Abstract
Concentrations of total Hg in sediments near six drilling sites in the Gulf of Mexico were elevated well above average background values of 40–80 ng/g. The excess Hg was associated with barite from discharged drilling mud. In contrast with total Hg, concentrations of methylmercury (MeHg) in these sediments did not vary significantly at nearfield (<100 m) versus farfield (>3 km) distances from the drilling sites. Observed variability in concentrations of MeHg were related to local differences in redox state in the top 10 cm of sediment. Low to non-detectable concentrations of MeHg were found in nearfield sediments that were anoxic, highly reducing and contained abundant H2S. At most drilling sites, nearfield samples with high concentrations of total Hg (>200 ng/g) had similar or lower amounts of MeHg than found at background (farfield) stations. Higher values of MeHg were found in a few nearfield sediments at one site where concentrations of TOC were higher and where sediments were anoxic and moderately reducing. Overall, results from this study support the conclusion that elevated concentrations of MeHg in sediments around drilling sites are not a common phenomenon in the Gulf of Mexico.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concentrations of total Hg in sediments adjacent to offshore drilling sites in the Gulf of Mexico are often 2–10 times higher than in nearby background sediment (Trocine and Trefry 1983; Neff 2002). This excess Hg is commonly associated with barite (BaSO4) as a component of spent drilling mud that is discharged during drilling. Present regulations set the upper limit for total Hg in barite at 1 μg/g (1,000 ng/g, United States Environmental Protection Agency 1993). Much less is known about the degree to which excess total Hg in sediment around offshore drilling sites is transformed into methylmercury (MeHg) and whether conditions in sediments near drilling sites are more conducive to the formation of MeHg than those in nearby ambient sediments.
Available evidence supports the claim that Hg associated with barite is segregated in separate sulfide minerals that are mined in trace amounts with barite ore (Kramer et al. 1980; Trefry and Smith 2003). If Hg is introduced to marine sediments with sulfides, its long-term fate depends on the stability of the sulfides over time. Sulfide stability is dependent on the redox state (Eh) and pH of the sediment. When redox potentials are below ∼0 mV, Hg is thermodynamically stable in various sulfide phases (Fleisher 1970). If these sulfide phases are subjected to a more oxidizing environment, thermodynamics favors the dissolution of sulfide phases that may contain Hg. Previous studies in various sedimentary environments show that optimum conditions for methylation of Hg occur in marine sediments that are enriched with organic carbon and nutrients, anoxic, yet low in concentrations of dissolved sulfide (Gilmour et al. 1992; Gagnon et al. 1996). The presence of sulfide is thought to inhibit methylation because Hg precipitates as HgS (Gilmour et al. 1998).
This study was carried out to determine whether concentrations of MeHg were enhanced in sediments around drilling sites where concentrations of total Hg were typically higher than those in background sediments. The Hg effort was a component of ongoing research on the environmental effects associated with the discharge of cuttings drilled with synthetic-based mud (SBM) in the Gulf of Mexico. Discharges of cuttings with SBM (olefin-rich organic substances) or increased deposition of organic matter from organisms inhabiting offshore structures may create a more reducing sedimentary environment and render it more favorable for methylation of Hg.
Methods
Field sampling and analysis
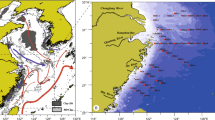
Sediments were collected from areas surrounding six offshore-drilling sites during 8–20 May 2002 (Fig. 1). Three of the sites were situated at water depths of 60–119 m on the continental shelf and three were at depths of 534–556 m on the upper slope (Fig. 1; Table 1). Platforms were present at three of the sites (MP299, MP288 and EI346) during sampling and seafloor templates or wellheads were in place at the remaining three sites. Surface sediments (0–2 cm) were collected at six random nearfield (NF) stations within 100 m of each drilling site and at two fixed (discretionary) stations within 100 m of each drilling site. The discretionary (D) stations were chosen based on results from previous sampling trips that showed elevated concentrations of Ba at specific locations. Data for the D stations were grouped with data for the NF stations from the same site in this paper. Subsurface samples (2–20 cm) were collected from two D stations at all six sites. Subsurface samples also were collected at one random NF station for three drilling sites (MP299, MP288 and MC496). Surface samples were collected from six random, farfield (FF) locations at >3 km from each drilling site and subsurface samples were collected at one FF location from each site. In addition, surface samples were collected from each drilling site at 2–6 midfield (MF) locations that were at distances of 100–250 m from the drill site.
