Abstract
Chemical weathering was investigated by collecting samples from five selected weathering profiles in a high elevation granitic environment located in Seoul, Korea. The overall changes of chemistry and mineralogical textures were examined reflecting weathering degrees of the samples, using polarization microscopy, X-ray diffraction (XRD), electron probe micro analysis (EPMA), X-ray fluorescence spectroscopy (XRF), and inductively coupled plasma–mass spectroscopy (ICP–MS). The chemical distribution in the weathering profiles shows that few trace elements are slightly immobile, whereas most major (particularly Ca and Na) and trace elements are mobile from the beginning of the granite weathering. On the other hand, there were mineralogical changes initiated from a plagioclase breakdown, which shows a characteristic circular dissolved pattern caused by a preferential leaching of Ca cation along grain boundaries and zoning. The biotite in that region is also supposed to be sensitive to exterior environmental condition and may be easily dissolved by acidic percolated water. As a result, it seems that some rock-forming minerals in the granitic rock located in Seoul are significantly unstable due to the environmental condition of acidic rainfall and steep slopes, where they are susceptible to be dissolved incongruently leading some elements to be highly depleted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical weathering of rocks is one of the major processes that modify the earth’s surface and is one of the vital processes in the geochemical distribution of elements. The rate and nature of chemical weathering are governed by many variables such as parent-rock type, topography, leaching conditions and biological activity (Nesbitt et al. 1980; Chen et al. 1997; Kirschbaum et al. 2005; Green et al. 2006). The mobilization and redistribution of major and trace elements during weathering is particularly complicated because these elements are affected by various processes such as dissolution of primary minerals, formation of secondary phases, coprecipitation and ion exchange on various minerals (Nesbitt 1979; Lee 1994; Islam et al. 2002; Oliva et al. 2004; Taboada et al. 2006).
The purpose of this study is to investigate the chemical weathering of the granite located in Seoul, a metropolitan city in Korea. The granite has several weathering profiles with thicknesses in the range of 1–4 m distributed sparsely in a mountainous area. These profiles include geological structures with mixed soils and rocks originating from weathered parent rocks and remaining in situ. We want to study the weathering of the granite with emphasis on two points. One is the role of acidic rainwater (pH < 5.5), which is initiated from the oxidation of carbon, sulfur, and nitrogen, which result from fossil fuel combustion around a big city like Seoul, to the weathering of rock. The other is the water–rock interaction resulting in the mobilization and redistribution of selected elements.
Study area
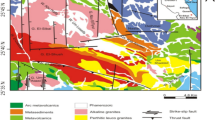
Bughan Mountain (837 m), located in the northern area of Seoul city, trending N 40°E (Fig. 1a), is part of the so-called Seoul granite of Jurassic age (Hong et al. 1982). It intruded into the Precambrian gneiss complex composed of banded biotite gneiss and limestone. The elevation of the area, including the investigated weathering profiles, ranges between 400 and 837 m, which is quite high when compared with some areas around the city. The granite shows a characteristic topography such as rounded hills and domes (Fig. 1b). It is medium- to coarse-grained equigranular biotite granite. It consists of quartz, plagioclase, microcline and biotite with minor muscovite, chlorite and opaque minerals (Table 1). Plagioclase occurs as subhedral phenocrysts with compositional zoning. Perthitic and myrmekitic intergrowths are commonly found.
a The location of the study area (Bughan Mt.) and its sampling sites (LS, LK, LY, SY, and FC), which are the weathering profiles of granite. The represented area wholly consists of a homogeneous granitic rock. The dotted lines mean several administrative divisions, and the solid lines indicate some stream lines. b The granite outcrops of the mountain top showing considerable steep slope. c A weathered saprolitic profile having some unweathered remnants
Mean annual temperature and precipitation of that region are 11.8°C and 1,369 mm, respectively. The mountain is characterized by thin acidic soils, steep slopes, and unreactive rock type (granitic rock), which produces surface or ground waters of low alkalinity (HCO3 − = ∼5.34 ppm). Owing to the limited soil–water contact in this area, the chemical weathering of bedrock minerals is the primary process by which incoming acidity is neutralized.
