Abstract
There is a growing public concern over the potential accumulation of heavy metals in soil, owing to rapid urban and industrial development in the last decade in India. Therefore, an attempt was made to investigate the pollution caused due to excessive accumulation of heavy metals in soils near Thane–Belapur industrial belt of Mumbai. Soil samples were collected from surrounding industrial areas and were analyzed for toxic/heavy metals by X-ray fluorescence spectrometer. The analytical results indicate that the soils in the study area were enriched with Cu, Cr, Co, Ni and Zn. The concentration ranges were: Cu 3.10–271.2 mg/kg (average 104.6 mg/kg), Cr 177.9–1,039 mg/kg (average 521.3 mg/kg), Co 44.8–101.6 mg/kg (average 68.7 mg/kg), Ni 64.4–537.8 mg/kg (average 183.6 mg/kg) and Zn 96.6–763.2 mg/kg (average 191.3 mg/kg). The visualization of spatial data is made by preparing distribution maps of heavy metal concentration in soils and co-relation diagrams. These results highlight the need for instituting a systematic and continuous monitoring of the study area for heavy metals and other forms of pollution to ensure that pollution does not become a serious problem in future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mumbai (Latitude 18°53′ to 19°04; Longitude 72°48′ to 72°53′E) is a heavily populated industrial island city on the west coast, which is known as the commercial capital of India. During the past 30 years, it has grown from a small city to an international city. In 1971, Mumbai had a population of 5,970,575, which increased to 8,227,382 in 1981, 9,990,000 in 1991 and 11,430,000 in 2001 (Dept of Public Health, Govt. of Maharashtra 2000). Congruent to this population expansion, the number of registered motor vehicles in Mumbai city has also increased. Fundamental to this rapid urbanization are the numerous industries at Thane–Belapur industrial area, which is one of the most rapidly developing industrial areas around Mumbai city, developed by Maharashtra State Government. This is also one of the contaminated sites identified by Central Pollution Control Board (CPCB), New Delhi. About 400 industries are in a 20 km area with all types of process industries, including chemical, pharmaceutical, textile, steel, paper, plastic, fertilizers, etc. Some of the well-known industries are E-Merck (I) Ltd., Hindustan Lever Ltd. and Pidilite industries Ltd., which have flourished in the area during recent years. Many of these industries discharge their treated or untreated effluents either on the open land or into unlined water streams. Average annual rainfall in the study area is about 2,500 to 3,500 mm, which falls during the period from of June to October. The rock formation in the area is derived from Deccan basalt and also from granites, gneisses and laterite. The soils of this region are highly saline. They are calcareous, neutral to alkaline (pH 7.5–8.5) and clays with a high amount of bases and have water-holding capacity (Shankar et al. 1994).
Pollution is a common concern in many modern, industrialized urban communities around the globe (Zhang et al. 1999; Moon et al. 2000). The connection between pollution and various health problems is now well documented and reasonably well known by the general public (Barzilay et al. 1999). Soils are usually the interface between human activities and other parts of the environment that should be preserved and protected (Krzyztof et al. 2004). Soil samples represent an excellent media to monitor heavy metal pollution because anthropogenic heavy metals are usually deposited on topsoils. Furthermore, soils not only serve as sources for certain metals but also function as sinks for metal contaminates (Alloway 1995; Govil et al. 2001). Therefore, analyses of heavy metals in soils offer an ideal means to monitor not only the pollution of soils itself, but also the quality of the overall environment as reflected in soil. Trace metal distribution is well documented for many countries (USA, Germany, Japan; Thuy et al. 2000); furthermore, a number of recent works (Manjunatha et al. 2001; Rawlins et al. 2002) evaluated the accumulation of heavy metals in various developed and developing countries. The situation is more serious in developing countries because of a sudden increase in industrialization and urbanization during the last decade.
The main objectives of the present investigation were: (1) to determine the concentration of heavy metals (Cu, Cr, Co, Ni and Zn) and to find the extent of their distribution in soils within the area, and (2) to provide a scientific basis for pollution control and further monitoring.