Map showing locations of offshore drilling sites in the Gulf of Mexico where sediment samples for this study were collected. Exact locations and water depths for each site are given in Table 1
Sediments were collected using a stainless steel box corer (50 cm × 50 cm × 50 cm). The surface 2 cm of sediment were removed using a Teflon spatula and ∼30 ml of sediment were placed in 50-ml, polystyrene vials (for total Hg) and ∼30 ml of sediment were placed in 60-ml Series 300 I-CHEM, cleaned glass containers (for MeHg) and frozen immediately. Subsurface samples were collected from the box core by carefully pushing a Teflon tube into the sediment. The sediment was extruded from the bottom in 2-cm increments. The outer few millimeters of sediment from each layer were removed and the remaining portion was placed in polystyrene and glass containers. All sampling equipment was cleaned with acid and rinsed with distilled-deionized water (DDW). The Teflon tubes were decontaminated between each use with soap, water, nitric acid, water, acetone and DDW.
One core from each station was immediately analyzed for oxygen using a 5-cm long microprobe (Microelectrodes, Inc. MI-730 O2 probe) that was mounted on a microscope stage fixed vertically above the core. By lowering the microscope stage in millimeter increments, oxygen measurements were taken to a depth with no detectable oxygen (<0.03 mg/l). Next, Eh and pH were measured through holes pre-drilled at 2-cm intervals in the wall of the core tube. The holes were covered with tape during shipboard sampling of the box core, and the tape was removed immediately before inserting a probe. The Eh was measured using an Orion Model 96-78-00 Platinum Redox Electrode and the pH and temperature measurements were carried out using a Sentron Red Line pH probe. All probes were calibrated prior to use for each core.
In addition to the data collected with oxygen, redox and pH probes, samples of interstitial water were collected at selected sites. Interstitial water was obtained by taking one 7-cm diameter subcore and transferring it to a whole core squeezer, similar to that described by Bender et al. (1987), for pore water extraction. Samples (∼10 ml) were collected directly in acid-washed glass syringes at 1-cm intervals in the uppermost 5 cm, 2-cm intervals from 5 to 13 cm, and 3-cm intervals below 13 cm. Interstitial water was analyzed for dissolved ammonia, total sulfide, iron and manganese using techniques described in Gu et al. (1987). Concentrations of nitrate and sulfate in interstitial water were determined using a Dionex DX-600 ion chromatograph (IC).
Laboratory analyses
In the laboratory, sediment samples were thawed, brought to room temperature and homogenized in the original vial using a Teflon rod. Wet sediments of 2–4 g were transferred to polypropylene-copolymer centrifuge tubes to determine the total Hg content. Separate samples were freeze-dried to determine water content. Concentrations of total Hg were determined for sediments that were heated with 4 ml nitric acid (HNO3) and 2 ml sulfuric acid (H2SO4) for ∼1 h in a 90°C water bath as described by Adeloju et al. (1994). A sediment certified reference material (CRM MESS-2), obtained from the National Research Council of Canada, was used to determine accuracy for concentrations of total Hg.
The final solutions and procedural blanks were analyzed by cold-vapor atomic absorption spectrometry (CVAAS) using a Laboratory Data Control Mercury Monitor with manual injection of 0.5 ml of solution.
