OA concluded that the level of evidence for a positive association between either BML or synovitis/effusion and pain is only moderate [159]. The odds ratio for having pain when BML was present ranged from 2.0 to 5.0 and for synovitis/effusion from 3.2 to 10.0.
In patients with radiographic knee OA, many studies consistently showed an association of BML with cartilage loss and OA progression [151, 154, 160–163], even in patients with minimal baseline cartilage damage [164]. Absence of BML was associated with a decreased risk of cartilage loss [154]. The risk for structural progression in the medial and lateral compartment was increased more than sixfold when medial or lateral BML were present (adjusted odds ratio 6.5 with medial BME present and 6.1 with lateral BML present) [160]. Varus or valgus limb malalignment is a confounder in the assessment of the relationship between BML and structural progression [151, 160]. A recent report showed that meniscal pathology also increases the risk of incident or enlarging BML over a period of 30 months [165] and may not be an independent predictor of structural progression.
A prospective cohort study in 271 healthy community-based adults with no clinical knee OA showed incident BML developing over the 2 year study period in 14% [153]. Incident BML were associated with knee pain (odds ratio 4.2) and with increased BMI. Of the BML present at baseline, 46% completely resolved. In a prospective cohort of 148 healthy women in middle age with no knee pain and no clinical knee OA, large BML were associated with progression of tibiofemoral cartilage defects [166]. These data suggest that the relationship between BML and cartilage loss is similar in knees with established OA and in knees without clinical knee OA or pain.
Synovitis
The optimal MRI evaluation of synovitis in knee OA requires contrast enhancement. Synovial enhancement by the contrast agent provides superior image quality for the assessment of synovial volume and differentiates synovitis from joint effusion by increased vascular perfusion and capillary permeability of the synovium [167]. Synovitis scores obtained by contrast-enhanced MRI showed good correlation with arthroscopic and microscopic synovitis scores [168]. Studies using nonenhanced MRI showed inconsistent results regarding an association between synovitis and knee pain. A recent report showed that synovitis detected by contrast-enhanced MRI had a strong association (adjusted odds ratio 9.2) with at least moderate severity of knee pain as assessed by the Western Ontario and McMaster Osteoarthritis Index pain scale [169]. A comprehensive whole-knee joint synovitis scoring system has been proposed recently, which showed a high reliability based on contrast-enhanced MRI [170]. Interventional studies assessing intraarticular corticosteroids or NSAIDs and paracetamol demonstrated associations between decreased synovitis and reduction of pain in patients with knee OA [171, 172]. Several semiquantitative and quantitative contrast-enhanced MRI assessment systems using different subregional approaches have been proposed to quantify synovitis [65, 167, 173–175].
Future directions
Future research in axial SpA should focus on further prospective assessment of the utility of structural lesions in more diverse settings and larger cohorts of patients suspected of having early SpA. It is also important for clinicians to know to what degree imaging of the spine contributes to diagnostic utility over and above assessment of the SIJ, which might in turn argue in favor of the use of WB MRI over conventional imaging. Finally, it is important to understand the prognostic significance of lesions observed on MRI and to what degree and which features portend an unfavorable radiographic outcome.
In addition to the documented utility for risk stratification for erosive progression in early RA and for early diagnosis of RA, other areas where MRI may be clinically important are emerging. Important research areas include whether adding MRI to routine clinical examination will improve clinical and radiographic outcome in RA patients. In particular, it is important to understand the prognostic significance of MRI findings in those patients achieving clinical remission with respect to the subsequent course of erosive progression, functional impairment, and disease activity. Technical advances, including the possibility of achieving overall assessment of the disease load by WB MRI and the potential clinical and scientific utility of improved cartilage assessment, e.g., by 3 T MRI, should be evaluated.
Research in OA should evaluate treatment interventions targeting BML and synovitis. These MRI signs of disease activity are potentially reversible, and there is some evidence that their improvement may also preserve joint integrity. Before using MRI for OA in daily routine, we need data that address whether a diagnosis of preradiographic OA by MR, which may facilitate early intervention, translates into a more favorable clinical outcome.
MRI is a very promising imaging modality not only for our understanding of the pathophysiology of axial SpA, RA, and OA and for confirming an early diagnosis before radiographic lesions appear, but also for monitoring disease upon treatment due to its noninvasive technique, lack of ionizing radiation, and its superior spatial and contrast resolution of all tissues involved in the disease process.
References
Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–1.
Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171:1151–3.
McQueen FM, Gao A, Østergaard M, King A, Shalley G, Robinson E, et al. High grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis. 2007;66:1581–7.
Jimenez-Boj E, Noebauer-Huhmann I, Hanslik-Schnabel B, Dorotka R, Wanivenhaus AH, Kainberger F, et al. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56:1118–24.
