Abstract
Coastal areas of Hong Kong Island are one of the most extensively urbanized areas in the world. Groundwater samples in natural slopes and developed spaces in the regions centered by the Mid-Levels area, Hong Kong Island, were collected and analyzed to investigate the natural and anthropogenic processes affecting the groundwater chemistry. The results presented may be of value to other coastal areas in the world for the identification of possible groundwater contamination sources. Groundwater samples in the natural slopes were in low total dissolved solid (TDS) (<100 mg/l), indicating that the waters were in the early evolutionary stage. Using chloride as a normalizing factor, the “non-marine” components of different major ions in the samples were calculated. The correlation analysis indicated the occurrence of weathering of plagioclase feldspars in the natural slopes. However, the breakdown of biotite and K-feldspar seems to be limited by short groundwater residence time and high resistance to weathering. The high variety in hydrochemical facies may suggest the presence of extremely heterogeneous subsurface geological conditions. In the developed spaces, groundwater samples exhibited a high range of TDS (~100–5300 mg/l) and were mainly dominated by Na–Cl and Na–Ca–Cl water types. Besides water-rock interactions, the groundwater chemistry was significantly affected by leakage from service pipes and the dissolution of concrete materials. Some chemicals were used as signatures to identify the leakage from various service pipes. The area generally suffered from widespread, but small amount of leakages, and no obvious leakage was discovered. The strong correlations among major cations and chloride suggested that even a small amount of leakage from salty flushing water pipes can significantly affect the groundwater chemistry. Groundwater is found to be highly aggressive toward concrete as supported by three commonly used aggressiveness indices. Additional Ca2+ may be released to groundwater by corrosion of subsurface concrete materials such as building foundations and basements. The strength of those subsurface engineering structures may be weakened. Besides, excess Ca2+ may deposit in the dewatering systems in the area, which may affect their performance in lowering high water tables. The findings regarding leakage from service pipes will be useful for various government organizations such as the Water Supplies Department and Drainage Services Department. Discussion of the behavior of Ca2+ is instructional to foundation and slope dewatering designs in the area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Few places in the world have experienced such intense urban growth as Hong Kong has over the last half century. This has created some environmental and engineering problems but at the same time also offered unprecedented opportunities for novel research. Urbanization of the rugged topography in Hong Kong has created thousands of cut slopes. Groundwater samples from the weepholes or drains installed in these slopes, which are otherwise extremely difficult or expensive to collect, provide an unique and economical chance to understand the physical and chemical natures of the subsurface flow system in the intensively urbanized hillslopes and the potential impacts of urbanization on groundwater chemical systems. However, in Hong Kong, groundwater chemical studies are few in number largely due to the fact that groundwater is not a major drinking water source. Over 70% of fresh water is imported from the Guangdong Province, China and the rest is supplied from local reservoirs (Water Supplies Department 2002). Previous local groundwater chemical studies have been restricted to subjects such as the evaluation of well water pollution in villages (Lam 1981, 1983) and the identification of leakage from service pipes by selected chemical tracers for slope stability studies (Geotechnical Control Office 1982).
In the last few years, studies have been carried out on the hydrogeochemistry in areas central to the Mid-Levels area, Hong Kong. Samples from natural springs and seepages from both cut and natural slopes in different seasons mainly in the Mid-Levels area were collected and analyzed, and provided valuable data for further environmental studies. Some of the results are presented and some of the fundamental processes such as water-rock interactions, and the issues of the impacts of urbanization on the subsurface environment are discussed. The results may be useful for coastal areas in other countries with a similar level of urban development to understand the potential threats to their groundwater resources.
Geology and hydrogeology of the study area
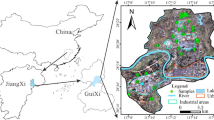
The Mid-Levels area, approximately 1.5 km2 in size, is situated on the northern slope of the Victoria Peak (550 mPD) on Hong Kong Island (Fig. 1). The study area can be divided into two parts with significantly different modes of development. The upper part of the area (>170 mPD) is essentially a natural slope with minimal development. In contrast, the lower part of the area has been extensively urbanized with numerous residential and office buildings and is regarded as one of the most highly urbanized areas in the world. Here, the original landscape has been greatly modified by continuous slope cutting and filling. Groundwater samples can be collected easily from the weepholes and drains installed in these slopes.
The geology and hydrogeology of the area have been described elsewhere in the Geotechnical Control Office (1982) and will only be briefly introduced here. The geology is dominated by two rock types, acidic volcanic rocks and a granitic intrusion. The volcanic rocks have been subject to low-grade regional metamorphism and deformation and affected by contact metamorphism where close to the granite. Both lithologies have been subsequently intruded by basaltic dykes. The irregular contact between the granite and volcanic rocks crosses the area and is disrupted by normal faults in several locations (Fig. 1).
Colluvium overlies several meters of decomposed rock above the bedrock. Granite underlies most of the developed area, composed of quartz (23%–42%), potassium feldspar (31%–42%), plagioclases (16%–35%), and biotite (~5%) according to Allen and Stephens (1971). Volcanic rock underlies the upper undeveloped slopes.
Although it is likely that the lithologies in the subsurface are very heterogeneous and anisotropic, the Geotechnical Control Office (1982) has grouped them into three aquifer units corresponding to: a) colluvium, b) decomposed volcanic and granite rocks, and c) volcanic and granite bedrocks. The colluvium contains transient and permanent perched water tables, whereas, as recently demonstrated by Jiao et al. (2003, 2004), the highly decomposed rock or saprolite below the colluvium is relatively impermeable due to its clay-rich content. The bedrock zone along the rockhead may be fairly permeable with confined groundwater contained within a well-developed fracture network.
Field and analytical methods
Groundwater and seepage samples were collected in September 2002 and January 2003 which represent the conditions of wet and dry seasons, respectively. The sampling and analytical techniques followed the standard methods (APHA 1998) and will be briefly introduced here. At each sampling site, pH and temperature were measured by a Hanna HI 98128 meter; electrical conductivity (EC) was measured by a Hanna HI 3292 ATC Conductivity Probe; and dissolved oxygen (DO) was measured by a WTW Oxi 330i meter with a Cellox 325 probe. Samples for geochemical analyses were filtered through a hand-held Hanna filter system using a 0.45-μm cellulose filter paper and collected in 500-ml HDPE bottles. A 125-ml sample for cation analysis was acidified to pH<2 using ultra-pure concentrated nitric acid. Except for Si, which was analyzed by inductively coupled plasma atomic emission spectrometer (ICP-AES), analyses for other cations were performed by inductively couple plasma mass spectrometer (ICP-MS). A three-point calibration curve was constructed for each cation. The NIST SRM 1640 was used to check for the reliability of the analysis. The measured values of SRM 1640 were all within 5% of the certified values. The results of three replicate analyses indicated that the precision of cation measurements was generally better than 5%. A second 125-ml sample was filtered and left unacidified to be used for anion analysis by HPLC Ion Chromatography. HCO −3 was measured by titration with standard hydrochloric acid (HCl) solution. A third 125-ml sample was collected for aggressive CO2 determination. Samples were stored in a refrigerator at 4°C prior to analysis.