Methylmercury in sediments was isolated by acid bromide/methylene chloride extraction and the aqueous phase was analyzed by ethylation, isothermal GC separation, and CVAFS detection following methods from Bloom and Crecelius (1983) and Bloom (1989). Concentrations of Al and Ba in sediments and the CRM MESS-2 were determined using 20 mg of sample that were digested with HClO4–HNO3–HF and diluted to 20 ml. For samples with very high concentrations of Ba, based on the presence of residual white solid after digestion, a smaller mass of sample (<10 mg of sediment) was digested and analyzed for Ba. Concentrations of Ba were determined by inductively-coupled plasma-mass spectrometry (ICP-MS) using a Perkin-Elmer ELAN 5000 instrument and analysis for Al was by flame atomic absorption spectrometry (FAAS) using a Perkin-Elmer Model 4000 instrument.
The total organic carbon (TOC) content of the sediments was determined following treatment with H3PO4 to remove inorganic carbon. Then, 200–400 mg of carbonate-free sediment were combusted, after addition of powdered vanadium pentoxide as a catalyst, at 900°C in a Shimadzu TOC-5050A instrument linked to a solid sampling module (SSM-5000A). A calibration curve was constructed with pure sucrose and checked every ten samples by analyzing the certified reference material (CRM) MESS-2.
Quality Control measures included balance calibration, instrument calibration (FAAS, CVAAS, ICP-MS, IC, TOC analyzer), matrix spike analysis for each metal, duplicate sample analysis, CRM analysis, procedural blank analysis and standard checks. With each batch of up to 40 samples, two procedural blanks, two CRMs, two duplicate samples and two matrix-spiked samples were analyzed. Results for concentrations of metals and TOC in the CRMs agreed within the 95% confidence interval of certified concentrations. Precision for the various analytes (expressed as a coefficient of variation, CV) was as follows: total Hg (1.5%), MeHg (8%), Al (1%), Ba (2%), TOC (2%).
Results and discussion
Total mercury in sediments
Concentrations of total Hg in surface sediments (0–2 cm) ranged from 11 to 92 ng/g for FF samples and 41 to 422 ng/g for NF samples (Table 2). Overall, concentrations of total Hg were significantly higher at NF stations relative to FF stations for all drilling sites except MP299 (t test at α = 0.05, double-tailed). For drilling sites EI346, EW963 and GC112, average concentrations of total Hg at NF stations were 3.9, 2.5 and 3.1 times greater than at FF sites. The distinctly higher concentrations of total Hg at the NF sites were observed whether using just the surface 2 cm of sediment or surface plus subsurface sediment. Only one subsurface sample from this study contained total Hg at a concentration that was greater than the maximum value of 422 ng/g found for surface sediments (site EW963, at 2–4 cm, total Hg 558 ng/g).
Concentrations of total Hg at MF and FF stations were not significantly different except at site MP288 where values for some sandy FF sediments distorted the mean value for total Hg. Overall, total Hg values in sediments at the six drilling sites from this study were representative of concentrations of total Hg in sediments at most sites where offshore activities have been carried out in the Gulf of Mexico as total Hg concentrations >1,000 ng/g are uncommon (Neff 2002).
Background concentrations of total Hg were higher in upper slope sediments (74 ± 7 ng/g) than in shelf sediments (45 ± 20 ng/g). The background ratio of total Hg/Al was 25% higher for upper slope sediments (9.1 ± 1.7 × 10−6) than for shelf sediments (7.3 ± 1.0 × 10−6). This difference is mostly due to the slower sedimentation rate on the upper slope and thus an increased time for particles to scavenge Hg from seawater. Concentrations of total Hg in background (FF) sediment from the Gulf of Mexico were positively correlated with concentrations of Al (Fig. 2). In some studies, the relationship between TOC and Hg has been strong and the natural relationship for total Hg in sediment has been developed using concentrations of Hg versus TOC or Hg versus TOC + Al (Lindberg and Harriss 1974; Kang et al. 2000). However, for sediments in this study, concentrations of TOC were <1% in all FF samples and a weak correlation was observed between TOC and total Hg (r = 0.38). Thus, a plot of Al versus Hg with a good correlation coefficient (Fig. 2a, r = 0.91) was used to normalize concentrations of total Hg for FF shelf sediment from this study. Data from FF shelf stations were plotted separately from slope stations because of previously described differences in the Hg/Al ratio between shelf and slope sediments. Most concentrations of total Hg from the NF stations at sites MP299 and MP288 fall within the 95% prediction interval because these sites contained only small amounts of drilling mud (Fig. 2a). In contrast, many of the data points from NF stations at site EI346 show enrichment of total Hg relative to background concentrations (Fig. 2a). Figure 2b shows that concentrations of total Hg in many NF samples from upper slope sites GC112 and EW963 were significantly greater than concentrations found in FF samples.