Appel H, Loddenkemper C, Grozdanovic Z, Ebhardt H, Dreimann M, Hempfing A, et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res Ther. 2006;8:R143.
Bollow M, Fischer T, Reisshauer A, Backhaus M, Sieper J, Hamm B, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis – cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135–40.
Bergman AG, Willen HK, Lindstrand AL, Pettersson HT. Osteoarthritis of the knee: correlation of subchondral MR signal abnormalities with histopathologic and radiographic features. Skeletal Radiol. 1994;23:445–8.
Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–40.
Saadat E, Jobke B, Chu B, Lu Y, Cheng J, Li X, et al. Diagnostic performance of in vivo 3-T MRI for articular cartilage abnormalities in human osteoarthritic knees using histology as standard of reference. Eur Radiol. 2008;18:2292–302.
Taljanovic MS, Graham AR, Benjamin JB, Gmitro AF, Krupinski EA, Schwartz SA, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 2008;37:423–31.
Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham 3rd CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8.
Østergaard M. Clarification of the role of ultrasonography, magnetic resonance imaging and conventional radiography in the ACR/EULAR 2010 rheumatoid arthritis classification criteria—comment to the article by Aletaha et al. Ann Rheum Dis. 2010;e-letter published online December 2.
Aletaha D, Hawker G, Neogi T, Silman A. Re: Clarification of the role of ultrasonography, magnetic resonance imaging and conventional radiography in the ACR/EULAR 2010 rheumatoid arthritis classification criteria—comment to the article by Aletaha et al. Ann Rheum Dis. 2011;e-letter published online January 11.
Hunter DJ, Neogi T, Hochberg MC. Quality of osteoarthritis management and the need for reform in the US. Arthritis Care Res. 2011;63:31–8.
Jang JH, Ward MM, Rucker AN, Reveille JD, Davis Jr JC, Weisman MH, et al. Ankylosing spondylitis: patterns of radiographic involvement—a re-examination of accepted principles in a cohort of 769 patients. Radiology. 2011;258:192–8.
Maksymowych WP, Weber U. Diagnostic utility of MRI in early spondyloarthritis. Curr Rheum Rep. 2011;in press.
Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8.
Mau W, Zeidler H, Mau R, Majewski A, Freyschmidt J, Stangel W, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol. 1988;15:1109–14.
Rudwaleit M, Haibel H, Baraliakos X, Listing J, Maerker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis. Results from the German spondyloarthritis inception cohort. Arthritis Rheum. 2009;60:717–27.
Barkham N, Keen HI, Coates LC, O’Connor P, Hensor E, Fraser AD, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging–determined early sacroiliitis. Arthritis Rheum. 2009;60:946–54.
Haibel H, Rudwaleit M, Listing J, Heldmann F, Wong RL, Kupper H, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis. Arthritis Rheum. 2008;58:1981–91.
Bennett AN, McGonagle D, O’Connor P, Hensor EMA, Sivera F, Coates LC, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum. 2008;58:3413–8.
Rudwaleit M, Jurik AG, Hermann KGA, Landewé R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–7.
Bollow M, Hermann KG, Biedermann T, Sieper J, Schontube M, Braun J. Very early spondyloarthritis: where the inflammation in the sacroiliac joints starts. Ann Rheum Dis. 2005;64:1644–6.
Weber U, Pfirrmann CWA, Kissling RO, MacKenzie CR, Khan MA. Early spondyloarthritis in an HLA B27-positive monozygotic twin pair: a highly concordant onset, sites of involvement, and disease course. J Rheumatol. 2008;35:1464–7.
Lambert RGW, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis. Arthritis Rheum. 2007;56:4005–14.
Maksymowych W, Dhillon SS, Chiowchanwisawakit P, Pedersen SJ, Martinez B, Østergaard M, et al. Development and validation of web-based training modules for systematic evaluation of active inflammatory lesions in the spine and sacroiliac joints in spondyloarthritis. J Rheumatol. 2009;36 Suppl 84:48–57.
Althoff CE, Feist E, Burova E, Eshed I, Bollow M, Hamm B, et al. Magnetic resonance imaging of active sacroiliitis: do we really need gadolinium? Eur J Radiol. 2009;71:232–6.
Madsen KB, Egund N, Jurik AG. Grading of inflammatory disease activity in the sacroiliac joints with magnetic resonance imaging: comparison between short tau inversion recovery and gadolinium contrast-enhanced sequences. J Rheumatol. 2010;37:393–400.