About 95% (36 out of 38) of values in the wet season fall in the range of +5 to −5% and the worst charge imbalance is about 7%. For the dry season results, about 85% (22 out of 26) of values fall in the range of +5 to −5% and the worst charge imbalance is about 7%. These results suggest that the analyses are of reasonably good accuracy.
Results and discussion
Groundwater and seepage samples were collected from both natural slopes and highly urbanized spaces in the Mid-Levels area. The results collected from the natural slopes serve two purposes: (1) to identify the natural processes controlling the groundwater chemistry; (2) to compare with the results collected from highly-urbanized spaces so that the impacts of urbanization on groundwater chemistry can be investigated.
Two sets of samples were collected, at the same location wherever possible, in wet and dry seasons to investigate the seasonal effects on groundwater in the area. However, some sampling points were missing in either season. This is because some sampling points cannot be accessed due to construction works (mainly slope maintenance) or the sampling points dried up (mainly in dry season). The sampling locations in wet and dry seasons are shown in Figs. 2 and 3, respectively.
Hydrogeochemical characteristics of the natural slopes
In Hong Kong, the pH of rainwater (the only recharge source for natural slopes) generally varies between 4 and 5 due to factors such as the dissolution of NO2 and SO2 emitted from motor vehicles and coal-burning power stations (Wong and Tanner 1997). Wong (1997) measured and calculated that the mean pH of rainwater in 1996 was about 4.6, which was similar to the measurement by the Environmental Protection Agency, Hong Kong (Environmental Protection Agency 1991–1994). These pollutants may contribute nitrates and sulphates to shallow groundwaters. Groundwater samples collected from the natural slopes in the wet season were slightly acidic (average pH = 6.08, and as low as 4.73) and with TDS less than 100 mg/l. This reflects their short residence time and low degree of water-rock interactions. In the dry season, groundwater became slightly more acidic, with an average pH of 5.68 but ranging as low as 4.63. Rainwater is generally more acidic in dry season than in wet season in Hong Kong (Tanner 1999a). Groundwater contained low levels of organic pollutants. The mean nitrate concentrations were about 1.70 mg/l and 2.14 mg/l in wet and dry seasons, respectively, and no PO 3−4 was detected in either season. A higher nitrate level was detected in the dry season, which may be related to the generally poorer air quality during the winter monsoon climate in Hong Kong.
Although groundwater samples from the natural slopes had similar TDS, their hydrochemical facies were quite different. Six hydrochemical facies (Na–Ca–Cl–HCO3–SO4, Na–Ca–HCO3–Cl, Na–Ca–HCO3–Cl–SO4, Na–HCO3–Cl, Na–Ca–Cl–HCO3, and Ca–Na–HCO3) were found among 18 samples in the wet season. Nearly the same patterns of hydrochemical facies (Na–Ca–Cl–HCO3–SO4, Na–Ca–HCO3–Cl, Na–Ca–HCO3–Cl–SO4, Na–HCO3–Cl, Na–Ca–Cl–SO4, and Ca–Na–Cl–HCO3–SO4) were observed among 12 samples in the dry season. No significant change in hydrochemical facies is observed in different seasons. Such high diversity of hydrochemical facies may reflect the highly heterogeneous geological condition of the area. Groundwater with different flow paths may undergo different natures and extents of water-rock interaction. Although rainwater chemistry in Hong Kong may vary from time to time as affected by such factors as weather condition (Tanner 1999b), rainwater is generally of marine origin due to the regional coastal environment and the “near-to-coast” locality of the area. Na+ and Cl− are more abundant in rainwater at coastal areas. Groundwater in the natural slopes still retained a partial coastal rainwater chemical signature as indicated by the dominance of Na+ and Cl− ions. However, the groundwater chemistry has been progressively modified by water-rock interactions along various flow paths in both seasons. Although about 80% of rain falls from May to September in Hong Kong (Hong Kong Observatory 2003), more sites exhibited higher TDS in wet season than in dry season in both natural slopes and developed areas (Table 1). A possible reason may be that more salts could be leached out due to the generally higher water table during the wet season.
The nature and the extent of water-rock interactions can be identified by investigating ion concentrations relative to chloride concentrations. Chloride is a useful normalizing factor because it does not enter into precipitation-dissolution processes except at brine concentrations and it rarely enters into oxidation-reduction or adsorption reactions (Feth 1981). This conservative nature makes it useful in comparison with the relative loss or gain of other ions, the evaluation of which can constrain possible water-rock interactions.
According to Figs. 4 and 5, all groundwater samples in the natural slopes are enriched in major cations compared to seawater in both seasons except for Mg2+ in certain sites, It appears that groundwater samples in the natural slope showed similar (for Ca2+) or even higher (for Na+, Mg2+, and K+) cation enrichment in wet season than in dry season. This is somehow consistent with the finding of generally higher TDS in wet season than in dry season. Part of the cation enrichment observed from groundwater may be contributed from the dissolution of aerosols, particulates, or dust in rainwater before falling on the ground (Wong 1997; Tanner and Tam 2000; Tanner and Wong 2000). The weathering of minerals such as plagioclase feldspars, K-feldspar, and biotite may also contribute certain ions to groundwater as showed by the following equations:
The excess “non-marine” component of each ion (Na+, Ca2+, K+, Mg2+, and SO 2−4 ) is calculated from the observed chloride value, assuming no chloride source in the study area other than precipitation characterized by seawater values. To further define possible sources for the ions, statistical techniques were applied to see whether there is any correlation among Na+, Ca2+, K+, Mg2+, SO 2−4 , HCO −3 , SiO2, and NO −3 . The statistical results are shown in Tables 2 and 3.
Na+ and Ca2+ are positively correlated with HCO −3 (R = 0.684 and 0.747, respectively, in wet season and R = 0.636, and 0.515, respectively, in dry season). This implies that Na+, Ca2+, and HCO −3 may be mainly derived from the weathering of plagioclase feldspar. The generally low HCO −3 (0.38 meq/l in wet season and 0.32 meq/l in dry season) indicates low degrees of water-rock interactions in the form of weathering. Na+ and SiO2 are linearly correlated (R = 0.760 in wet season and 0.724 in dry season), which further confirms that the source of Na+ and SiO2 is likely to be the weathering of sodic feldspar (Eq. 1).