Concentrations of Al versus total Hg in a all shelf sediments from sites MP299, MP288 and EI346, and b all upper slope sediments from sites MC496, EW963 and GC112. The solid lines are from linear regressions for farfield samples from the a shelf and b upper slope and dashed lines show 95% prediction intervals
A linear relationship between concentrations of Ba and total Hg sometimes can be used to calculate concentrations of total Hg associated with the barite used in drilling mud at a particular site. Barite has been specifically identified by X-ray diffraction in many NF samples from this study (Isphording et al. 2004). A strong linear relationship was observed for Ba versus total Hg in NF sediment from site EI346 (Fig. 3a). Background data for Ba and total Hg plot at <0.1% Ba. The strong correlation supports the previous statement that Ba (i.e., barite) is the primary variable correlated with the excess amount of total Hg in NF sediment from site EI346. If the line in Fig. 3a is extrapolated to pure barite at 58.8% Ba, the concentration of total Hg is 653 ng/g (obtained by substituting 58.8% Ba for x in the equation in Fig. 3a). Typical “industrial barite” contains 85–95% barite (i.e., 50–56% Ba). Based on the range of Ba levels in industrial barite (50–56% Ba, Trefry and Smith 2003), the average total Hg content of barite used at site EI346 is estimated at 594 ng/g (from the average of 563 ng/g at 50% Ba and 624 ng/g at 56% Ba). These estimated values are in line with regulations set by the United States Environmental Protection Agency (EPA) that allow a maximum Hg level of 1,000 ng/g in barite (United States Environmental Protection Agency 1993). Concentrations of Ba in sediment from NF stations at sites MP299 and MP288 were too low to permit a reasonable extrapolation to the total Hg content of any barite that may be present. The estimated average concentrations of total Hg in the industrial barite used at the upper slope sites (using an average of 53% Ba for industrial barite) are as follows: 413 ng/g for site MC496, 978 ng/g for site EW963 and 698 ng/g for site GC112 (Fig. 3), values that also are in line with EPA regulations. As previously stated, the excess total Hg in these samples is present as a separate sulfide phase that is an impurity associated with the original barite deposit (Trefry and Smith 2003).
Methylmercury in sediment
Concentrations of MeHg in surface sediments from this study ranged from 0.11 to 1.05 ng/g for FF samples and <0.03 to 2.7 ng/g for NF samples (Table 2). The grand mean ± standard deviations for MeHg in all samples (surface and subsurface) from this study were as follows: FF = 0.44 ± 0.27 (n = 62), MF = 0.49 ± 0.17 (n = 25) and NF = 0.43 ± 0.41 ng/g (n = 109). No statistically significant differences in concentrations of MeHg in surficial sediment between NF and FF stations were observed for any of the six sites studied (α = 0.05, double-tailed). When surface and subsurface data were combined, no significant differences in concentrations of MeHg at NF versus FF stations were observed for sites MP299, EI346, MC496, EW963 and GC112; however, MeHg concentrations were significantly higher at the NF versus FF stations for site MP288 due to the sandy composition of some FF samples (Table 2). No subsurface samples from any FF or NF stations contained higher concentrations of MeHg than found for surface sediments.