Baraliakos X, Hermann KG, Landewe R, Listing J, Golder W, Brandt J, et al. Assessment of acute spinal inflammation in patients with ankylosing spondylitis by magnetic resonance imaging: a comparison between contrast enhanced T1 and short tau inversion recovery (STIR) sequences. Ann Rheum Dis. 2005;64:1141–4.
Madsen KB, Jurik AG. Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res. 2010;62:11–8.
Algin O, Gokalp G, Ocakoglu G. Evaluation of bone cortex and cartilage of spondyloarthropathic sacroiliac joint: efficiency of different fat-saturated MRI sequences (T1-weighted, 3D-FLASH, and 3D-DESS). Acad Radiol. 2010;17:1292–8.
Weber U, Pedersen SJ, Hodler J, Østergaard M, Lambert RGW, Maksymowych WP. Does fat infiltration in the sacroiliac joint contribute to the diagnostic utility of MRI in ankylosing spondylitis? Arthritis Rheum. 2009;60(suppl 10):54 [abstract].
Braun J, Bollow M, Eggens U, Koenig H, Distler A, Sieper J. Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum. 1994;37:1039–45.
Hanly JG, Mitchell MJ, Barnes DC, MacMillan L. Early recognition of sacroiliitis by magnetic resonance imaging and single photon emission computed tomography. J Rheumatol. 1994;21:2088–95.
Bollow M, Braun J, Hamm B, Eggens U, Schilling A, Koenig H, et al. Early sacroiliitis in patients with spondyloarthropathy: evaluation with dynamic gadolinium-enhanced MR imaging. Radiology. 1995;194:529–36.
Blum U, Buitrago-Tellez C, Mundinger A, Krause T, Laubenberger J, Vaith P, et al. Magnetic resonance imaging (MRI) for detection of active sacroiliitis—a prospective study comparing conventional radiography, scintigraphy, and contrast enhanced MRI. J Rheumatol. 1996;23:2107–15.
Puhakka KB, Jurik AG, Egund N, Schiottz-Christensen B, Stengaard-Pedersen K, van Overeem Hansen G, et al. Imaging of sacroiliitis in early seronegative spondylarthropathy. Assessment of abnormalities by MRI in comparison with radiography and CT. Acta Radiol. 2003;44:218–29.
Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–43.
Weber U, Lambert RGW, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis. An international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62:3048–58.
Weber U, Lambert RGW, Pedersen SJ, Hodler J, Østergaard M, Maksymowych WP. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res (Hoboken). 2010;62:1763–71.
Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum (Arthritis Care Res). 2005;53:703–9.
Maksymowych WP, Dhillon SS, Chiowchanwisawakit P, Pedersen SJ, Martinez B, Østergaard M, et al. Development and validation of web-based training modules for systematic evaluation of active inflammatory lesions in the spine and sacroiliac joints in spondyloarthritis. J Rheumatol. 2009;36 suppl 84:48–57.
Lambert RGW, Pedersen SJ, Maksymowych WP, Chiowchanwisawakit P, Østergaard M. Active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis—definitions, assessment system, and reference image set. J Rheumatol. 2009;36 suppl 84:3–17.
Østergaard M, Maksymowych WP, Pedersen SJ, Chiowchanwisawakit P, Lambert RGW. Structural lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis—definitions, assessment system, and reference image set. J Rheumatol. 2009;36 suppl 84:18–34.
Pedersen SJ, Østergaard M, Chiowchanwisawakit P, Lambert RGW, Maksymowych WP. Validation of definitions for active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis. J Rheumatol. 2009;36 suppl 84:35–8.
Chiowchanwisawakit P, Østergaard M, Pedersen SJ, Lambert RGW, Conner-Spady B, Maksymowych WP. Validation of definitions for structural lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis. J Rheumatol. 2009;36 suppl 84:39–47.
Weber U, Hodler J, Kubik RA, Rufibach K, Lambert RGW, Kissling RO, et al. Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum (Arthritis Care Res). 2009;61:900–8.
Bennett AN, Rehman A, Hensor EMA, Marzo-Ortega H, Emery P, McGonagle D. Evaluation of the diagnostic utility of spinal magnetic resonance imaging in axial spondylarthritis. Arthritis Rheum. 2009;60:1331–41.
Bennett AN, Rehman A, Hensor EMA, Marzo-Ortega H, Emery P, McGonagle D. The fatty Romanus lesion: a non-inflammatory spinal MRI lesion specific for axial spondyloarthropathy. Ann Rheum Dis. 2010;69:891–4.
Rennie WJ, Dhillon SS, Conner-Spady B, Maksymowych WP, Lambert RGW. MRI assessment of spinal inflammation in ankylosing spondylitis: standard clinical protocols may omit inflammatory lesions in thoracic vertebrae. Arthritis Rheum. 2009;61:1187–93.