Methodology
Sample collection
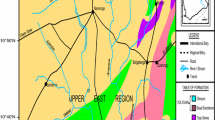
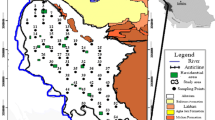
A total of 28 soil samples were collected, mostly from the upper surface (0–50 cm) to differentiate between natural and anthropogenic sources of pollutants. Normally industrial pollutants contaminate the upper layer of the soil (0–40 cm). In case of natural pollutant, the entire soil shows high metal enrichment at all depths. The samples were collected in self-locking polythene bags and sealed in double bags. Use of metal tools was avoided and a plastic spatula was used for sample collection. Figure 1 shows the location of the soil samples collected from the area.
Sample preparation
Soil samples were dried for 2 days at 60°C. The dry soil sample was disaggregated with mortar pestle. The sample was finely powdered to −250 mesh size (US standard) using a swing grinding mill. Sample pellets were prepared for analysis by X-ray fluorescence (XRF) spectrometry, using a backing of boric acid and pressing at 25 tons of pressure. A hydraulic press was used to prepare the pellets for XRF analysis to determine trace elements.
Instrumentation
Along with a 4 kW X-ray generator, a Philips PW-2440 fully automatic, microprocessor- controlled, 168-position automatic PW 2540 vrc sample changer X-ray Spectrometer was used for the determination of major and trace metals (Cu, Cr, Co, Ni and Zn) in soil samples. Suitable software ‘Super Q’ was used to define dead-time correction and inter element matrix-effects. Reference samples from the US Geological Survey, Canadian Geological Survey, International Working Group France (Govindaraju 1994), and NGRI India (Govil and Narayana 1999; Govil 1993) were used to prepare calibration curves for major oxides and trace metals (for details of XRF procedure, see Govil 1985; Rao and Govil 1995). The trace- element data is reported in Table 1 and major-element data in Table 2.
Processing of analytical data
Summary statistics of the heavy metal data (Table 3) set were first calculated to evaluate the distribution of elements. Frequency distribution of each of the elements was examined based on comparison with the threshold values, cluster analysis and calculation of skewness. Skewness measures the asymmetry of the observations. Geochemical variables, especially trace elements, have low concentrations in the environment, while the anomalies resulting from natural processes and anthropogenic activities cause the occurrence of high concentration in data sets. This makes the probability distribution positively skewed (Webster and Oliver 2001). The correlation between the elements was estimated by the scatter plots (Huisman et al. 1997).
Results and discussions
Total heavy metals concentrations
To determine the variability of the content of heavy metals in soils, comparative analysis of the elements was first performed. Table 3 summarizes the statistics of the data. Maximum permitted threshold soil concentrations of potentially toxic metals prescribed by WHO guidelines (WHO 1996) are also given. Distributions of Cu, Cr, Co, Ni and Zn are skewed (the skewness coefficient exceeded 1.0). Regardless of skewed distributions, the mean value and median are similar for most metals, considering that the median value was lower (Table 3). The highest skewness is found in Zinc, with the g1 value of 2.97. However, after the logarithmic transformation, the mean value and the median equalize, g1=0.47 indicating that these values can be further tested by applying more complex statistical methods. The environmental geochemical phenomena, including heavy metal concentrations in soils, commonly correlated, are caused by the physico-chemical features of elements and geochemical processes (Zhang and Selinus 1998). Also, some of the major oxide data, as shown in Table 2 revealed high concentrations of Fe and Mn in the area. The study also showed concentrations of the metals that are to a large extent linearly correlated. Table 4 is the correlation matrix. It shows strong correlation between Cr and Co (r=0.75361), Cu and Zn (r=0.71477), Co and Ni (r=0.79984).