It is suggested that plagioclase feldspar is usually the first primary mineral to be weathered significantly, followed by K-feldspar and biotite at a later stage, while quartz should be the most stable of all the minerals (Awoleye 1991). K-feldspars usually appear as large crystals in the groundmass in the volcanics in the area. Their lower surface area to volume ratios may retard the weathering rate. Given the short residence time of shallow groundwater, K-feldspar and biotite may not be significantly weathered. This is further reflected by the poor correlation among Mg2+, K+, and SiO2. Other sources may also contribute Mg2+ and K+ to groundwater. Mg2+ is positively correlated with SO 2−4 (R = 0.798 in wet season and 0.745 in dry season) but since neither can be correlated with NO −3 , they are not likely from anthropogenic sources such as fertilizers, but may be derived naturally from lithological sources or from soils. In fact, the low nitrate level measured in the natural slopes confirms that fertilizers are not a likely source. Another possible explanation of the SO 2−4 excess in shallow groundwater might be acid rainfall. Few sites have molar Mg/Cl smaller than that of the seawater, which may imply that ion exchange is taking place on a localized scale in both seasons. K+ is positively correlated with NO −3 (R = 0.538 in wet season), and although no fertilizer is used in this area, potassium nitrate, KNO3, appears to form in this hot and humid climate by bacterial action during the decomposition of organic materials such as plant debris. In dry season, such reaction may become inactive as indicated by the weak correlation between K+ and NO −3 .
Hydrogeochemcial characteristics of the developed area
Twenty and fourteen seepage samples were collected in the built-up area in wet and dry seasons, respectively. The pH ranged from 5.63 to 7.52 with a mean of 6.75 in wet season. In dry season, based on the samples collected, the pH ranged from 5.64 to 7.10 with a mean of 6.38. The average nitrate concentrations were 18.27 mg/l and 16.26 mg/l in wet and dry seasons, respectively, which are about 11 times and 8 times higher than that of the natural slopes. Seepage samples in the developed area exhibited significantly larger variation in TDS than that in the natural slopes. Unlike the great variety of hydrochemical facies observed in the natural slopes, seepage samples in the developed area were generally dominated by Na–Cl and Na–Ca–Cl water types. More sites showed a higher TDS in wet season than in dry season. However, urban hydrological systems may be far more complex than rural ones because many additional recharge sources like leakage from service pipes can be found (Lerner et al. 1990). Some sites were diluted by rainwater in the form of leakage from stormwater drains in the built-up area during wet season.
In the study area, the built-up space is located downstream, while the natural slope is upstream. The TDS of groundwater usually increase as flow path increases. In this case, the TDS suddenly jumped from <100 mg/l in the natural slopes to as high as 5,300 mg/l in the developed area over less than one kilometer horizontal distance. It seems unlikely that such TDS elevation over a short distance could be caused solely by natural processes. Seepage waters in the developed area are possibly mixed with high-salinity waters. Since all the sampling locations are situated well above the mean sea level and there is virtually no groundwater pumping activity in the area, the elevated salinity in some of the groundwater samples should not be related to seawater intrusion. Possible factors affecting the groundwater chemistry in highly urbanized spaces are discussed in the following section.
Leakage from service pipes
It is generally believed that leakage from water mains occurs in every urban area (Lerner 1986; Pokrovsky et al. 1999; Norin et al. 1999; Ofwat 2000). In Hong Kong, about 22.5% of fresh water in water mains is leaked out (Environmental Protection Department 2002). Leaking conditions could vary considerably in different areas depending on factors such as the age of the water distribution system and the frequency of replacement and repair work. It is reasonable to assume that seepage samples collected in the built-up area may contain a certain amount of leakage from service pipes. Four types of service pipes have been installed in the area: sewage pipes, flushing water pipes, drinking water pipes, and stormwater drains. Focus will be placed on the first three types of service pipes. Water carried in each of them has a unique chemical signature, which is helpful for the identification of leakage.
Sewage usually contains high levels of nitrate, phosphate, and/or saline ammonia. It is suggested by Lerner and Halliday (1993) that phosphate concentration is sensitive to pH, and it can only be used as a marker when the pH is higher than 7 in all samples. However, in this study, few groundwater samples were of pH higher than 7. Therefore, phosphate concentration should be omitted during consideration. Table 4 shows the results of nitrate and saline ammonia measured in the developed area in wet and dry seasons.
As demonstrated by the levels of nitrate (NO −3 ) and saline ammonia (NH +4 ), it seems that some seepage waters in the developed area contained leakage from sewage pipes. However, sewage leakage seems to be insignificant as supported by the generally low maximum concentrations of each of the above parameters.
Fluoride is added into the drinking water in Hong Kong to the level of 0.5 mg/l for dental protection purpose (World Health Organization 1994). Such 0.5 mg/l of fluoride and the TDS of about 120 mg/l could be regarded as references for the identification of leakage from drinking water pipes. No site in the developed area had both fluoride and TDS similar to that of the drinking water. Certain sites had similar fluoride levels; however, they had a much higher TDS than drinking water. Therefore, it seems that there is no obvious leakage from fresh water pipes in the area. However, small-scale leakage is considered to be occurring because zero leakage from water mains is hardly possible in any urban area.
Seawater has been used for flushing in Hong Kong since 1955, and after the mid-1960s in the Western Hong Kong Island (Ho 2001). In 2001, about 80% of the population was supplied with seawater for flushing in Hong Kong (Water Supplies Department 2002). Many seepage samples in the built-up area were characterized by high TDS and dominated by Na+ and Cl− ions, suggesting that they are possibly contaminated by salt water in different extents. Because more samples could be collected in wet season, the extent of leakage from flushing water pipes in the area would be evaluated largely based on the wet season results. The chloride level of seepage samples ranged from 15 mg/l to 2,840 mg/l in wet season, which represents a concentration of 0.08%–15% of seawater in which the Cl− level is about 19,000 mg/l. The mean chloride level of groundwater in the developed area was about 480 mg/l (2.53% of seawater). No seepage was found to be chemically comparable to seawater in the area in both seasons. In this sense, the built-up area is generally free from any major leakage from flushing water pipes. However, small-scale leakage was common. As demonstrated in Tables 5 and 6, groundwater chemistry in the developed area seems to be significantly affected by seawater as indicated by the strong correlation among the major cations and chloride and sulphate, respectively.
