Abstract
The complex depositional pattern of clay and sand in most of the areas controlled the vertical and lateral movement of nitrate in groundwater. The variation of nitrate concentration at different groundwater levels and the lateral distribution of nitrate in the groundwater at two sites indicated the filtration of nitrate by clayey formations. A rural agricultural district located in the Vamsadhara river basin, India was selected for studying the lateral and vertical distribution of nitrate in the groundwater and the association of nitrate with other chemical constituents. The nitrate concentrations in the groundwater are observed to vary between below detectable limit and 450 mg NO3/L. The sources for nitrate are mainly point sources (poultry farms, cattleshed and leakages from septic tanks) and non-point sources (nitrogenous fertilisers). The nitrate concentrations are increased after fertiliser applications. However, very high concentrations of nitrate are derived from animal wastes. Relatively better correlations between nitrate and potassium are observed (R = 0.74 to 0.82). The better relationship between these two chemical constituents in the groundwater may be due to the release of potassium and nitrate from both point and non-point sources. The nitrate and potassium concentrations are high in the groundwater from clayey formations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrite derived from nitrate in vivo with amines and amides to form N-nitroso compounds, which may have carcinogenic properties (VanMaanen et al. 1996). In Colombia and Italy, high levels of nitrate in well waters are associated with an increased risk of gastric cancer (Cuello and Correa 1976; Gilli et al. 1984). In a cross-sectional study in an area with a high incidence of gastric cancer in north eastern China, an association between high levels of nitrate in drinking water supplies and neoplastic changes in the stomach was observed (Xu et al. 1992). The association of stomach cancer and high nitrate levels were also reported by Boeing (1991). VanMaanen et al. (1996) reported here, basing on their work in the Netherlands, that urinary excretion of N-nitrosamines was observed during high nitrate exposure. They concluded that drinking water contamination by nitrate implies a genetic risk. WHO (1984) prescribed a drinking water standard of 45 mg/L (or 10 mg NO3-N/L) for nitrate. In India, there were no reports correlating nitrate with reported diseases. However, the author observed the indirect effects of nitrate like reduction of efficiency of drug used for treatment of urinary disorders. This work is communicated.
The groundwater pollution due to nitrates is increasing in India. The water quality assessment studies carried out in 17 Indian states by NEERI showed that out of 4,696 water samples, 1,290 samples (27%) have nitrate exceeding the drinking water standard (Bulusu and Pande 1990). The author (Rao 1998, 2002) has observed nitrate concentrations reaching a maximum of about 450 mg NO3/L in the lower portions of Vamsadhara and Godavari river basins.
The main objective of the present paper is to study the lateral distribution of nitrate and its fluctuation in different hydrogeological conditions during different periods. Some preliminary studies in this area were conducted by the author (Rao 1998).
Study area
The study area represents the lower portion of Vamsadhara river basin occupying the major part of Srikakulam district, Andhra Pradesh, India (Fig. 1). The area has a typical rural background and is an economically backward district in the Andhra Pradesh state. Agriculture is the major economic activity in the area. The Vamsadhara river is a moderate and perennial river flowing through the Eastern Ghat regions of Orissa and Northern Andhra Pradesh. Originating at Belagad (600 m above MSL) of Pulbhani district of Orissa the river joins the sea at Kalingapatnam of Srikakulam district in Andhra Pradesh. The Vamsadhara river basin spreads over an area of 10,600 sqkm.
The study area, based on the topographic characteristics, can be divided into (1) hilly regions and (2) plains. The hilly regions in the area form a part of the Eastern Ghat ranges of South India. The hills occurring along the northern and western boundaries are of higher altitude while those along the eastern margin area of relatively lower latitude. The southern region is devoid of hills. The highest point reaches a height of 750 m above MSL in the north western part of the area. Most of the study area lies below 45 m MSL. The area receives an average annual rainfall of 1,000 ± 50 mm, both from southwest and northeast monsoons.
Most of the area is characterised by wide spread alluvial cover underlain by granite gneisses. The lower Vamsadhara basin consists of Eastern Ghat super group of rocks belonging to Archean age (Devvarma 1986). These rocks are subjected to granulite facies of metamorphism. The rock type of this area belong to Khondalite suit of rocks, Charnockites, Granites, Mafic dykes, quartz veins and pegmatite dykes. Most of the pegmatite bodies are associated with granitic rocks throughout the area (Devvarma 1986 and N.S. Rao, personal communication). The mineralogical assemblages of the pegmatites are Vermiculite, fluorapatite, magnetite, allanite, zircon, sphene, barytes and barium feldspars. Fluorite mineral, in general, is observed to occur in granitic pegmatites.