The data in Table 2 show the within-site and site-to-site variability in concentrations of MeHg and the generally higher amounts of MeHg at the upper slope stations. The mean value for MeHg in all FF sediment from the shelf was 0.28 ± 0.17 ng/g relative to a mean of about two times more MeHg (at 0.59 ± 0.26 ng/g) for FF sediments from the upper slope (Table 2). A similar trend was observed for all NF stations where average concentrations of MeHg were 0.28 ± 0.16 and 0.59 ± 0.52 ng/g, for shelf and upper slope sediments, respectively (Table 2). These trends in concentrations of MeHg are consistent with general trends for concentrations of total Hg (Table 2) with ∼60–70% higher values of total Hg at both NF and FF stations on the upper slope relative to the shelf. Concentrations of total Hg alone do not explain the variance in the data for MeHg as will be discussed in more detail below. However, concentrations of both MeHg and total Hg increase from shelf to upper slope sediments for the locations studied.
Concentrations of MeHg were below the detection limit of 0.03 ng/g in surface sediments from three NF stations at site EI346 (Table 2; Fig. 4a), even though concentrations of total Hg were >200 ng/g. High concentrations of total H2S (>1 mM) in interstitial water and low values for Eh (<−100 mV) suggest that excess Hg in NF sediments from site EI346 was not being converted to MeHg. Data for MeHg in samples from NF stations at site MC496 group close to data for the FF samples from the upper slope (Fig. 4b). However, the NF samples from site EW963, with high concentrations of total Hg, have MeHg values that are similar to or lower than those for FF sediments (Fig. 4b). This observation for site EW963 is similar to that made for site EI346 on the shelf and supports the argument that excess Hg associated with barite at site EW963 was not being converted to MeHg due to the presence of H2S in the sediment.
Concentrations of total Hg versus MeHg for sediments at a all farfield (FF) stations from the shelf and nearfield (NF) sediments from sites MP299, MP288 and EI346 and b all FF stations from the slope and NF stations from sites MC496 and EW963 and c all FF stations from the slope and NF stations from site GC112. The two lines on each graph show reference lines for concentrations of MeHg as a percent of total Hg
In contrast to the observations for NF samples from MC496 and EW963, seven NF samples from site GC112 contained higher concentrations of both total Hg and MeHg than observed at FF sites from the upper slope (Fig. 4c). However, an additional four NF samples from site GC112, with total Hg concentrations >100 ng/g, contained MeHg at values that were below the average of ∼0.6 ng/g for background sediments from the upper slope. The above trends show that enhanced concentrations of barite-related total Hg in NF sediments around drilling sites do not generally lead to higher values of MeHg, with the possible exception of several stations at site GC112. However, even at site GC112, the highest concentration of MeHg (2.7 ng/g) was only ∼3% of the ambient concentration of total Hg (79 ng/g) and thus the additional MeHg could be from either an anthropogenic or natural source of Hg. The grand average for the fraction of total Hg that was present as MeHg was 0.60 ± 0.31% for all shelf sediment, the same as the value of 0.60 ± 0.32% for all upper slope sediment.
For comparison, concentrations of MeHg ranged from 0.03 to 10 ng/g and total Hg values were as high as 783 ng/g in the sediments from Lavaca Bay, Texas, where a chlor-alkali facility was in operation during the late 1960s (Bloom et al. 1999). Kannan et al. (1998) reported a range of <0.001–0.32 ng/g for MeHg in various estuaries along the Gulf of Mexico coast of Florida. In studies of sediment cores from the Saguenay Fjord, Canada, Gagnon et al. (1996) showed that concentrations of MeHg rose from <0.1 ng/g in the oxic surface layers of sediment to ∼2 ng/g at 1–2 cm and >10 ng/g at 6–8 cm where total Hg values were 145, 175 and 700–800 ng/g, respectively. Concentrations of MeHg in sediment from this study in the Gulf of Mexico were below values from Lavaca Bay and were within the lower range of values reported for the Saguenay Fjord in Canada.
As previously discussed, production of MeHg may be enhanced by increased concentrations of TOC in the sediments. Values for TOC in surface sediments from this study ranged from 0.08 to 1.42% for FF stations and 0.67 to 4.1% for NF stations (Table 3). Concentrations of TOC at FF stations on the upper slope (1.15 ± 0.20%) were about 40% higher than on the shelf (0.80 ± 0.41%). Variability in TOC concentrations at FF stations (average CV 8%, excluding site MP288) was much lower than in the NF stations (average CV 37%) showing both the relative uniformity in TOC in background sediments and the patchiness in TOC values near drilling sites, most likely due to the irregular distribution of drilling discharges.