Five weathering profiles were selected for the study of granitic rock weathering. Most of them have heights of 1–4 m with unweathered remnants in parts (Fig. 1c). The studied sites are roughly homogeneous and have not had any catastrophic and dynamic events. There is no significant difference between them, because they have their homogeneous rock component without any fault, dikes, and slight metamorphism. They have similar rock-forming minerals, geostructure, and vegetation, except for the FC profile, which has been highly weathered, containing considerable secondary clay minerals. Samples were collected from each profile with a different weathering degree.
Analytical methods
For a textural investigation of the fresh and weathered rocks, air-dried samples were impregnated with Araldite epoxy resin under vacuum. The impregnated samples were air-dried for 2 weeks of air-drying, and then polished with 0.05 μm alumina paste for microscopic observation and electron microprobe analysis. Microprobe analysis was carried out by wavelength dispersive X-ray analysis at an acceleration voltage of 15 kV using a JEOL JCXA 733 superprobe instrument.
For analysis of bulk rock chemical compositions, each sample was crushed, pulverized, homogenized, and then analyzed by X-ray fluorescence spectroscopy (XRF) for major elements, and by inductively coupled plasma–mass spectroscopy (ICP–MS) for trace elements.
To analyze the concentration of some major elements in streams of that region, water samples from different streams were taken and analyzed by the ICP–MS.
X-ray diffraction (XRD) analyses were carried out for the identification of constituent minerals using a Rigaku RAD3-C automatic horizontal goniometer diffractometer equipped with a scintillation counter and a Cu X-ray tube operating at 40 kV/30 mA in a continuous scan mode. Slit set was 1°–0.15 mm–1°, and the scanning speed was 2° min−1.
Results
Microscopic features of weathered primary minerals
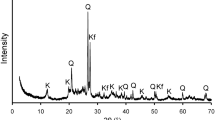
Examination by electron microprobe revealed that, in our acidic environment, the mineral dissolution proceeds from the edges, especially in the case of layered silicates such as biotite (Fig. 2), where thin and amorphous ferrihydrite precipitates are preferably formed on the primary mineral surface. As weathering proceeds, various clay minerals are developed as secondary products of primary minerals in granite. At initial, 2:1 clay minerals such as smectite are prevailed on the rock surface, resulting from the dissolution and transformation of feldspar and biotite (Fig. 3a). In highly weathered granite, however, poorly crystallized 1:1 kaolinite is predominant as secondary mineral (Fig. 3b). The smectite is further transformed to kaolinite or halloysite if the microenvironment underwent more intense leaching.
X-ray diffraction patterns of the clays, which were separated from slightly weathered granite (a) and intensively weathered one (b). In a, smectite (S) is predominant with minor vermiculite (V), but poorly crystalline hydrated kaolinite which was dried to 115°C is prevailed in b. EG ethylene glycol treatment
The weathering product of feldspar is commonly smectite. Textures of weathered plagioclase at the early alteration stage are circular or square outlined along structural boundaries with compositional differences (Fig. 4). The plagioclase phenocrysts in the microphotographs are discontinuously zoned, having a calcic core mantled by a more sodic rim. The crystals are discontinuously zoned and have multiple zoning. As the weathering becomes more progressive, the widths of dissolved parts are gradually enlarged, and several microfractures are developed around them. Water penetrates and diffuses into the compositional zoning resulting in the formation of dissolved stripes through a structural destruction. These dissolved stripes gradually extend to fresh parts of the mineral and eventually become much large voids.
Weathering of chlorite in acid soils proceeds through a transformation to vermiculite with a loss of Fe and Mg. Muscovite is almost not altered with only slight weathering margin of grains.