Maksymowych WP, Crowther SM, Dhillon SS, Conner-Spady B, Lambert RGW. Systematic assessment of inflammation by magnetic resonance imaging in the posterior elements of the spine in ankylosing spondylitis. Arthritis Care Res (Hoboken). 2010;62:4–10.
Weber U, Maksymowych WP, Jurik AG, Pfirrmann CWA, Rufibach K, Kissling RO, et al. Validation of whole-body against conventional magnetic resonance imaging for scoring acute inflammatory lesions in the sacroiliac joints of patients with spondylarthritis. Arthritis Rheum (Arthritis Care Res). 2009;61:893–9.
Weber U, Hodler J, Jurik AG, Pfirrmann CWA, Rufibach K, Kissling RO, et al. Assessment of active spinal inflammatory changes in patients with axial spondyloarthritis: validation of whole body MRI against conventional MRI. Ann Rheum Dis. 2010;69:648–53.
Rudwaleit M, Schwarzlose S, Hilgert ES, Listing J, Braun J, Sieper J. MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis. 2008;67:1276–81.
Wang YF, Teng MMH, Chang CY, Wu HT, Wang ST. Imaging manifestations of spinal fractures in ankylosing spondylitis. Am J Neuroradiol. 2005;26:2067–76.
Koivikko MP, Kiuru MJ, Koskinen SK. Multidetector computed tomography of cervical spine fractures in ankylosing spondylitis. Acta Radiol. 2004;7:751–9.
Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18:145–56.
Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther. 2008;10:R104.
Van der Heijde D, Landewé R, Baraliakos X, Hermann K, Houben H, Hsu B, et al. MRI-inflammation of the vertebral unit (vu) only marginally contributes to new syndesmophyte formation in that unit: a multi-level analysis. Ann Rheum Dis. 2008;67(Suppl II):130 [abstract].
Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RGW. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60:93–102.
Heuft-Dorenbosch L, Landewé R, Weijers R, Wanders A, Houben H, van der Linden S, et al. Combining information obtained from magnetic resonance imaging and conventional radiographs to detect sacroiliitis in patients with recent-onset inflammatory back pain. Ann Rheum Dis. 2006;65:804–8.
König H, Sieper J, Wolf KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990;176:473–7.
Østergaard M, Stoltenberg M, Løvgreen-Nielsen P, Volck B, Jensen CH, Lorenzen I. Magnetic resonance imaging-determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum. 1997;40:1856–67.
Østergaard M, Stoltenberg M, Løvgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovitis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998;16:743–54.
Ostendorf B, Peters R, Dann P, Becker A, Scherer A, Wedekind F, et al. Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis Rheum. 2001;44:2492–502.
Perry D, Stewart N, Benton N, Robinson E, Yeoman S, Crabbe J, et al. Detection of erosions in the rheumatoid hand; a comparative study of multidetector computerized tomography versus magnetic resonance scanning. J Rheumatol. 2005;32:256–67.
Døhn U, Ejbjerg BJ, Court-Payen M, Hasselquist M, Narvestad E, Szkudlarek M, et al. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8:R110.
Døhn U, Ejbjerg BJ, Hasselquist M, Narvestad E, Møller J, Thomsen HS, et al. Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res Ther. 2008;10:R25.
Døhn UM, Ejbjerg B, Boonen A, Hetland ML, Hansen MS, Knudsen LS, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis. 2011;70:252–8.
Savnik A, Malmskov H, Thomsen HS, Bretlau T, Graff LB, Nielsen H, et al. MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high-field MRI (1.5 T). Eur Radiol. 2001;11:1030–8.
Lindegaard H, Vallø J, Hørslev-Petersen K, Junker P, Østergaard M. Low field dedicated magnetic resonance imaging in untreated rheumatoid arthritis of recent onset. Ann Rheum Dis. 2001;60:770–6.
Crues JV, Shellock FG, Dardashti S, James TW, Troum OM. Identification of wrist and metacarpophalangeal joint erosions using a portable magnetic resonance imaging system compared to conventional radiographs. J Rheumatol. 2004;31:676–85.
Taouli B, Zaim S, Peterfy CG, Lynch JA, Stork A, Guermazi A, et al. Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. AJR Am J Roentgenol. 2004;182:937–43.
Ejbjerg BJ, Narvestad E, Jacobsen S, Thomsen HS, Østergaard M. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis. 2005;64:1280–7.
Chen TS, Crues III JV, Ali M, Troum OM. Magnetic resonance imaging is more sensitive than radiographs in detecting change in size of erosions in rheumatoid arthritis. J Rheumatol. 2006;33:1957–67.