Figure 6a and 6b presents semi-log plots of sodium and chloride concentrations in the Mid-Levels area in wet and dry seasons, respectively. There are two distinct slopes (shown as dotted gray lines) on each of the plots, a steep slope at low concentrations and a gentle slope at higher concentrations. The weight Na/Cl for the steep slope is approximately unity, greater than the seawater ratio of 0.55. High ratios are very typical of fresh groundwater. The bimodal slope on these plots may imply a transition from fresh water to water possessing a saline component (Nordstrom et al. 1989). This reflects one of the major changes in the hydrogeochemical system from the natural slope to the developed area. The plots further support that groundwater in the built-up area is obviously affected by seawater.
Water-rock interactions
Owing to the exceptionally high salinity of flushing water, a small amount of leakage could effectively mask the original groundwater chemistry. Seepage waters in the developed area may also have undergone certain kinds of modifications, not just mixed with the leakage from service pipes, as indicated by their compositions. If groundwater is solely dominated by salty flushing water, then the next most abundant cation and anion should be magnesium and sulphate, after sodium and chloride. Also, the observed molar Na/Cl should be close to 0.85. However, these are not the cases for most samples in the developed area.
As indicated by different cation concentrations relative to Cl−, the weathering of minerals and ion exchange processes seem to be taking place in the developed area. The molar Na/Cl of some seepage points in the developed area were slightly higher than that in the natural slopes. This suggests that plagioclase feldspar weathering continues to take place and Na+ is added into the water. The Na/Cl could be lowered by mixing with the leaked salty flushing water in the developed area. Some localities even had molar Na/Cl lower than that of the seawater, confirming that ion exchange is occurring. Many sites had molar Mg/Cl lower than seawater and some of them even showed great deficiency of Mg2+ compared with seawater. These values represent that ion-exchange processes may significantly “remove” Mg2+ ions from groundwater in some localities. It is also likely that Mg2+ is lost in the geological environment as secondary magnesium silicates (Banks et al. 1998). The weathering of minerals and ion exchange are still process in the developed slope. However, it seems that ion-exchange processes became more significant in the lower part of the developed area especially during dry season. More cations could be washed out and contribute to the seepage chemistry during wet season and/or ion-exchange processes may be more dominant in dry season than in wet season. The generally slower groundwater movement in dry season may provide a more favorable environment for ion-exchange processes.
Dissolution of concrete materials
When groundwater flows from the natural slopes down to the developed area, different reactions may take place. Some processes may lower the overall degree of Ca2+ enrichment. These processes include (a) the loss of Ca2+ (and HCO −3 ) by calcite precipitation (Eq. 5), as calcium deposits in the form of “tufa” are discovered in many of the weepholes and horizontal drains in retaining walls; and (b) dilution by leakage from water mains in the built-up area.
However, many seepage samples from the developed slopes were found to be enriched in Ca2+ to nearly the same extent as those from the natural slopes. Therefore, some processes may also be taking place to add Ca2+ into the groundwater. As mentioned, some sites with molar Na/Cl and Mg/Cl lower than that of seawater may indicate ion exchange taking place. It may be possible that Ca2+ is exchanged by Na+ or Mg2+ on clay particles and this process may contribute part of the observed Ca2+ in groundwater. However, according to Figs. 4 and 5, it seems that the positive deviation of Ca2+ from the seawater dilution line is significantly larger than that of the negative deviation of either Na+ or Mg2+ . This indicates that the ion-exchange process alone may not be able to account for the observed amount of Ca2+ .
Another possible source of Ca2+ could be the dissolution of concrete in the developed area where much of the in-situ materials have been largely replaced by construction materials. The high-rise buildings in the Mid-Levels area contain basements and deep foundations (with piers anchored 5 m below the rockhead) constructed by concrete. Some of these structures are permanently or periodically beneath water tables. Concrete slowly degenerates when water soaked by leaching of cement-paste compounds. Calcium hydroxide, which constitutes some 25% of cement paste, is found in zones on aggregate surfaces and may precipitate in voids and cracks. It is readily soluble in water to about 1,200 mg/l (French 1992). The leaching of concrete by groundwater may become more obvious if the water is acidified. Three widely used indices were employed to assess the potential aggressiveness of water towards concrete. They are the Aggressiveness Index (AI), the Langelier Saturation Index (LSI), and the Ryznar Stability Index (RSI).
The aggressiveness index is defined as pH + log10[Ca2+][Alk CaCO3] (French 1992). LSI is an equilibrium model derived from the theoretical concept of saturation and provides an indicator of the degree of saturation of water with respect to calcium carbonate (Langelier 1946). LSI is calculated by the following method:
where pH = −log[H+], a = [log10(TDS) − 1]/10, b = −13.12 × log10 (°C + 273) + 34.55, c = log10 (Ca2+ as CaCO3, mg/l) − 0.4, d = log10 (alkalinity as CaCO3, mg/l)
RSI attempts to correlate an empirical database of scale thickness observation in water systems to the water chemistry (Roberge 2000). Like the LSI, the RSI has its basis in the concept of saturation level. The Ryznar index is calculated by:
where pH is the measured water pH and pHs is the pH at saturation in calcite or calcium carbonate.
From Table 7, it is clear that groundwater changes from highly corrosive to concrete materials from the natural slopes to less corrosive in the built-up area. This is because the highly corrosive groundwater from the natural area would eventually flow to the built-up area, and concrete in foundations and other underground structures may be attacked, releasing Ca2+ which in turn causes the water to become less corrosive. The calculated results seem to suggest that groundwater in dry season was generally slightly more corrosive to concrete whether in the natural slope or developed area. This may be because the pH of rainwater in dry season is generally lower than that in wet season, as stated previously. The more acidic rainwater in dry season may increase the corrosiveness of groundwater and thus more Ca2+ may be leached from concrete. In dry season, the highly corrosive groundwater and the generally low groundwater table might cause corrosion of deep foundations, which may negatively affect the structure of high-rise buildings in the study area accordingly. Groundwater usually moves more slowly in dry season because of the lower hydraulic gradient. This may increase the time of contact between groundwater and concrete and increase the amount of Ca2+ leached out. Moreover, as suggested, ion-exchange processes seem to be more effective in dry season and more Ca2+ may be released from clay particles in this way.
Conclusion
By investigating the major ion chemistry of groundwater collected from natural slopes and highly urbanized spaces in a coastal area in Hong Kong, the major natural and anthropogenic processes affecting the groundwater chemical system may be defined.