Behavior of elements
The choice of a reference frame is the most important assumption for any mass-balance calculation of an open system (Middelburg et al. 1988). Ti can be selected as the element for normalization on the basis of its low solubility and its presence in several stable primary minerals such as rutile and ilmenite. The percentage changes of elements relative to TiO2 as a function of the degree of weathering are presented graphically in Fig. 5. The percentage increase or decrease of any element X in a sample, relative to fresh parent rock can be calculated according to the following formula (Nesbitt 1979):
In order to compare various weathering profiles to one another, we need an independent measure for the degree of weathering of each sample. During weathering, the chemistry of a rock is modified. Many investigators have developed chemical techniques to quantify and classify the mineralogical and chemical characteristics of weathered rocks (Ruxton 1968; Parker 1970; Vogel 1973; Nesbitt and Young 1982; Middelburg et al. 1988; Gupta and Rao 2001; Kim and Park 2003; Lee and Yi 2007). Indeed, some of these weathering indexes have been specifically developed for granites. We introduced the parameter weathering degree (WD) suggested by Middelburg et al. (1988), which is suitable to apply in our acidic environment and defined as:
where R = (CaO + Na2O + K2O)/(Al2O3 + H2O).
The ratio R is a measure for the degree of feldspar breakdown, including the accumulation and formation of clay minerals. The parameter WD approaches 1 when minerals such as gibbsite and kaolin prevail, and is equal to 0 for fresh rocks. Chemical analyses were performed on each of the levels in the profiles studied; the concentration of major and trace elements present in the profile samples is shown in Tables 2 and 3.
Behavior of major elements
At the initial stage of the granitic rock weathering, the leaching of Ca and Na is relatively higher as compared with that of other constituent major elements (Fig. 5). The migration trends showing the significant loss of Ca and Na in the profiles probably result from first and rapid breakdown of plagioclase. However, other major elements such as Si, Al, K and Mg are almost similar in their behavior patterns, displaying a slow loss from the parent rock during the weathering (Fig. 5). Fe is considered to be essentially immobile (Nesbitt 1979), and may be precipitated as Fe-oxides or -oxyhydroxides on the primary mineral surfaces. The percentage loss on ignition (LOI) (Table 2) can be used to determine the degree of weathering (Suoeka et al. 1985). It is likely that LOI may be presumably related with H2O and correlated with the amount of clay developed during weathering. The H2O component (LOI) increases in the profiles during the weathering progress, presenting a possibility to be used as one of weathering criteria. The general trend of the elemental mobility in our profiles follows the order: Ca > Na > Mg ≥ Si ≥ Al ≥ K > Fe > Ti.
Behavior of trace elements
In all the weathering profiles investigated, some heavy metals such as Ni, Cu and Co are, at least with respect to Ti, considerably immobile and accumulated (Fig. 5). U shows initial enrichments, and later progressive depletions (Fig. 5). On the other hand, alkaline earth (e.g., Be, Sr) and rare earth (e.g., La, Nd, Sm, Eu) elements are slightly depleted with weathering progress (Table 3). Most of them seem to be slightly leached out without being largely incorporated into, or precipitated on iron-oxides and clay minerals. It is assumed that the phenomena result from the lack of soil layers containing secondary minerals sufficiently developed in situ to retard the trace elements to be removed from parent rocks. Koons et al. (1980) insisted that degree of association of trace metals with Fe-oxides and clay minerals is important to be enriched in profile during rock weathering. The presence or absence of secondary minerals in weathering suites may be, therefore, a factor of major importance for the behavior of trace elements in our study area.
Discussion
The Bughan Mountain is characterized by thin acidic soils, steep slopes, and unreactive granitic rock (Lee 1994). According to Velbel (1992), the most sensitive landscape to acidification is that underlain by crystalline silicate bedrock, especially granitic bedrock. This is because the silicate minerals do not react with percolating solutions quickly enough to neutralize the added acidity or to contribute base cations and acid-neutralizing capacity to the soil exchange complex and natural waters, respectively. The immediate contact of acidic rainfall to the rock will cause the surface state to be polarized and weaken for a moment, instead of leading to the formation of mature secondary minerals in situ. In frequent exposures to acid rainfall, the cation release rates or amounts from primary minerals would be higher as compared with the case of normal neutralized condition. There is a significant loss of most major elements, in particular Ca, Na and Si, in the Bughan Mountain due to the acidic environmental condition (Table 4). However, few trace elements (e.g., Cd and U) are incorporated in or adsorbed on secondary minerals (Fig. 6). The elements are largely enriched in the clays fraction and depleted in the sand fractions. This means that the weathering process occasionally involves the selective leaching of ions and their subsequent concentration in secondary phases. The development of secondary minerals is important, rather than that of sandy soils, for attracting and retaining considerable trace elements released from parent rocks (Nesbitt and Markovics 1997; Taboada et al. 2006). However, the thin soils with limited amounts of secondary minerals in the study area did not sufficiently play an important role in terms of restraining the elemental release from the profiles.