Concentrations of TOC for NF sediments at sites MP299 and MP288 (excluding sandy sediments at site MP288) were not significantly different than for FF samples (Table 3) as also observed for MeHg at these two sites (Table 2). Furthermore, the low concentrations of MeHg (<0.1 ng/g) in the sandy, TOC-poor sediment from three FF stations at site MP288 show that sediments with very low values of TOC (i.e., <0.2%) are likely to have low concentrations of MeHg. In NF sediment from site EI346, elevated concentrations of total Hg coincided with elevated concentrations of TOC due to the presence of both total Hg (with barite) and TOC (as SBM) in the drilling discharges. However, the sediments with higher amounts of TOC at site EI346 contained lower concentrations of MeHg (Fig. 5a). These lower amounts of MeHg at higher concentrations of TOC in these sediments were most likely the result of higher amounts of total H2S (>1 mM) that inhibit methylation of Hg.
Some NF sediments at upper slope sites MC496, EW963 and GC112 contained 2–4 times more TOC than the other NF stations; however, concentrations of MeHg were within the same range as the other NF and FF stations from these sites (Fig. 5b). In contrast, sediment from several stations at site GC112 with elevated values of TOC (about double ambient TOC concentrations), were marked by the highest concentrations of MeHg (as much as 2–3 times higher than ambient concentrations). The redox environment in the sediments, as previously discussed and as presented below, plays a role in stimulating or inhibiting the production of MeHg.
Vertical distributions of total mercury and methylmercury and relationships to redox conditions
Vertical profiles for total Hg, MeHg, dissolved oxygen and Eh were extremely variable from site to site (Figs. 6, 7, 8). Concentrations of dissolved oxygen were generally non-detectable (<0.03 mg/l) in many NF sediments and decreased to non-detectable values between 3 and 5 cm at FF stations. The measured Eh values represent the sum of all oxidation and reduction reactions that are occurring in the sediment and can be given an approximate correlation with various redox reactions. For example, the onset of bacterial reduction of nitrate (a replacement oxidizing agent for oxygen) to ammonia was observed to occur at an Eh of ∼200 mV. Based on results from this study, the onset of sulfate reduction to H2S occurred as Eh values fell below 0–100 mV. The redox environment defined in this paper is mainly based on the Eh; however, in several cases, supporting concentrations for interstitial water were available to confirm conclusions based on the Eh values.
Vertical profiles for site EI346 show that concentrations of MeHg were below detection limits (<0.03 ng/g) in the top 5 cm of sediment from a NF station, even though concentrations of total Hg were almost eight times greater than background values (Fig. 6). Oxygen was depleted in the top mm of the NF core and the Eh was about −150 mV (Fig. 6). As predicted from the Eh data, sulfate reduction was occurring in the top few centimeters of the core from NF station where dissolved total H2S in the interstitial water was >1 mM. Such conditions are consistent with observations by other investigators that methylation of Hg is inhibited under such conditions (Gagnon et al. 1997). In contrast with the MeHg profile for NF station at site EI346, oxygen penetrated to a depth of ∼2.5 cm in the sediment at the FF station, a point below which the Eh approached 0 mV and an increase in sediment concentrations of MeHg was observed (Fig. 6).
At site EW963, low Eh values (about −100 mV), oxygen depletion in the top 1 mm of sediment, and low concentrations of MeHg, despite high concentrations of total Hg, were observed at the NF station (Fig. 7). In contrast, at the FF station, oxygen persisted to a depth of 3 cm, a modest redoxcline was observed with nitrate reduction and concentrations of MeHg were ∼1 ng/g at the top of the core (Fig. 7).