Schirmer C, Scheel AK, Althoff CE, Schink T, Eshed I, Lembcke A, et al. Diagnostic quality and scoring of synovitis, tenosynovitis and erosions in low-field MRI of patients with rheumatoid arthritis: a comparison with conventional MRI. Ann Rheum Dis. 2007;66:522–9.
Freeston JE, Conaghan PG, Dass S, Vital E, Hensor EM, Stewart SP, et al. Does extremity-MRI improve erosion detection in severely damaged joints? A study of long-standing rheumatoid arthritis using three imaging modalities. Ann Rheum Dis. 2007;66:1538–40.
Duer-Jensen A, Vestergaard A, Døhn UM, Ejbjerg B, Hetland ML, Albrecht-Beste E, et al. Detection of rheumatoid arthritis bone erosions by two different dedicated extremity MRI units and conventional radiography. Ann Rheum Dis. 2008;67:998–1003.
Duer-Jensen A, Ejbjerg B, Albrecht-Beste E, Vestergaard A, Døhn UM, Hetland ML, et al. Does low-field dedicated extremity MRI (E-MRI) reliably detect bone erosions in rheumatoid arthritis? A comparison of two different E-MRI units and conventional radiography with high-resolution CT scanning. Ann Rheum Dis. 2009;68:1296–302.
Østergaard M, Conaghan PG, O'Connor P, Szkudlarek M, Klarlund M, Emery P, et al. Reducing invasiveness, duration, and cost of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous contrast injection—does it change the assessment of inflammatory and destructive joint changes by the OMERACT RAMRIS? J Rheumatol. 2009;36:1806–10.
Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6.
Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for outcome measures in rheumatology. J Rheumatol. 1998;25:198–9.
Østergaard M, Klarlund M, Lassere M, Conaghan P, Peterfy C, McQueen F, et al. Interreader agreement in the assessment of magnetic resonance images of rheumatoid arthritis wrist and finger joints—an international multicenter study. J Rheumatol. 2001;28:1143–50.
Conaghan P, Lassere M, Østergaard M, Peterfy C, McQueen F, O’Connor P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Exercise 4: an international multicenter longitudinal study using the RA-MRI Score. J Rheumatol. 2003;30:1376–9.
Lassere M, McQueen F, Østergaard M, Conaghan P, Shnier R, Peterfy C, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Exercise 3: an international multicenter reliability study using the RA-MRI Score. J Rheumatol. 2003;30:1366–75.
Østergaard M, Conaghan P, O'Connor P, Ejbjerg BJ, Szkudlarek M, Peterfy C, et al. Reducing costs, duration and invasiveness of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous gadolinium injection—does it affect assessments of synovitis, bone erosions and bone edema? Ann Rheum Dis. 2003;62(suppl I):67 [abstract].
Haavardsholm EA, Østergaard M, Ejbjerg BJ, Kvan NP, Uhlig TA, Lilleas FG, et al. Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum. 2005;52:3860–7.
Bird P, Joshua F, Lassere M, Shnier R, Edmonds J. Training and calibration improve inter-reader reliability of joint damage assessment using magnetic resonance image scoring and computerized erosion volume measurement. J Rheumatol. 2005;32:1452–8.
Østergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64 suppl 1:i2–55.
Haavardsholm EA, Østergaard M, Ejbjerg BJ, Kvan NP, Kvien T. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66:1216–20.
Østergaard M, Bøyesen P, Eshed I, Gandjbakhch F, Lillegraven S, Bird P, et al. Development and preliminary validation of an MRI joint space narrowing score for use in rheumatoid arthritis: a potential adjunct to the OMERACT RA MRI scoring system. J Rheumatol. 2011;in press.
Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:27–35.
Zikou AK, Argyropoulou MI, Voulgari PV, Xydis VG, Nikas SN, Efremidis SC, et al. Magnetic resonance imaging quantification of hand synovitis in patients with rheumatoid arthritis treated with adalimumab. J Rheumatol. 2006;33:219–23.
Argyropoulou MI, Glatzouni A, Voulgari PV, Xydis VG, Nikas SN, Efremidis SC, et al. Magnetic resonance imaging quantification of hand synovitis in patients with rheumatoid arthritis treated with infliximab. Joint Bone Spine. 2005;72:557–61.
Døhn UM, Skjødt H, Hetland ML, Vestergaard A, Møller JM, Knudsen LS, et al. No erosive progression revealed by MRI in rheumatoid arthritis patients treated with etanercept, even in patients with persistent MRI and clinical signs of joint inflammation. Clin Rheumatol. 2007;26:1857–61.
Østergaard M, Duer A, Nielsen H, Johansen JS, Narvestad E, Ejbjerg BJ, et al. Magnetic resonance imaging for accelerated assessment of drug effect and prediction of subsequent radiographic progression in rheumatoid arthritis: a study of patients receiving combined anakinra and methotrexate treatment. Ann Rheum Dis. 2005;64:1503–6.