During weathering of crystalline rocks, rock-forming minerals are partly dissolved and hydrolysis and hydration take place (Islam et al. 2002). Dissolution and weathering of minerals play an important role in element transport in granitic rocks because they redistribute elements between solid and solution. Some authors (Jeong 2000; White and Brantley 2003; Wilson 2004) have suggested that the mineral dissolution is selective in natural condition. In our samples, plagioclase feldspar is much more weathered than K-feldspar in all the sections. Furthermore, the plagioclase in the samples frequently shows circularly or squarely dissolved outlines developed in its interior as low weathering degree prevails (Fig. 4). Long time ago, Clayton (1988) reported a possibility for the preferential weathering of anorthite for the explanation of large Ca2+ source in Idaho. Some authors also observed that plagioclase feldspars are commonly zoned and the more calcic core could be often easily weathered (Inskeep et al. 1991; Williams et al. 1993; Oliva et al. 2004). However, few studies have directly investigated the selective weathering patterns of calcic plagioclases, which have quite distinctive dissolved pathways along Ca component (Fig. 4) as well as obvious intrusive features by water (Fig. 7). The intrusive diffusion of water presumably begins at a certain defect or dislocation site existing on the mineral surface or grain boundary and causes soluble components (e.g., preferentially Ca cation) to be easily displaced by H+ ions in water, resulting in the formation of the characteristic dissolved pattern. Similarly, Banfield and Eggleton (1990) observed that feldspar destabilization starts on the crystal surface at already structurally disturbed microsites (i.e., dislocations and cleavages). Consequently, the compositionally different region in the plagioclase with higher dislocation densities, for instance, may influence the grain to be selectively dissolved by the intrusive diffusion of hydrogen ions, and the cation leaching would increase from there.
Conclusions
The chemical composition and mineralogy of the studied profiles show characteristic changes with the weathering progress. The migration of major and trace elements is considerable in the acidic environment and exhibits the less accumulation of them onto the clay fraction. In addition, the textures of weathered silicates are much selectively dissolved and appear as sensitive as the exterior acidic environment, forming much more amorphous phases rather than crystallized ones. In addition, the steep slopes of the geographic site also interfere with the mineral–water interaction to be sufficient. As a result, some rock-forming minerals in the granitic rock located in Seoul are significantly unstable due to the abnormal environmental condition, where they are susceptible to be dissolved incongruently leading some elements to be highly depleted.
References
Banfield JF, Eggleton RA (1990) Analytical transmission electron microscope studies of plagicoclase, muscovite and K-feldspar weathering. Clays Clay Miner 38:77–89
Chen P, Lin M, Zheng Z (1997) On the origin of the name kaolin and the kaolin deposits of the Kauling and Dazhou areas, Kiangsi, China. Appl Clay Sci 12:1–25
Clayton JL (1988) Some observations on the stoichiometry of feldspar hydrolysis in granitic soil. J Environ Qual 17:153–157
Green EG, Dietrich WE, Banfield JF (2006) Quantification of chemical weathering rates across an actively eroding hillslope. Earth Planet Sci Lett 242:155–169
Gupta AS, Rao KS (2001) Weathering indices and their applicability for crystalline rocks. Bull Eng Geol Env 60:201–221
Hong SH, Lee BJ, Hwang SK (1982) Explanatory text of the geological map of Seoul sheet (1:50,000). Korea Institute of Energy and Resources, Seoul
Inskeep WP, Nater EA, Bloom PR, Vandervoort DS, Erich MS (1991) Characterization of laboratory weathered labradorite surfaces using X-ray photoelectron spectroscopy and transmission electron microscopy. Geochim Cosmochim Acta 55:787–800
Islam MR, Stuart R, Risto A, Vesa P (2002) Mineralogical changes during intense chemical weathering of sedimentary rocks in Bangladesh. J Asian Earth Sci 20:889–901