Materials and methods
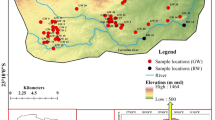
A total of about 350 water samples were collected during August 1998, February 1999, December 1999 and March 2000. The location of wells where water samples were collected are shown in Fig. 2. One hundred and twenty villages were selected for the present study. Throughout the study, the observation wells were not changed. All the observation well waters were sampled in two periods only (i.e. August 1998—‘before fertiliser application’ and February 1999—‘after fertiliser application‘). One well was sampled from each village. However, additional samples were also collected at few villages. In the remaining two periods, only 40 selected observation well waters were sampled to study the fluctuation of nitrate in the groundwater. Waterlevels in the open wells were measured. One water sample in 500 ml capacity and three more samples in 125 ml capacity polyethylene bottles were collected from each well. The water sample in 500 ml capacity bottle was used for the determination of chloride, sulphate, sodium, potassium, fluoride and silica determinations only. The other three water samples in 125 ml capacity bottles were preserved with concentrated HCl, HNO3 and HgCl2 for iron, manganese, and phosphate and nitrate determinations, respectively, in the laboratory. Dissolved oxygen, pH, calcium, total hardness, bicarbonate and residual chlorine were determined at the well site immediately after drawing the water for each test separately. Electrical conductivity (EC) was determined on the same day at the base station. The APHA (1989) standard methods were followed for determination of all the constituents mentioned earlier. The reference number of the standard method of APHA (1989) was given in the parenthesis of the method used. The iron was determined using 1,10-phenanthroline method (method D of 3500-Fe). The iron reported in this paper was total iron. Nitrate was determined by cadmium reduction method using HACH chemicals and visual spectrophotometer (Systronics make). Manganese was determined by persulfate method (method D of 3500-Mn). The calcium and hardness, bicarbonate, chloride were determined by EDTA (method D of 3500-Ca and 2340 C), standard acid (2320 B), argentometric (method B of 4500-Cl) titration methods, respectively. Magnesium concentrations were calculated from hardness and calcium concentrations (method E of 3500-Mg). Sodium and potassium concentrations were determined using flame photometric methods (methods D of 3500-Na and 3500-K). Sulfate was determined using turbidimetric method (method E of 4500−SO 4). Silica and phosphate were determined using molybdosilicate method (method D of 4500-Si) and vanadomolybdophosphoric acid method (method C of 4500-P), respectively. Fluoride was determined by both Alizarin Red S (visual method) and SPADNS (spectrophotometric) methods (method D of 4500-F). Except in very few samples, the similar values were obtained from both the methods. SPADNS solution was imported from HACH (USA). The standard solutions for fluoride, nitrate, iron and manganese were obtained from HACH (USA) and SISCO laboratories (India). During December 1999, only pH, EC, calcium, magnesium, chloride, bicarbonate and silica were determined besides nitrate, while the samples collected during May 2000 were analysed only for nitrate, calcium, magnesium, bicarbonate and pH since the main emphasis is given only to study the nitrate fluctuation.
Results
The groundwater over most of the area occurs at shallow depths. Of about 125 wells sampled, 101 wells were open wells. The groundwater levels were measured in all open wells. The groundwater level was observed to vary between 0.20 and 10.10 m below ground level (bgl) during August 1998, while it varied between 0.45 and 8.70 m bgl during February 1999. The mean and median waterlevels during August 1998 were 3.1 and 2.7 m bgl, respectively, while 3.6 m bgl was observed as mean and median level for February 1999. The groundwater levels in more number of villages were within 4.0–5.0 m bgl during February 1999. From August 1998 to February 1999, rise in waterlevel was observed in 65% villages and fall in waterlevel was obseved in 35% villages. The waterlevel fluctuation between the two periods was less than 2.0 m in about 74 villages. Since rainfall was the only source for groundwater recharge, the rainfall distribution during the study period over the area was shown in Fig. 3. The data were collected from the only IMD (Indian Meteorological Department) station which was nearer to the well No. 106 (Fig. 2). The total number of sampling days during August 1998 were 12 and they were not rainy. Only some parts of the area got rainfall during the sampling period, and the occurrence of rainfall was not uniform throughout the area.