Lisbona MP, Maymo J, Perich J, Almirall M, Perez-Garcia C, Carbonell J. Etanercept reduces synovitis as measured by magnetic resonance imaging in patients with active rheumatoid arthritis after only 6 weeks. J Rheumatol. 2008;35:394–7.
Døhn UM, Østergaard M, Bird P, Boonen A, Johansen SJ, Møller JM, et al. Tendency towards erosive regression on magnetic resonance imaging at 12 months in rheumatoid arthritis patients treated with rituximab. Ann Rheum Dis. 2009;68:1072–3.
Haavardsholm EA, Østergaard M, Hammer HB, Bøyesen P, Boonen A, van der Heijde D, et al. Monitoring anti-TNFalpha treatment in rheumatoid arthritis: responsiveness of magnetic resonance imaging and ultrasonography of the dominant wrist joint compared with conventional measures of disease activity and structural damage. Ann Rheum Dis. 2009;68:1572–9.
Østergaard M, Stoltenberg M, Henriksen O, Lorenzen I. Quantitative assessment of synovial inflammation by dynamic gadolinium-enhanced magnetic resonance imaging. A study of the effect of intra-articular methylprednisolone on the rate of early synovial enhancement. Br J Rheumatol. 1996;35:50–9.
Kubassova O. Automatic segmentation of blood vessels from dynamic MRI datasets. Med Image Comput Comput Assist Interv. 2007;10:593–600.
Kubassova OA, Boyle RD, Radjenovic A. Quantitative analysis of dynamic contrast-enhanced MRI datasets of the metacarpophalangeal joints. Acad Radiol. 2007;14:1189–200.
Hodgson RJ, Connolly S, Barnes T, Eyes B, Campbell RS, Moots R. Pharmacokinetic modeling of dynamic contrast-enhanced MRI of the hand and wrist in rheumatoid arthritis and the response to anti-tumor necrosis factor-alpha therapy. Magn Reson Med. 2007;58:482–9.
Hodgson RJ, Barnes T, Connolly S, Eyes B, Campbell RS, Moots R. Changes underlying the dynamic contrast-enhanced MRI response to treatment in rheumatoid arthritis. Skeletal Radiol. 2008;37:201–7.
Tripoliti EE, Fotiadis DI, Argyropoulou M. Automated segmentation and quantification of inflammatory tissue of the hand in rheumatoid arthritis patients using magnetic resonance imaging data. Artif Intell Med. 2007;40:65–85.
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999;58:156–63.
Østergaard M. Magnetic resonance imaging in rheumatoid arthritis. Quantitative methods for assessment of the inflammatory process in peripheral joints. Dan Med Bull. 1999;46:313–44.
Klarlund M, Østergaard M, Jensen KE, Madsen JL, Skjødt H, Lorenzen I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. Ann Rheum Dis. 2000;59:521–8.
Backhaus M, Burmester GR, Sandrock D, Loreck D, Hess D, Scholz A, et al. Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis. 2002;61:895–904.
Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen HS, Østergaard M. The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: a comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum. 2005;52:2300–6.
Jarrett SJ, Conaghan PG, Sloan VS, Papanastasiou P, Ortmann CE, O’Conner PJ, et al. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54:1410–4.
Durez P, Malghem J, Nzeusseu TA, Depresseux G, Lauwerys BR, Westhovens R, et al. Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum. 2007;56:3919–27.
Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–309.
Østergaard M, Emery P, Conaghan PG, Fleischmann RM, Xu W, Hsia EC, et al. Golimumab and methotrexate combination therapy significantly improves synovitis, osteitis and bone erosion compared to methotrexate alone—a magnetic resonance imaging study of methotrexate-naïve rheumatoid arthritis patients. Arthritis Rheum. 2010;62(suppl):S952 [abstract].
Sugimoto H, Takeda A, Hyodoh K. Early stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. 2000;216:569–75.
Boutry N, Hachulla E, Flipo RM, Cortet B, Cotten A. MR imaging involvement of the hands in early rheumatoid arthritis: comparison with systemic lupus erythematosus and primary Sjogren syndrome. Eur Radiol. 2005;15 suppl 1:262. (B-561) [abstract].
Solau-Gervais E, Legrand JL, Cortet B, Duquesnoy B, Flipo RM. Magnetic resonance imaging of the hand for the diagnosis of rheumatoid arthritis in the absence of anti-cyclic citrullinated peptide antibodies: a prospective study. J Rheumatol. 2006;33:1760–5.