In the natural slopes, groundwater chemistry is mainly controlled by water-rock interactions, particularly plagioclase weathering. The weathering of K-feldspar and biotite may take place to a lesser degree due to the short groundwater residence time and the high resistance of these minerals towards weathering. Limited and localized ion-exchange processes and the decomposition of plant materials also occur. In general, the TDS in groundwater samples collected in wet season was higher than that in dry season. The highly diverse hydrochemical facies in both seasons may imply the high heterogeneity and complex geological environment of the slope.
In the developed area, many groundwater samples were contaminated to various extents by leakage from service pipes, including sewage, fresh water, and flushing water pipes. Small-scale leakage from service pipes was common in the area but no major leakage was noticed. This finding is useful for the Water Supplies Department of the Hong Kong Government to further reduce the amount of leakage in the area. Using chloride as a normalizing factor, it seems that the weathering of plagioclase feldspar occurred in the developed area, adding Na+ ions to the groundwater. As indicated by Na+ and Mg2+ ions, ion-exchange processes could also change the water chemistry significantly. The ion-exchange process appears more significant in the lower part of the developed area and in the dry season. Ca2+ ions may add to groundwater through the process of dissolution of subsurface concrete materials, since groundwater in the natural slopes is strongly corrosive to concrete in the developed area. Water samples in dry season were slightly more corrosive to concrete. Such corrosive groundwater may attack the deep foundations of buildings since the water table is relatively low during dry season. This finding may be instructive for foundation designs in the area. Excess Ca2+ may deposit in dewatering systems such as horizontal drains installed in cut slopes. This may adversely affect their performance in lowering high water tables.
Besides the implications stated, the results presented may also have a reference value to other highly urbanized coastal areas in the world for the identification of possible groundwater contamination sources. This may aid in groundwater resources management.
References
Allen PM, Stephens EA (1971) Report on the Geological Survey of Hong Kong. Hong Kong Government Press, p 116 plus 2 maps
(APHA) American Public Health Association, American Water Works Association and Water Environment Federation (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, USA
Awoleye OA (1991) Weathering and iron oxide mineralogy of Hong Kong Granite. PhD Thesis, University of Glasgow
Banks D, Reimann C, Skarphagen H (1998) The comparative hydrochemistry of two granitic island aquifers: The Isles of Scilly, UK and the Havler Islands, Norway. Sci Total Environ 209:169–183
Environmental Protection Agency (EPA) (1991–1994) Air quality in Hong Kong: results from the EPA air quality monitoring network for Hong Kong 1991–1994, Environmental Protection Agency, Hong Kong
Environmental Protection Department (EPD) (2002) Sustainable Development for the 21st Century: Environmental Baseline Report. Environmental Protection Department, Hong Kong Government Press
European Community (EC) (1988) Quality of Water intended for Human Consumption Regulations. S. I. No. 81 of 1988
Feth JH (1981) Chloride in natural continental water: a review. U. S. Geological Survey Water-Supply Paper 2176, p 30
French WJ (1992) Influence of groundwater chemistry and motion on highway construction materials. Ground chemistry: implications for construction. In: Hawkins AB (ed) Proceedings of the international conference on the implications of ground chemistry and microbiology for construction, University of Bristol, UK
Geotechnical Control Office (GCO) (1982) Mid-levels Study: report on geology, hydrology and soil properties. Geotechnical Control Office, Hong Kong Government
Ho PY (2001) Water for a barren rock—150 years of water supply in Hong Kong. Commercial Press, Hong Kong
Hong Kong Observatory (HKO) (2003) Summary of meteorological observations in Hong Kong 2002. Hong Kong Observatory, Hong Kong
Jiao JJ, Leung CM, Ding GP (2003) Confined Groundwater at No. 52, Hollywood Road, Hong Kong. In: Lee CF, Tham LG (eds) International conference on slope engineering, 8–10 December 2003, Hong Kong
Jiao JJ, Wang XS, Nandy S (2004) Preliminary assessment of the impacts of deep foundations and land reclamation on groundwater flow in a coastal area in Hong Kong, China. Hydrogeol J, DOI: 10.1007/s10040-004-0393-6