The statistical summary of groundwater quality was presented in Table 1 for understanding the general quality of groundwater in the study area. Nitrate concentrations as low as BDL to a maximum of 275 mg NO3/L were observed in the groundwater during August 1998. The minimum concentrations in February 1999 were the same as in August 1998 and the maximum observed in this period is 450 mg NO3/L. In most of the villages, the concentrations were less than 50 mg NO3/L (Fig. 4a). The spatial distribution of nitrate during August 1998 and February 1999 was shown in Fig. 5a, b. Increase in nitrate concentrations was observed from August 1998 to February 1999 in most of the well waters (Fig. 4b). The highest increase of 333 mg NO3/L (i.e. the difference of 450 and 117 mg/L) was observed from August 1998 to February 1999 at well No. 25.
Discussion
It is well known that the nitrogenous fertilisers are one of the important sources for groundwater nitrate for the past two decades. The approximate nitrogen inputs applied to the study area are presented in Table 2. The consumption of huge quantities of nitrogenous fertilisers per year is supposed to increase the nitrate levels in the groundwater. Contrary to this expectation, most of the area records less nitrate (Figs. 4, 5a, b). Seventy two villages in August 1998 and more or less similar number of villages during February 1999 registered nitrate concentrations less than 25 mg/L. These low nitrate (< 25 mg/L) concentrations are attributed to be derived from nitrogenous fertilisers, since many investigators (Malik and Banerji 1981; Handa 1983; Sankaranarayana et al. 1989; Sehgal et al. 1989; Bulusu and Pande 1990; Mehta et al. 1990; Kondratas and Mikalauskas 1973; Klimas and Paukstys 1993; Hamilton and Shedlock 1992; Kolpin et al. 1994) have reported that the contribution of nitrate from the fertiliser to the groundwater can vary from as little as 3 mg/L to as much as 1,800 mg/L. Further, nitrogenous materials are rare in geologic system (Uma 1993).
More or less uniform dosages of nitrogenous fertiliser consumption and prevalence of low nitrate concentrations in the majority of the area indicates that the groundwater is being polluted slowly by the fertiliser application. This indication is supplemented by the figures (Fig. 4a, b). It is clear in Fig. 4b that there is an increase in the nitrate after fertiliser application. Increase in nitrate, particularly at low levels, in more number of samples is attributed to the pollution from fertilisers. However, high concentrations of nitrate observed at few places are attributed to other sources rather than fertiliser. A thorough observation at the wells, where high concentrations of nitrate are observed, indicated that there is an association between high nitrate concentrations and animal wastes at cattlesheds which are located near the well sites. The animal wastes at cattlesheds acted as point sources. High concentrations of nitrate are also observed in the groundwater nearer to the markets where animal trading was taking place once in a week.
When the cattlesheds are situated on the places where the soil is dominated by clay, the ammonium (released from animal excrement and urine) is sorbed on clay during dry periods and this sorbed ammonium creates and releases nitrate into the groundwater when it comes into contact with the groundwater (Rao 1998). The slow denitrification process in clayey soils may also be responsible for high nitrate concentration in clayey formations (Klimas and Paukstys 1993). The dominance of clay in the surface soil is observed in a major part of the area.
It was observed from the geoelectrical soundings and litholog information over the area that the groundwater in most of the study area fluctuates in any one or two of the identified subsurface formations viz. clayey formation, sandy formation and weathered rock formation during different periods of the year. If the groundwater is present in the sandy and weathered rock formations, the TDS (total dissolved salts) of water is low as compared to the same in clayey formations. The water from the clayey formations is rich in salts. For example, an electrical conductivity of 9,350 μS/cm is observed in the water from a clayey formation at Kunajammannapeta village (No. 16, Fig. 1). The people of this village get drinking water from the neighbouring villages. The groundwater in the surrounding five villages (well Nos. 12, 13, 16A, 17 and 22; Fig. 1) have an electrical conductivity ranging from 1,411 to 2,382 μS/cm at 25°C (Table 3).
The vertical and lateral spread of nitrate from point source is controlled by hydrogeological setting of the area.
A field example for lateral spread is shown in Fig. 6a. Close to well No. 52 (‘D’ in Fig. 6a), a cluster of cattle sheds are observed in the thick clayey formation. Two more wells away from the well No. 52 are also sampled to study the lateral spread of nitrate from point sources. The data for well ‘B’ in Fig. 6a are collected from the earlier work (March 1991) of the author (Rao 1996). The groundwater of wells A and C (Fig. 5a) which are located in a sandy formation has low salt content as compared to the same of clayey formations. The nitrate is not reached from the surroundings of well No. 52 to the groundwater of wells A and C. Hence they have very less nitrate in their groundwater.