Copper
The average concentration of copper in the investigated area is 104.6 mg/kg and lies between 3.10 mg/kg and 271.2 mg/kg (Table 1). The normal threshold value prescribed in soils is 30 mg/kg. Figure 2 shows a marked copper accumulation in soils around the industrial area, which may be from application of agrochemicals in the agro-based industry in the area. Copper is, however, characterized by the so-called point sources of contamination, namely uncontrolled, active or untended waste dumps. Such places imply great contamination risks for not only soil but also groundwater (Romic et al. 2001; Govil et al. 1999). From the aspect of groundwater hazards in this area, only the quantities of metals leached to the sorum/gravel substratum contact are dispersed in the aquifer, thus potentially contaminating it. The transfer of contamination and its dispersion in the aquifer is a very slow process, and changes in metal concentration in the upper aquifer layer are not manifested in a short time. However, the smaller the sorum depth, the higher the risk.
Chromium
Chromium levels around the study area ranged from 177.9 mg/kg to 1,039 mg/kg, with an average of 521.3 mg/kg (Table 2), which is considerably high as shown in the distribution map of chromium (Fig. 3). The normal range of Cr in soils is 100 mg/kg (Ferguson 1990). Chromium and its compounds are primarily used in the production of steel and other alloys, chrome plating and pigment production, which is the major industrial activity in the Thane–Belapur study area. However, inadequate disposal of waste containing chromium at industrial sites in the past decade has contaminated the land and groundwater. Although small amounts of naturally occurring trivalent chromium (Cr+ 3) are essential for nutrition, the presence of industry produced (Cr+ 6), in water or the food chain is considered to be highly hazardous. The source of Cr appears to be anthropogenic—from some industries producing steel, textiles in the area. Ansari et al. (1999) found very high Cr levels in soil, i.e. up to 1,220 mg/kg in some industrial areas of India.
Cobalt
The concentrations of cobalt in the analyzsed samples of soil in the study area are generally high. In almost all the samples the concentrations were above the normal threshold value of 17 mg/kg with average concentration of 68.7 mg/kg ranging from 44.8 mg/kg to 101.6 mg/kg. Cobalt usually occurs in association with other metals, such as copper, nickel, manganese and arsenic. One of the most important soil properties is its acidity. The more acidic the soil, the greater the potential for cobalt toxicity (Ferguson 1990; Govil et al. 1998). The source of cobalt to the environment could be the burning of coal and the industrial processes that use the metal or its compounds. The highest concentration, 101.6 mg/kg, in the study area was determined at a location in the vicinity of the metal industries (Fig. 4), indicating the source as anthropogenic.
Nickel
The geochemical map in Fig. 5 shows the spatial distribution of Ni in the study region. The high average concentration of nickel (183.6 mg/kg) is related to some anthropogenic input. However, the highest concentrations up to 537.8 mg/kg are found in the area which is higher than the normal threshold content of Ni in soils from 1 mg/kg to 80 mg/kg (Aswathanarayana 1995). Nickel accumulation in the soil is obviously influenced by the Thane–belapur ring road and some of the industries. Anthropogenic sources, such as combustion of fuel or waste released from industries where nickel is extracted from ore, may have some effect on increase in Ni concentrations, as confirmed in this study.
Nickel is known for its considerable spatial variation in soil, even on a small scale (Proctor and Baker 1994), but mention should also be made of its temporal variability, characteristic of this metal which are not typical of heavy metals, in general (Xiangdong Li et al. 2004). In such cases, the fluctuation of metals in soil can be monitored and their bioavailability and mobility estimated solely by stationery trials. Nickel concentrations available to plants are much lower than the total, but still there is no universally accepted method for evaluation of its bioavailabilty, the main reason being a great variability in Ni concentrations in soils.
Zinc
Zinc belongs to a group of trace metals, which is an essential element for the growth of humans and plants. The main sources of pollution are industry and the use of liquid manure, composed materials and agrochemicals such as fertilizers and pesticides in agriculture. The average zinc concentration in the soil of Thane–belapur is 191.3 mg/kg, ranging from 96.6 mg/kg to 763.2 mg/kg. Zinc showed a distribution pattern similar to copper in soils. There are clearly marked locations having higher concentrations of zinc in soils in the vicinity of the industrial area (Fig 6). However, only two samples showed zinc concentrations higher than the normal threshold value (Fig 7).