In NF sediment at site GC112, the Eh (Fig. 8) was generally higher than observed in NF sediments from sites EI346 (Fig. 6) and EW963 (Fig. 7). Under this condition, production of MeHg was enhanced by >25% relative to the FF station at site GC112. As previously discussed with Fig. 4, several NF samples from station GC112 had elevated concentrations of MeHg relative to FF stations whereas the opposite was observed at sites EI346 and EW963.
Conclusions
Concentrations of total Hg in sediments from reference sites for this study ranged from 11 to 92 ng/g relative to values of 48–558 ng/g for sediment collected within 100 m of drilling sites. Strong linear relationships (r values of 0.89–0.97) were observed between concentrations of Ba and total Hg in sediment from NF stations where total Hg values exceeded background values by a factor of 3–10. Barium values in these NF sediments ranged from 20 to 28%, relative to ambient Ba concentrations of ∼0.05–0.15%. The strong linear relationships between total Hg and Ba, coupled with the high levels of Ba (as barite) in these sediments, support barite as a common source for both metals and facilitate estimation of the total Hg content of the industrial barite used during drilling. Average concentrations of total Hg in the industrial barite used at each site, as calculated from the Hg/Ba ratio, were below the limit of 1,000 ng/g set by EPA for Hg in barite.
The results from this investigation show that concentrations of MeHg in surficial (0–2 cm) sediments did not vary significantly between NF and FF stations at any of the six sites studied. When subsurface samples (2–20 cm) were included, no significant differences between MeHg concentrations at NF and FF stations were observed at five of six sites. At a sixth site, significantly higher concentrations of MeHg were found at NF than at FF stations due to the presence of sandy sediment with low values of total Hg and MeHg at three FF stations from this one site. The average concentration of MeHg for all FF sediments (0.44 ± 0.27 ng/g) was nearly identical to the average for all NF sediments (0.45 ± 0.41 ng/g), although greater variability in values was found at NF stations due to the patchiness of drilling discharges.
Results from several stations at one shelf site (EI346) and one site from the upper slope (EW963) show that redox conditions in sediment at NF stations (especially production of H2S) can lead to values of MeHg at NF stations that were 3–10 times lower than in background (FF) sediment. In contrast, at site GC112 on the upper slope, concentrations of MeHg were higher by a factor of 1.2 to about 2.5 in a few NF samples relative to FF samples. The combination of factors that led to higher values in the sediments from site GC112 seems to be higher values of TOC and total Hg, along with what may be optimal redox conditions. In contrast, however, four NF samples from the GC112 site had lower than background concentrations of MeHg. Results for site GC112 were somewhat ambiguous in that concentrations of MeHg were enhanced at a few stations; however, the higher MeHg values were equivalent to ∼3% or less of natural concentrations of total Hg. Therefore, these anomalously high values of MeHg could have either a natural or an anthropogenic source of Hg.
The data presented in this study make a reasonable initial argument for the conclusion that Hg introduced with barite during offshore drilling cannot be directly linked to enhanced values of MeHg in NF sediments. The opposing argument that sediment values of MeHg are attributable to Hg introduced during offshore drilling is certainly weak based on results from this study. The results from this study also suggest that, in most instances, changes in redox environment associated with the presence of drilling mud and cuttings do not lead to higher concentrations of MeHg in sediments. Relative to background sediments, much lower concentrations of MeHg were found in NF sediment adjacent to drilling sites where the sediments were anoxic, highly reducing and enriched with dissolved H2S. In a few cases, more MeHg was found in NF sediment than FF sediment when the sediments were less sulfidic.