Similar phenomena is observed in the areas where surface clayey formation was followed by a sandy formation (Fig. 6b). When the waterlevel drops down from top clayey formation to the sandy formation, the nitrate levels are reduced. Generally, the waterlevels during monsoon period are raised and the groundwater concentrations are decreased due to the dilution by rainwater. The groundwater during pre-monsoon period may have more nitrate since it receives continuous supply of nitrate from point source without any dilution as the groundwater exist at shallow depths at majority of the places. In case of Fig. 6b, the top clayey formation may be acted as a filter for nitrate. When a three phase system of two solutions of electrolyte is separated by a clay membrane, the effect can be described by the equation of a perfect electrode (Degens and Chillingar 1967). A typical situation for the role of clay on vertical and lateral spread of nitrate is shown in Fig. 7.
In addition to the above example (Fig. 6b), the vertical distribution of nitrate in some wells observed during the four periods is shown in Fig. 8a–l and the data for some more wells are presented in Table 4 to understand the nitrate fluctuation. The chloride, EC are also presented along with nitrate as these are indicators of dilution and TDS. The scale for water levels and nitrate is same for all cross-sections presented in Fig. 8. However, the scale for EC and chloride is changed for the sake of clarity. Increase in nitrate concentrations with depth is observed at wells 2, 101, 81 (Fig. 8a, e, h), while decrease in nitrate was observed at well No. 110 with depth (Fig. 8i). In the remaining crosssections of Fig. 8, mixed relations of nitrate with depth are observed. During December, the groundwater occurs in most of the wells at shallow depths due to the groundwater recharge by rainwater. These shallow waterlevels exist for some time and hence the waterlevels during February in most of the wells are nearer to the levels of December. However, much difference in nitrate concentrations can be observed between these two periods (Fig. 8). During December, the NO3 concentrations at majority of the places are diluted, while the continuous supply of nitrate from point sources reached the shallow depths during February increased the nitrate concentration. If the nitrate source is solely derived from fertiliser which is applied seasonally, the concentrations may be decreased with depth due to possible denitrification. However, the perennial supply of nitrate from point sources reached the groundwater at a depth of about 10 m (Fig. 8d, k, l).
The adsorption/rentention properties of clay and the complex depositional pattern of sand and clay control the lateral and vertical distribution of nitrate in the groundwater. The three open wells (Fig. 8d, k, l) are located near the hillocks and are relatively at a higher elevation. The diameter of these circular wells is about 3 m. The top sediment is porous and followed by weathered rock formations. At well Nos. 26 and 94 (Fig. 8d, l), septic tank leakages from the surrounding residential area are also suspected. It is also suspected at these wells that the nitrate was reaching slowly and directly from the top layers, along the well walls, to the water column which was at about 10 m depth.
For discussing both vertical and lateral distribution of nitrate from a point source (poultry farms), another field example is presented in Fig. 9. The waste from the poultry farms was leached into the groundwater at well No. 75. During rainy season, the rainwater may dilute the nitrate concentration. Interestingly, the pollution had not reached to well No. 75A which is about 80 m away from well No. 75. It is suspected that the wells may be separated vertically by atleast one clay layer which might have filtered the nitrate and hence high nitrate concentrations are not reached to well No. 75A. However, 30 mg NO3/L at 1.0 m depth in well 75A and 45 mg NO3/L at a depth of 1.8 m in well 75 indicates that a small layer comprised of porous formation may join both the wells. The area is not feasible for geoelectrical soundings to trace the exact subsurface configuration and the author was not permitted for conducting any subsurface investigations at this place. Tacon (1987) reported that the nitrogen, phosphorus and potassium in poultry manure are 3.77, 1.89 and 1.76, respectively. The values are expressed as percent by weight (percentage free moisture-free basis). The cattle manure has less phosphorous as compared to poultry manure (N, P and K in cattle manure: 1.91, 0.56 and 1.40). Further, the cattle/buffalo urine had almost nil phosphorous levels (Tacon 1987). The poultry waste was dumped near the well (No. 75). There is no additional source besides poultry waste for nitrate, except a small quantity of nitrogenous fertiliser applied to the surrounding small bit of agricultural field. The phosphate concentrations observed at wells 75 and 75A are 2.30 mg/L and BDL, respectively (Table 5) during August 1998. The median phosphate concentration for the entire study area is about 0.45 mg/L (Table 1). Hence, the occurrence of relatively high concentrations of PO4 at well 75 is an indication of contamination from chicken faeces.