Lam KC (1981) Groundwater pollution south of Yuen Long: a summary report. In: Paper presented at environmental conservation in the 80’s, an exhibition by the Conservancy Association in the City Hall, 21–22 February 1981, Hong Kong
Lam KC (1983) The chemical quality and use of well water in the New Territories. Department of Geography, Chinese University of Hong Kong
Langelier WF (1946) Chemical equilibria in water treatment. J Am Water Works Assoc 38:169
Lerner DN (1986) Leaking pipes recharge groundwater. Ground Water 24:654–662
Lerner DN, Halliday D (1993) The impacts of sewers on groundwater quality, Groundwater problems in urban areas. In: Wilkinson WB (ed) Proceedings of the international conference organized by the Institution of Civil Engineers, 2–3 June 1993, Thomas Telford, London, pp 64–75
Lerner DN, Issar AS, Simmers I (1990) Groundwater recharge: a guide to understanding and estimating natural recharge. In: Lerner DN, Issar AS, Simmers I (eds) International contribution to hydrogeology 8. Verlag Heinz Heise, Hannover IAH
Nordstrom DK, Ball JW, Donahoe RJ, Whittemore D (1989) Groundwater chemistry and water-rock interactions at Stripa. Geochimica et Cosmochimica Acta 53:1727–1740
Norin M, Hultén A-M, Svensson C (1999) Groundwater studies conducted in Göteborg, Sewden. In: Chilton J (ed) Groundwater in the urban environment—selected city profiles. AA Balkema, Rotterdam, pp 209–216
Ofwat (2000) 1999–2000 report on leakage and water efficiency. Office of Water Services, Birmingham
Pokrovsky DS, Rogov GM, Kuzevanov KI (1999) The impact of urbanization on the hydrogeological conditions of Tomsk, Russia. In: Chilton J (ed) Groundwater in the urban environment—selected city profiles. AA Balkema, Rotterdam, pp 217–223
Roberge PR (2000) Handbook of corrosion engineering. McGraw-Hill, New York
Tanner PA (1999a) Analysis of Hong Kong daily bulk and wet deposition data from 1994 to 1995. Atmos Environ 33:1757–1766
Tanner PA (1999b) Relationships between rainwater composition and synoptic weather systems deduced from measurement and analysis of Hong Kong daily rainwater data. J Atmosp Chem 33:219–240
Tanner PA, Wong AYS (2000) Soluble trace metals and major ionic species in the bulk deposition and atmosphere of Hong Kong. Water Air Soil Pollut 122:261–279
Tanner PA, Tam WF (2000) Small-scale horizontal variations in ionic concentrations of bulk deposition from Hong Kong. Water Air Soil Pollut 122:433–448
Water Supplies Department (WSD) (2002) Annual Report (2001–2002). Water Supplies Department, Hong Kong Government
Wong AYS, Tanner PA (1997) Monitoring environmental pollution in Hong Kong: trends and prospects. Trend Anal Chem 16(4):180–190
Wong YS (1997) Analysis of major and trace components in Hong Kong rainwater. The City University of Hong Kong, Hong Kong, MPhil Thesis
World Health Organization (WHO) (1994) Fluorides and oral health: report of a WHO expert committee on oral health status and fluoride use, WHO Technical Report Series, No 846
Acknowledgements
This study is partially supported by the Seed Funding within the Faculty of Science in The University of Hong Kong, the Hong Kong Research Grants Council (RGC) (HKU 7013/03) of the Hong Kong Special Administration Region, China, and Development Budget for Area of Excellence in Water Environment Engineering, the University of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Summary of chemical results of samples from the Mid-Levels area in wet season. (Temp is in °C, E.C. is in mS/cm, Flow is in mL/s and the rest are in mg/l). “n.d.” represents not determined
Sample ID | pH | Temp | E.C. | DO | Flow | Na+ | Mg2+ | K+ | Ca2+ | SiO2 | F− | Cl− | NO −3 | PO 3−4 | SO 2−4 | HCO −3 | TDS | % error |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Natural slope | ||||||||||||||||||
13 | 6.95 | 26.0 | 0.06 | 5.59 | – | 10.70 | 1.07 | 2.49 | 4.58 | 9.75 | 0.12 | 11.18 | 1.42 | n.d. | 9.78 | 17.79 | 69.01 | 0.75 |
15 | 6.71 | 24.7 | 0.06 | 5.68 | – | 8.71 | 0.94 | 1.60 | 6.44 | 11.26 | 0.14 | 9.96 | 1.01 | n.d. | 7.09 | 24.63 | 71.97 | −1.78 |
16 | 6.83 | 25.8 | 0.06 | 5.63 | – | 9.63 | 1.01 | 2.18 | 5.62 | 9.63 | 0.12 | 11.09 | 1.26 | n.d. | 9.61 | 20.07 | 70.38 | −1.39 |
17 | 6.71 | 24.6 | 0.06 | 5.63 | – | 10.74 | 1.11 | 2.43 | 5.65 | 9.85 | 0.11 | 11.21 | 1.23 | n.d. | 9.80 | 20.07 | 72.35 | 1.89 |
18 | 6.16 | 22.4 | 0.05 | 5.34 | 93 | 10.85 | 0.82 | 3.23 | 2.77 | 14.60 | 0.13 | 10.69 | 2.13 | n.d. | 3.46 | 25.54 | 74.52 | −4.16 |
19 | 6.33 | 22.4 | 0.06 | 5.38 | 321 | 10.75 | 0.79 | 2.67 | 4.70 | 17.33 | 0.30 | 10.10 | 1.29 | n.d. | 3.66 | 33.75 | 85.58 | −5.64 |