Jeong GY (2000) The dependence of localized crystallization of halloysite and kaolinite on primary minerals in the weathering profile of granite. Clays Clay Miner 48:196–203
Kim S, Park HD (2003) The relationship between physical and chemical weathering indices of granites around Seoul, Korea. Bull Eng Geol Env 62:207–212
Kirschbaum A, Martínez E, Pettinari G, Herrero S (2005) Weathering profiles in granites, Sierra Norte (Córdoba, Argentina). J South Am Earth Sci 19:479–493
Koons RD, Helmke PA, Jackson ML (1980) Association of trace elements with iron oxides during rock weathering. Soil Sci Soc Am J 44:155–159
Lee CH, Yi JE (2007) Weathering damage evaluation of rock properties in the Bunhwangsa temple stone pagoda, Gyeongju, Republic of Korea. Environ Geol 52:1193–1205
Lee SY (1994) Environmental mineralogy of granite weathering in the Bughan mountain area. Seoul National University, MS, Korea
Ministry of Environment (Korea) (2006) Annual report for the air quality, Korea. http://www.me.go.kr
Middelburg JJ, Van Der Weijden CH, Woittiez JRW (1988) Chemical processes affecting the mobility of major, minor and trace elements during weathering of granitic rocks. Chem Geol 68:253–273
Nesbitt HW (1979) Mobility and fractionation of rare earth elements during weathering of a granodiorite. Nature 279:206–210
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 299:715–717
Nesbitt HW, Markovics G (1997) Weathering of granodioritic crust, long-term storage of elements in weathering profiles, and petrogenesis of siliciclastic sediments. Geochim Cosmochim Acta 61:1653–1670
Nesbitt HW, Markovics G, Price RC (1980) Chemical processes affecting alkalis and alkaline earths during continental weathering. Geochim Cosmochim Acta 44:1659–1666
Oliva P, Dupré B, Martin F, Viers J (2004) The role of trace minerals in chemical weathering in a high-elevation granitic watershed (Estibère, France): chemical and mineralogical evidence. Geochim Cosmichim Acta 68:2223–2244
Parker A (1970) An index of weathering for silicate rocks. Geol Mag November:501–504
Ruxton PP (1968) Measures of the degree of chemical weathering of rock. J Geol 76:518–527
Suoeka T, Lee IK, Hurmatsu M, Imamura S (1985) Geomechemical properties and engineering classification for decomposed granite soils in Kaduna district, Nigeria. In: Proceedings of 1st international conference on geomechanics in tropical lateritic and saprolitic soils, Brasilia, Publ 1, pp 175–186
Taboada T, Cortizas AM, García C, García-Rodeja E (2006) Uranium and thorium in weathering and pedogenetic profiles developed on granitic rocks from NW Spain. Sci Total Environ 356:192–206
Velbel MA (1992) Geochemical mass balances and weathering rates in forested watersheds of the Southern Blue Ridge. III. Cation budgets and the weathering rate of amphibole. Am J Sci 292:58–78
Vogel DE (1973) Precambrian weathering in acid metavolcanic rocks from the Superior Province, Villebond Township, south central Quebec. Can J Earth Sci 12:2080–2085
White AF, Brantley SL (2003) The effect of time on the weathering of silicate minerals: why do weathering rates differ in the laboratory and field? Chem Geol 202:479–506
Williams MW, Brown AD, Melack JM (1993) Geochemical and hydrologic controls on the composition of surface water in a high-elevation basin, Sierra Nevada, California. Limnol Oceanogr 38:775–797
Wilson MJ (2004) Weathering of the primary rock-forming minerals: processes, products and rates. Clay Miner 39:233–266
Acknowledgments
The authors express a gratitude to Dr. Seok Hoon Lee for his trouble to analyze our samples using ICP–MS and XRF in the KBSI (Korea Basic Science Institute), and sincerely appreciate the constructive reviews and suggestions by the reviewers (Dr. T. Martín-Crespo and A. Kirschbaum).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.Y., Kim, S.J. & Baik, M.H. Chemical weathering of granite under acid rainfall environment, Korea. Environ Geol 55, 853–862 (2008). https://doi.org/10.1007/s00254-007-1037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1037-7