An attempt has been made to establish relationships between nitrate and other constituents in the groundwater. The details are presented in Table 6. The x–y plots between nitrate and 12 other constituents for August 1998 are presented in Fig. 10. Since relatively better correlations are observed within the nitrate range of 25–150 mg/L, only those samples fall within this nitrate range are plotted in Fig. 10. At low nitrate range, no better correlations are observed (Table 6). It is observed from Table 6 that relatively better correlations are observed between nitrate (NO3) and potassium (K) for both periods in the entire nitrate range. More or less similar R-value is observed for K and NO3 for both periods (Table 6). However, the R-value is further increased to 0.82 during August 1998 if the samples having more than 25 mg NO3/L are only considered. The relatively better correlation betwen K and NO3 may be explained by the fact that both point sources (i.e. wastes at poultry farms and cattle sheds) and non-point fertiliser sources (Table 7) release nitrate and potassium into the groundwater of the study area. Further, the K concentrations at majority of the places are increased after fertiliser application (Table 8). For example, the groundwater at some wells (Nos. 2, 7, 9 and 25) existing in the clayey formations where the point sources are dominant have more nitrate and potassium (NO3 range: 60 to 450 mg/L and K range: 150 to 575 mg/L) than the similar well water present in the same formation without any point source (well No. 16, Table 3). This observation also hints that high concentrations of K in the water of clayey formations may also be derived from animal wastes in the study area. Increase of chloride concentrations with the increase of nitrate due to contamination by septic tank systems was reported from 18 data points by McQuillan (2004). The author (Rao 1998) also observed a better relation between Cl and NO3 in the waters (N = 12) which was polluted by point sources during November 1992. However, during the present study, weak postive correlations are obtained for nitrate and chloride (Table 6). For example, at well No. 25, the variation in chloride concentrations with respect to depth (i.e. at different water levels) is narrow (i.e. 590 to 633 mg/L), while the nitrate varies from 87 to 450 mg/L (Fig. 8b).
Conclusion
The sources for nitrate in the groundwater of Srikakulam district are mainly point sources (wastes at cattlesheds and poultry farms, leakages from septic tanks) and non-point sources (urea and NPK fertilisers). The groundwater is being slowly polluted by nitrogenous fertilisers. The very high concentrations of nitrate reaching 450 mg NO3/L are received from point sources. The complex depositional pattern of sand and clay controlled the lateral and vertical movement of nitrate in the groundwater. The adsorption/retention properties of clay render high salinity to groundwater in the clayey formations. When the cattlesheds are situated on the places where the soil is dominated by clay, the ammonium (released from animal exrrement and urine) is sorbed on clay during dry periods and this sorbed ammonium creates and releases nitrate into the groundwater when it comes into contact with groundwater. The slow denitrification process in clayey soils may also be responsible for high nitrate concentration in clayey formations. The clay acted as a filter for nitrate when the water moves from clayey formation to another relatively porous formation. Presently, the nitrate contamination from fertiliser source is relatively less and this source is a seasonal one. Hence, the nitrate concentrations are decreased with depth may be due to the possible denitrification, if the fertiliser is the sole source of nitrate. In case of point source, the supply is continuous and hence high nitrate concentrations are also observed at relatively deeper level. Relatively better correlation (R = 0.74 to 0.82) between nitrate and potassium is observed. The release of both potassium and nitrate into the groundwater from both point and non-point sources may be the main reason for relatively better relationship between them. After fertiliser application, the concentrations of nitrate (NO3) and potassium (K) are increased in most of the samples. Further, the NO3 and K are high in the groundwater from clayey formations.