20 | 6.41 | 22.2 | 0.08 | 5.78 | 59 | 11.67 | 1.17 | 3.01 | 8.38 | 14.38 | 0.51 | 10.55 | 1.73 | n.d. | 6.73 | 37.10 | 95.51 | 1.19 |
21 | 6.16 | 21.9 | 0.07 | 5.38 | 315 | 10.92 | 1.05 | 3.12 | 8.10 | 13.15 | 0.26 | 10.41 | 1.87 | n.d. | 7.86 | 34.21 | 91.19 | −0.16 |
22 | 6.14 | 22.4 | 0.05 | 5.72 | 33 | 8.16 | 1.25 | 2.52 | 6.00 | 6.84 | 0.13 | 8.71 | 0.99 | n.d. | 10.61 | 20.52 | 65.90 | 0.17 |
23 | 6.71 | 25.1 | 0.08 | 5.68 | 12 | 8.38 | 1.09 | 2.70 | 14.44 | 7.08 | 0.13 | 8.48 | 1.10 | n.d. | 7.98 | 43.70 | 95.24 | 4.40 |
24 | 6.81 | 23.4 | 0.05 | 5.92 | – | 7.59 | 1.09 | 2.46 | 4.19 | 6.76 | 0.10 | 9.06 | 2.79 | n.d. | 10.35 | 13.23 | 57.70 | −2.88 |
26 | 6.75 | 24.6 | 0.05 | 5.72 | – | 8.20 | 1.07 | 2.52 | 6.51 | 5.43 | 0.08 | 10.87 | 2.18 | n.d. | 8.87 | 17.79 | 63.65 | 0.98 |
D009 | 4.88 | 21.1 | 0.05 | 5.49 | 81 | 7.33 | 1.19 | 2.90 | 3.56 | 7.62 | 0.20 | 9.42 | 2.22 | n.d. | 8.92 | 12.32 | 55.91 | −1.51 |
D012 | 4.73 | 21.5 | 0.04 | 5.52 | 36 | 6.96 | 1.26 | 2.59 | 3.52 | 6.56 | 0.26 | 9.67 | 2.22 | n.d. | 8.30 | 11.86 | 53.42 | −2.10 |
D116A | 5.11 | 21.5 | 0.05 | 4.96 | 71 | 8.46 | 0.73 | 2.43 | 4.29 | 11.39 | 0.14 | 9.64 | 1.78 | n.d. | 4.12 | 23.72 | 67.02 | −4.81 |
D118 | 5.09 | 21.7 | 0.06 | 5.36 | 60 | 10.60 | 0.99 | 3.85 | 4.20 | 11.65 | 0.11 | 14.31 | 3.57 | n.d. | 2.21 | 21.90 | 73.76 | −0.90 |
Drain | 4.66 | 22.3 | 0.05 | 4.11? | 159 | 8.21 | 0.86 | 2.66 | 3.68 | 9.69 | 0.11 | 10.19 | 0.58 | n.d. | 4.57 | 19.16 | 60.17 | −1.91 |
PS#1 | 6.33 | 22.2 | 0.05 | 5.74 | – | 10.12 | 0.80 | 3.17 | 3.51 | 11.44 | 0.21 | 11.34 | 1.23 | n.d. | 5.42 | 21.90 | 69.39 | −3.14 |
Developed area | ||||||||||||||||||
30 | – | 23.2 | 0.13 | 5.97 | 98 | 18.08 | 1.89 | 3.54 | 11.29 | 10.40 | 0.50 | 14.75 | 1.14 | n.d. | 15.70 | 56.01 | 133.46 | −2.55 |
7 | 7.39 | 29.9 | 0.26 | 5.27 | – | 20.80 | 2.76 | 8.53 | 34.36 | 7.65 | 0.60 | 25.23 | 18.84 | n.d. | 27.60 | 80.27 | 227.14 | 2.66 |
35 | 7.05 | 26.0 | 0.53 | 5.66 | – | 89.67 | 7.09 | 9.24 | 21.15 | 4.14 | 0.36 | 106.05 | 8.07 | n.d. | 57.55 | 65.13 | 370.28 | 3.48 |
37 | 6.80 | 25.7 | 0.33 | 5.51 | 28 | 38.01 | 3.79 | 8.85 | 32.11 | 8.74 | 0.28 | 48.96 | 21.12 | 1.25 | 32.15 | 79.36 | 275.39 | 1.37 |
39 | 6.80 | 27.4 | 0.71 | 5.29 | – | 87.86 | 8.64 | 18.17 | 68.23 | 9.78 | 0.64 | 99.83 | 32.33 | 1.20 | 62.82 | 169.67 | 561.07 | 6.16 |
No.2 | 6.94 | 26.1 | 0.54 | 5.31 | 15 | 59.23 | 17.52 | 18.43 | 41.84 | 0 | 0.07 | 81.82 | 2.79 | n.d. | 35.87 | 218 | 476.26 | −0.71 |
36 | 5.63 | 24.7 | 0.93 | 4.03 | 70 | 137.09 | 13.84 | 13.52 | 40.40 | 5.80 | 0.14 | 263.85 | 11.00 | n.d. | 60.69 | 43.51 | 593.03 | −0.70 |
43 | 6.59 | 25.9 | 0.99 | 5.39 | – | 154.67 | 16.11 | 14.08 | 35.40 | 6.11 | 0.27 | 262.57 | 15.22 | n.d. | 77.00 | 103.99 | 688.26 | −3.69 |
44 | 6.70 | 26.4 | 0.99 | 5.63 | 116 | 140.11 | 16.21 | 14.17 | 52.78 | 6.11 | 0.33 | 260.61 | 17.18 | n.d. | 65.83 | 88.48 | 664.80 | −0.12 |
8 | 7.52 | 26.7 | 1.22 | 5.67 | 265 | 171.30 | 10.30 | 21.14 | 83.18 | 9.04 | 0.38 | 289.71 | 34.87 | n.d. | 75.22 | 106.27 | 806.29 | 3.79 |
27 | 5.75 | 25.2 | 1.35 | 3.17 | 35 | 234.77 | 7.72 | 14.46 | 39.38 | 5.53 | 0.53 | 361.90 | 21.03 | n.d. | 73.77 | 40.05 | 804.51 | 1.70 |
42 | 6.35 | 27.8 | 1.36 | 4.95 | – | 237.30 | 20.20 | 18.06 | 32.73 | 8.85 | 0.29 | 319.78 | 19.11 | n.d. | 123.15 | 55.19 | 838.64 | 4.77 |
45 | 6.96 | 25.8 | 1.27 | 5.56 | 32 | 189.10 | 20.83 | 12.69 | 57.23 | 4.44 | 0.20 | 312.42 | 12.18 | n.d. | 69.85 | 116.31 | 799.07 | 2.94 |
46 | 6.34 | 25.1 | 1.43 | 4.83 | 35 | 234.88 | 22.61 | 15.47 | 28.58 | 1.09 | <0.01 | 331.15 | 25.81 | n.d. | 101.16 | 91.68 | 856.62 | 1.95 |
47 | 7.11 | 26.3 | 1.25 | 5.61 | – | 178.67 | 13.85 | 16.42 | 70.28 | 5.38 | 0.29 | 295.70 | 13.86 | n.d. | 84.84 | 148.51 | 831.38 | 0.28 |
48 | 6.20 | 25.6 | 1.47 | 3.94 | 63 | 223.03 | 35.56 | 30.64 | 30.42 | 2.26 | <0.01 | 419.49 | 25.16 | n.d. | 83.65 | 68.87 | 923.78 | −0.60 |
28 | 7.15 | 26.7 | 2.49 | 5.69 | 32 | 396.91 | 46.75 | 31.65 | 90.75 | 3.67 | <0.01 | 722.80 | 28.15 | n.d. | 139.76 | 200.69 | 1673.30 | −1.11 |
29 | 6.95 | 25.8 | 2.91 | 5.63 | – | 488.44 | 48.23 | 33.45 | 55.07 | 2.04 | <0.01 | 742.55 | 36.60 | n.d. | 124.70 | 125.89 | 1671.13 | 4.76 |
38 | 7.02 | 25.8 | 7.94 | 5.73 | 256 | 1448.45 | 186.88 | 94.52 | 277.08 | 4.84 | <0.01 | 2844.39 | 9.00 | n.d. | 362.61 | 63.86 | 5331.71 | 3.07 |
40 | 6.93 | 27.8 | 4.22 | 5.16 | – | 894.38 | 100.76 | 47.47 | 171.56 | 6.01 | 0.30 | 1766.42 | 11.90 | n.d. | 210.16 | 127.25 | 3353.81 | 0.43 |
Appendix 2
Summary of chemical results of samples from the Mid-Levels area in dry season. (Temp is in °C, E.C. is in mS/cm, Flow is in mL/s and the rest are in mg/l). “n.d.” represents not determined.