Tamai M, Kawakami A, Uetani M, Takao S, Rashid H, Tanaka F, et al. Early prediction of rheumatoid arthritis by serological variables and magnetic resonance imaging of the wrists and finger joints: results from prospective clinical examination. Ann Rheum Dis. 2006;65:134–5.
Duer A, Østergaard M, Hørslev-Petersen K, Vallø J. Magnetic resonance imaging and bone scintigraphy in the differential diagnosis of unclassified arthritis. Ann Rheum Dis. 2008;67:48–51.
Tamai M, Kawakami A, Uetani M, Takao S, Arima K, Iwamoto N, et al. A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis Rheum. 2009;61:772–8.
Duer-Jensen A, Hørslev-Petersen K, Hetland ML, Bak L, Ejbjerg B, Hansen MS, et al. MRI bone edema is an independent predictor of development of rheumatoid arthritis in patients with early undifferentiated arthritis. Arthritis Rheum. 2011;63:in press.
Suter LG, Fraenkel L, Braithwaite RS. The role of magnetic resonance imaging in the diagnosis and prognosis of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;doi:10.1002/acr.20409.
Machado PM, Koevoets R, Bombardier C, van der Heijde D. The value of magnetic resonance imaging and ultrasound in undifferentiated arthritis: a systematic review. J Rheumatol. 2010;38 suppl 87:31–7.
Savnik A, Malmskov H, Thomsen HS, Graff LB, Nielsen H, Danneskiold-Samsøe B, et al. MRI of the wrist and finger joints in inflammatory joint diseases at 1-year interval: MRI features to predict bone erosions. Eur Radiol. 2002;12:1203–10.
McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1814–27.
Palosaari K, Vuotila J, Takalo R, Jartti A, Niemelae RK, Karjalainen A, et al. Bone oedema predicts erosive progression on wrist MRI in early RA—a 2-yr observational MRI and NC scintigraphy study. Rheumatol (Oxford). 2006;45:1542–8.
Tanaka N, Sakahashi H, Ishii S, Sato E, Hirose K, Ishima T. Synovial membrane enhancement and bone erosion by magnetic resonance imaging for prediction of radiologic progression in patients with early rheumatoid arthritis. Rheumatol Int. 2005;25:103–7.
Lindegaard HM, Vallø J, Hørslev-Petersen K, Junker P, Østergaard M. Low-cost, low-field dedicated extremity magnetic resonance imaging in early rheumatoid arthritis: a 1-year follow-up study. Ann Rheum Dis. 2006;65:1208–12.
Haavardsholm EA, Bøyesen P, Østergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67:794–800.
Hetland ML, Ejbjerg B, Hørslev-Petersen K, Jacobsen S, Vestergaard A, Jurik AG, et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis. 2009;68:384–90.
Hetland ML, Stengaard-Pedersen K, Junker P, Østergaard M, Ejbjerg BJ, Jacobsen S, et al. Radiographic progression and remission rates in early rheumatoid arthritis—MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis. 2010;69:1789–95.
Bøyesen P, Haavardsholm EA, Østergaard M, van der Heijde D, Sesseng S, Kvien TK. MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis. 2011;70:428–33.
Bøyesen P, Haavardsholm EA, van der Heijde D, Østergaard M, Hammer HB, Sesseng S, et al. Prediction of MRI erosive progression: a comparison of modern imaging modalities in early rheumatoid arthritis patients. Ann Rheum Dis. 2011;70:176–9.
Benton N, Stewart N, Crabbe J, Robinson E, Yeoman S, McQueen FM. MRI of the wrist in early rheumatoid arthritis can be used to predict functional outcome at 6 years. Ann Rheum Dis. 2004;63:555–61.
Zheng S, Robinson E, Yeoman S, Stewart N, Crabbe J, Rouse J, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65:607–11.
McQueen F, Beckley V, Crabbe J, Robinson E, Yeoman S, Stewart N. Magnetic resonance imaging evidence of tendinopathy in early rheumatoid arthritis predicts tendon rupture at six years. Arthritis Rheum. 2005;52:744–51.
Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73.
Martinez-Martinez MU, Cuevas-Orta E, Reyes-Vaca G, Baranda L, Gonzalez-Amaro R, Abud-Mendoza C. Magnetic resonance imaging in patients with rheumatoid arthritis with complete remission treated with disease-modifying antirheumatic drugs or anti-tumour necrosis factor alpha agents. Ann Rheum Dis. 2007;66:134–5.
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67.
Roemer FW, Eckstein F, Guermazi A. Magnetic resonance imaging-based semiquantitative and quantitative assessment in osteoarthritis. Rheum Dis Clin N Am. 2009;35:521–55.
Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90.
Kornaat PR, Ceulemans RYT, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)—inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102.
Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston-Leeds Osteoarthritis Knee Score). Ann Rheum Dis. 2008;67:206–11.
Biswal S, Hastie T, Andracchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–92.
Lynch JA, Roemer FW, Nevitt MC, Felson DT, Niu J, Eaton CB, et al. Comparison of BLOKS and WORMS scoring systems. Part I. Cross-sectional comparison of methods to assess cartilage morphology, meniscal damage and bone marrow lesions on knee MRI: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18:1393–401.
Roemer FW, Lynch JA, Niu J, Zhang Y, Crema MD, Tolstykh I, et al. A comparison of dedicated 1.0 T extremity MRI versus large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2010;18:168–74.
Wildi LM, Raynauld JP, Martel-Pelletier J, Beaulieu A, Bessette L, Morin F, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011; doi:10.1136/ard.2010.140848.
Garnero P, Peterfy C, Zaim S, Schoenharting M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis. A three-month longitudinal study. Arthritis Rheum. 2005;52:2822–9.
Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–35.
Kornaat PR, Kloppenburg M, Sharma R, Botha-Scheepers SA, Le Graverand MPH, Coene LNJ, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol. 2007;17:3073–8.
Davies-Tuck ML, Wluka AE, Wang Y, English DR, Gilles GG, Cicuttini F. The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann Rheum Dis. 2009;68:904–8.
Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68:1461–5.
Wildi LM, Raynauld JP, Martel-Pelletier J, Abram F, Dorais M, Pelletier JP. Relationship between bone marrow lesions, cartilage loss and pain in knee osteoarthritis: Results from a randomised controlled clinical trial using MRI. Ann Rheum Dis. 2010;69:2118–24.
Felson DT, Chaisson CE, Hill CL, Totterman SMS, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–9.
Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92.
Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–9.
Yusuf E, Kortekaas MC, Watt I, Huizinga T, Kloppenburg M. Do knee abnormalities visualized on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2011;70:60–7.
Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6.
Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21.
Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74.
Kothari A, Guermazi A, Chmiel JS, Dunlop D, Song J, Almagor O, et al. Within-subregion relationship between bone marrow lesions and subsequent cartilage loss in knee osteoarthritis. Arthritis Care Res. 2010;62:198–203.
Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the Multicenter Osteoarthritis Study. Radiology. 2009;252:772–80.
Englund M, Guermazi A, Roemer FW, Yang M, Zhang Y, Nevitt MC, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST study. Ann Rheum Dis. 2010;69:1796–802.
Wluka AE, Hanna F, Davies-Tuck M, Wang Y, Bell RJ, Davis SR, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68:850–5.
Roemer FW, Javaid MK, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–74.
Loeuille D, Rat AC, Goebel JC, Champigneulle J, Blum A, Netter P, et al. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2009;17:1186–92.
Baker K, Grainger A, Niu J, Clancy M, Guermazi A, Crema M, et al. Relation of synovitis to knee pain using contrast-enhanced MRI. Ann Rheum Dis. 2010;69:1779–83.
Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. 2011; doi:10.1136/ard.2010.139618.
Østergaard M, Stoltenberg M, Gideon P, Sorensen K, Henriksen O, Lorenzen I. Changes in synovial membrane and joint effusion volumes after intraarticular methylprednisolone. Quantitative assessment of inflammatory and destructive changes in arthritis by MRI. J Rheumatol. 1996;23:1151–61.
Brandt KD, Mazzuca SA, Buckwalter KA. Acetaminophen, like conventional NSAIDs, may reduce synovitis in osteoarthritic knees. Rheumatol (Oxford). 2006;45:1389–94.
Rhodes LA, Grainger AJ, Keenan AM, Thomas C, Emery P, Conaghan PG. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MR in knee osteoarthritis. Rheumatology. 2005;44:1569–73.
Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–501.
Fotinos-Hoyer AK, Guermazi A, Jara H, Eckstein F, Ozonoff A, Khard H, et al. Assessment of synovitis in the osteoarthritic knee: comparison between manual segmentation, semiautomated segmentation, and semiquantitative assessment using contrast-enhanced fat-suppressed T1-weighted MRI. Magn Res Med. 2010;64:604–9.
Funding sources
The Canadian Arthritis Society: National Research Initiative Award; Alberta Heritage Foundation for Medical Research; Walter L. and Johanna Wolf Foundation, Zurich, Switzerland; Foundation for Scientific Research at the University of Zurich, Switzerland
Competing interests
The authors declare that they have no competing interests in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, U., Østergaard, M., Lambert, R.G.W. et al. The impact of MRI on the clinical management of inflammatory arthritides. Skeletal Radiol 40, 1153–1173 (2011). https://doi.org/10.1007/s00256-011-1204-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-011-1204-5