References
APHA (American Public Health Association) (1985) Standard methods for the examination of water and waste water, 16th edn. APHA, American Water Works Association and Water Pollution Control Federation, Washington DC
Boeing H (1991) Epidemiological research in stomach cancer: progress over the last ten years. J Cancer Res Clin Oncol 117:113–143
Bulusu KR, Pande SP (1990) Nitrates—a serious threat to groundwater pollution. Bhu-Jal News 5:39–43
Cuello C, Correa P, Haenszel W (1976) Gastric cancer in Colombia. I. Cancer risk and suspect environmental agents. J Natl Cancer Inst 57:1015–1020
Degens ET, Chillingar GV (1967) Diagenesis of subsurface waters. In: Larson G, Chillingar GV (eds) Diagenesis in sediments. Developments in sedimentalogy. Elsevier, Amsterdam
Devvarma D (1986) Some aspects of texture, mineralogy and geochemistry of the Vamsadhara river sediments, East Coast of India. PhD, Andhra University, Andhra Pradesh, India
Gilli G, Corrao G, Favilli S (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas: first descriptive study of the Piemonte Region, Italy. Sci Total Environ 3:35–48
Hamilton AP, Shedlock JR (1992) Are fertilisers and pesticides in the groundwater? A case study of the Delmarva Peninsula, Delaware, Maryland, Virginia. USGS Circular 1080:1–16
Handa BK (1983) Effect of fertiliser on groundwater quality in India. In: Symposium on groundwater development—a perspective for the year 2000 AD. University of Roorkee, India
Klimas A, Paukstys B (1993) Nitrate contamination of groundwater in the Republic of Lithuania. NGU Bull 424:75–85
Kolpin DW, Burkart MR, Thurman EM (1994) Herbicides and nitrate in near-surface aquifers in the mid continental United States, 1991. USGS Water Supply Pap 2413:1–34
Kondratas AR, Mikalauskas VV (1973) An example of regional groundwater contamination. Nedra, Moscow, pp 280
Malik S, Banerji S (1981) Nitrate pollution of groundwater as a result of agriculture development in Indo-Ganga plain, India. In: Proceedings of international symposium on quality of groundwater, The Netherlands
McQuillan D (2004) Groundwater quality impacts from on-site septic systems. Paper presented at 13th annual conference on National on-site wastewater Recycling Association, Albuqueraque, N.M. held during November 7–10 http://www.nmenv.state.nm.us/food/LiquidWaste/NOWRA.paper.pdf. Cited 10 June2005
Mehta BC, Singh RV, Srivatsava MM, Das S (1990) Impact of fertiliser use on groundwater quality in parts of Ganjam District, Orissa. Bhu-Jal News 5:44–48
Rao NS (1996) Geohydrological and hydrochemical properties of the Lower Vamsadhara River Basin, Andhra Pradesh, India. PhD, Andhra University, India
Rao NS (1998) Impact of clayey soils on nitrate pollution in the groundwater of the lower Vamsadhara River basin, India. Hydrol Sci J 43:701–714
Rao NS (2002) Nitrate occurrence and its behaviour in the groundwater of Godavari Delta, India. The Indian Geogr J 77:31–38
Sankaranarayana G, Sudarsan V, Narsimulu C (1989) Nitrate pollution in the groundwater of Sangareddy Area, AP, India. In: Proceedings of the international workshop on appropriate methodologies for development and management of groundwater resources in developing countries, vol 2, IBH-Oxford, New Delhi
Sehgal VK, Sahgal RK, Kakar YP (1989) Nitrate pollution of groundwater in Lucknow Area, U.P. In: Proceedings of the international workshop on appropriate methodologies for development and management of groundwater resources in developing countries, vol 2, IBH-Oxford, New Delhi
Tacon AGJ (1987) The nutrition and feeding of farmed fish and shrimp—a training manual-3. Nutrient sources—composition of feedstuffs and fertilizers. FAO, Brazil. http://www.fao.org/docrep/field/003/AB468E/AB468E05.html. cited 6 March 2006
Uma KO (1993) Nitrates in shallow (regolith) aquifers around Sokoto Town, Nigeria. Environ Geol 21:70–76
VanMaanen JMS, Irene JW, Geja H, Dallinga JW, Paul LJM, Mertens, Kleinjans JCS (1996) Nitrate contamination of drinking water: relationship with HPRT variant frequency in Lymphocyte DNA and urinary excretion of N-Nitrosamines
WHO (World Health Organization) (1984) Drinking water quality control in small community supplies. In: Guidelines for drinking water quality, vol 3
Xu G, Song P, Reed PI (1992) The relationship between gastric muscosal changes and nitrate intake via drinking water in a high-risk populations for gastric cancer in Moping county, China. Eur J Cancer Prev 1:437–443
Acknowledgements
The work was funded by the Department of Science and Technology (DST), New Delhi under the Young Scientist Scheme (HR/SY/A-07/97). The financial support from DST for carrying out this study is gratefully acknowledged. The author is also grateful to Dr. B.K. Handa, Supdt. Chemist (Retired), Water Research Centre, Chandigarh for his valuable suggestions for initiating the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, N.S. Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 51, 631–645 (2006). https://doi.org/10.1007/s00254-006-0358-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0358-2