References
Adeloju SB, Dhindsa HS, Tandon RK (1994) Evaluation of some wet decomposition methods for mercury determination in biological and environmental materials by cold vapour atomic absorption spectrometry. Anal Chim Acta 285:359–364
Bender ML, Martin W, Hess J, Sayles F, Ball L, Lambert C (1987) A whole-core squeezer for interfacial pore water sampling. Limnol Oceanogr 32:1214–1225
Bloom NS (1989) Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapour atomic fluorescence detection. Can J Fish Aq Sci 46:1131–1140
Bloom NS, Crecelius EA (1983) Determination of mercury in seawater at sub-nanogram per liter levels. Mar Chem 14:49–59
Bloom NS, Gill GA, Cappelino S, Dobbs C, McShea L, Driscoll C, Mason R, Rudd JWM (1999) Speciation and cycling of mercury in Lavaca Bay, Texas, sediments. Environ Sci Technol 33:7–13
Fleisher M (1970) Summary of the literature on the inorganic chemistry of mercury. US Geological Survey Prof Paper 713
Gagnon C, Pelletier E, Mucci A, Fitzgerald WF (1996) Diagenetic behavior of methylmercury in organic-rich coastal sediment. Limnol Oceanogr 41:428–434
Gagnon C, Pelletier E, Mucci A (1997) Behavior of anthropogenic mercury in coastal marine sediments. Mar Chem 59:159–176
Gilmour CC, Henry EA, Mitchell R (1992) Sulfate stimulation of mercury methylation in freshwater sediments. Environ Sci Technol 26:2281–2287
Gilmour CC, Riedel, Ederington MC, Bell JT, Benoit JM, Gill GA, Stordal MC (1998) Methylmercury concentrations and production rates across a trophic gradient in the northern Everglades. Biogeochemistry 80:327–345
Gu D, Iricanin N, Trefry JH (1987) The geochemistry of interstitial water for a sediment core from the Indian River Lagoon, Florida. Fla Sci 50:99–110
Isphording WC, Clark MW, Hart AD (2004) In: Gulf of Mexico comprehensive synthetic based muds monitoring program, vol II. American Petroleum Institute, Washington, DC
Kang WJ, Trefry JH, Nelsen TA, Wanless HR (2000) Direct atmospheric inputs versus runoff fluxes of mercury to the lower Everglades and Florida Bay. Environ Sci Technol 34:4058–4063
Kannan K, Smith RG, Lee RF, Windom HT, Heitmuller JM, Summers JK (1998) Distribution of total and methyl mercury in water, sediment, and fish from south Florida estuaries. Arch Environ Contam Toxicol 34:109–118
Kramer JR, Grundy HD, Hammer LG (1980) Occurrence and solubility of trace metals in barite for ocean drilling operations. In: Research on environmental fate and effects of drilling fluids and cuttings, vol II. Courtesy Associates, Washington, DC, pp 789–798
Lindberg SE, Harriss RC (1974) Geochemistry of mercury in the estuarine sediments and interstitial water. Environ Sci Technol 8:459–462
Neff JM (2002) Fates and effects of mercury from oil and gas exploration and production operations in the marine environment. Report to the American Petroleum Institute, Washington, DC, 135 pp
Trefry JH, Smith JP (2003) Forms of mercury in drilling fluid barite and their fate in the marine environment: a review and synthesis. SPE paper 80571 presented at 2003 SPE/DOE/EPA exploration and production environmental conference, San Antonio, 10–12 March 2003
Trocine RP, Trefry JH (1983) Particulate metal tracers of petroleum drilling mud dispersion in the marine environment. Environ Sci Technol 17:507–512
United States Environmental Protection Agency (1993) Oil and gas extraction point source category, offshore subcategory; effluent limitations guidelines and new source performance standards. Fed Regist 58(41):12454–12512
Acknowledgments
The authors thank Steve Viada, Al Hart, Frank Johnson and Woody Powell from Continental Shelf Associates, Inc., for logistical support in the field and with data compilation and statistics. Special thanks to Joe Smith, Mary Boatman, Mike Parker, Jim Ray and other reviewers of this study. This paper was originally presented as SPE 80569 at the SPE/EPA/DOE Exploration and Production Environmental Conference, held in San Antonio, Texas, USA, 10–12 March, 2003. This research was funded through the Synthetic Based Muds Research Group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trefry, J.H., Trocine, R.P., McElvaine, M.L. et al. Total mercury and methylmercury in sediments near offshore drilling sites in the Gulf of Mexico. Environ Geol 53, 375–385 (2007). https://doi.org/10.1007/s00254-007-0653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-0653-6