Sample ID | pH | Temp | E.C. | DO | Flow | Na+ | Mg2+ | K+ | Ca2+ | SiO2 | F− | Cl− | NO −3 | PO 3−4 | SO 2−4 | HCO −3 | TDS | % error |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Natural slope | ||||||||||||||||||
13 | 6.43 | 15.7 | 0.06 | 9.07 | – | 10.17 | 1.05 | 2.14 | 5.55 | 13.08 | 0.09 | 11.99 | 3.67 | n.d. | 8.79 | 15.05 | 71.59 | 2.00 |
16 | 6.43 | 15.3 | 0.06 | 8.92 | – | 10.07 | 1.02 | 1.98 | 4.17 | 5.92 | 0.08 | 11.77 | 3.34 | n.d. | 8.56 | 16.42 | 63.32 | −3.29 |
17 | 6.36 | 16.0 | 0.06 | 8.66 | – | 9.51 | 1.01 | 1.79 | 6.47 | 14.34 | 0.09 | 11.99 | 3.34 | n.d. | 8.73 | 15.51 | 72.78 | 2.20 |
18 | 5.14 | 21.8 | 0.06 | 6.60 | 108 | 9.65 | 0.69 | 2.33 | 4.48 | 18.29 | 0.18 | 10.74 | 2.35 | n.d. | 3.38 | 21.44 | 73.52 | −0.19 |
19 | 5.21 | 21.9 | 0.06 | 5.90 | 134 | 10.21 | 0.72 | 2.82 | 6.37 | 20.30 | 0.17 | 9.74 | 0.90 | n.d. | 2.66 | 26.91 | 80.79 | 6.38 |
20 | 5.62 | 21.2 | 0.08 | 7.44 | 26 | 9.56 | 1.02 | 2.08 | 8.32 | 18.67 | 0.53 | 10.45 | 1.33 | n.d. | 6.07 | 34.66 | 92.69 | −2.16 |
21 | 5.20 | 21.7 | 0.07 | 7.51 | 170 | 8.97 | 0.88 | 2.08 | 6.19 | 14.42 | 0.13 | 10.23 | 1.32 | n.d. | 6.47 | 26.91 | 77.61 | −3.53 |
24 | 5.91 | 16.1 | 0.05 | 8.41 | 26 | 6.90 | 1.02 | 1.59 | 4.77 | 7.85 | 0.07 | 9.89 | 2.62 | n.d. | 9.87 | 7.30 | 51.87 | 1.23 |
26 | 6.52 | 16.5 | 0.09 | – | – | 8.40 | 1.06 | 2.05 | 9.64 | 8.76 | 0.12 | 12.59 | 2.27 | n.d. | 9.18 | 20.07 | 74.14 | 3.91 |
D012 | 5.50 | 18.6 | 0.05 | 8.31 | 6 | 6.39 | 1.15 | 1.83 | 3.66 | 8.73 | 0.12 | 9.03 | 2.21 | n.d. | 8.55 | 11.86 | 53.53 | −4.79 |
D116A | 5.22 | 21.2 | 0.05 | 6.97 | 51 | 7.64 | 0.69 | 1.72 | 4.51 | 13.99 | 0.15 | 9.02 | 1.27 | n.d. | 4.26 | 22.81 | 66.05 | −5.68 |
LFS | 4.63 | 21.9 | 0.28? | 6.35 | 95 | 7.41 | 0.52 | 3.00 | 2.24 | 7.15 | 0.11 | 9.13 | 1.08 | n.d. | 3.05 | 15.96 | 49.69 | −4.01 |
Developed area | ||||||||||||||||||
MM | 5.85 | 21.30 | 0.15 | 8.20 | 371 | 10.50 | 2.27 | 2.47 | 16.74 | 6.50 | 0.47 | 20.07 | 6.31 | n.d. | 19.22 | 31.47 | 116.04 | −1.32 |
30 | 6.44 | 21.70 | 0.14 | 7.81 | 83 | 15.91 | 1.74 | 2.59 | 12.32 | 17.40 | 0.50 | 14.56 | 1.54 | n.d. | 15.23 | 49.72 | 131.51 | −1.67 |
No.2 | 6.66 | 24.80 | 0.43 | – | 12 | 42.04 | 11.64 | 10.86 | 25.57 | 3.08 | 0.03 | 36.98 | 3.84 | n.d. | 25.57 | 188.83 | 348.58 | −4.33 |
8 | 7.07 | 20.20 | 1.10 | 7.88 | 106 | 147.87 | 8.79 | 16.00 | 86.74 | 7.15 | 0.33 | 282.15 | 23.10 | n.d. | 59.84 | 98.06 | 732.32 | 3.07 |
27 | 6.31 | 23.60 | 0.96 | 4.25 | 10 | 130.94 | 9.23 | 12.17 | 50.57 | 3.22 | 0.23 | 243.12 | 16.06 | n.d. | 62.84 | 89.40 | 619.62 | −3.13 |
43 | 6.35 | 23.30 | 0.94 | 7.49 | – | 131.10 | 14.62 | 10.87 | 69.12 | 5.95 | 0.19 | 234.14 | 14.06 | n.d. | 70.42 | 77.54 | 629.15 | 5.26 |
44 | 6.48 | 22.80 | 0.97 | 7.23 | 89 | 129.73 | 15.45 | 10.29 | 49.81 | 5.97 | 0.12 | 247.60 | 14.58 | n.d. | 61.79 | 67.50 | 604.41 | 0.26 |
45 | 7.10 | 23.70 | 0.53 | 7.29 | 70 | 67.40 | 8.41 | 5.32 | 33.10 | 5.55 | 0.19 | 115.46 | 6.73 | n.d. | 32.81 | 74.80 | 350.70 | 1.28 |
46 | 5.90 | 25.50 | 1.57 | 6.16 | 32 | 226.34 | 25.39 | 13.69 | 59.50 | 1.13 | <0.01 | 425.61 | 26.60 | n.d. | 95.11 | 82.56 | 959.31 | −1.66 |
48 | 5.95 | 24.00 | 1.23 | 4.94 | 29 | 174.22 | 22.81 | 13.98 | 38.76 | 1.97 | <0.01 | 332.35 | 21.68 | n.d. | 74.76 | 71.61 | 753.87 | −2.93 |
LLR | 5.64 | 23.90 | 1.49 | 2.33 | 14 | 217.05 | 8.00 | 10.54 | 55.88 | 5.99 | 0.42 | 425.49 | 14.18 | n.d. | 70.24 | 36.94 | 847.93 | −4.16 |
28 | 7.01 | 21.80 | 2.65 | 6.72 | 11 | 386.08 | 45.84 | 28.07 | 118.10 | 3.97 | <0.01 | 780.03 | 26.52 | n.d. | 139.06 | 181.99 | 1715.93 | −2.04 |
29 | 6.73 | 22.80 | 3.36 | 7.39 | 46 | 581.82 | 61.98 | 28.80 | 191.88 | 2.33 | <0.01 | 1063.28 | 31.88 | n.d. | 173.55 | 124.06 | 2259.57 | 5.94 |
42 | 5.84 | 21.80 | 2.38 | – | Drops | 372.59 | 39.76 | 18.08 | 111.00 | 11.77 | <0.01 | 723.13 | 20.51 | n.d. | 156.27 | 42.87 | 1495.99 | 1.58 |
Rights and permissions
About this article
Cite this article
Leung, Cm., Jiao, J.J., Malpas, J. et al. Factors affecting the groundwater chemistry in a highly urbanized coastal area in Hong Kong: an example from the Mid-Levels area. Environ Geol 48, 480–495 (2005). https://doi.org/10.1007/s00254-005-1290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-005-1290-6