Cluster analysis
R type cluster analysis is used to elucidate the complex relationships between the different heavy metals studied. Numerous factors control their relative abundance, including the original concentrations in rocks, during the process of soil formation, as well as anthropogenic factors such as pollution. The correlation coefficient “r” was used as the measurement parameter of relationships, and the single linkage clustering method was used to divide the elements into clusters (Davis 1986). All the samples were used for the calculation of the correlation coefficient of the elements using the following equation
where j, k = 1–5 are the number of elements, i is the number of soil samples; \(0 \leq\vert r \vert\leq1. \)
The results of the calculations are listed in the Table 4 and plotted in Fig. 8.
Table 4 and Fig. 8 indicate that Cr–Co and Cr–Ni are closely related with a correlation coefficient of 0.75361 and 0.72041; Co–Ni also have a close relationship as shown by a correlation coefficient of 0.79948, respectively. Cu–Zn also showed correlation with the correlation coefficient of 0.71477. Overall all elements, Cr, Co, Cu, Ni and Zn, are grouped together, indicating that the anthropogenic sources of these heavy metals are closely related in soil of the study area.
In a recent study, Romic and Romic (2003) prepared cluster analysis on numerous heavy metals present in the agriculture topsoil in an urban area of the Croatian capital of Zagreb. They identified the group of clustered elements, including Cd, Pb, Cu, Zn and Ni as heavily influenced by anthropogenic means. However, in this study area the cluster group of heavy metals shows the influence of the anthropogenic input from industries such as steel, agrochemicals, fertilizers, waste released from industry.
Conclusion
Soils in the Thane–Belapur industrial development area were found to be significantly contaminated by Cu, Cr, Co, Ni, Zn and some of the major elements such as Fe and Mn. The detected levels of total metal contamination in many of the samples were found to exceed international threshold values. By the cluster analysis applied to determine associations of heavy metals in soils of Thane–Belapur, the groups of Cu, Cr, Co, Ni and Zn showed a strong anthropogenic influence. High nickel concentrations are also connected to morphogetic characteristics of the greater region, primarily basic rocks of the surrounding mountain range. However, it has been assumed that the anomalous concentrations of nickel in the vicinity of the highway and industry are of anthropogenic input. Copper is characterized by strongly scattered anthropogenic influence. This particularly relates to the uncontrolled waste dumps and liquid waste from industries. With the analytical results of Cr, Co and Zn obtained in the soils of the study area it is also indicated that the pollution of these elements in the area originated mainly from industries in the area, either from dumping of industrial waste or from release of untreated effluents on to the ground which contaminate the underground water table. High values of Fe and Mn in the area also indicate anthropogenic origin from some petrochemical-based industry. According to the environmental quality criteria for soils, the site would require remediation. It is obvious that systematic and continuous monitoring for heavy metal pollution should be instituted, and certain steps (like phyto-remediation by growing some plants in the area) should be carried out as soon as possible to minimize the rate, and extent of future pollution problems.
References
Alloway BJ (1995) Heavy metals in soils. Blackie, London
Ansari AA, Singh IB, Tobschall HJ (1999) Status of anthropogenic ally induced metal pollution in the kanpur-unnao industrial region of the Ganga plain, India. Environ Geol 38(1):25–33
Aswathanarayana U (1995) Geoenvironment: an introduction. A.A.Balkema Publishers, Rotterdam, p 270
Barzilay JI, Weinberg WG, Eley JW (1999) The water we drink: water quality and its effects on health. Rutgers University Press, New Brunswick
Davis JC (1986) Statistics and data analysis in geology, 2nd edn. Wiley, New York
Department of Public Health, Government of Maharashtra (2000) State population policy; G.R.No.P.P 2000/Sr.No.57/00/FW1, Govt. of Maharashtra, Mumbai
Ferguson JE (1990) The heavy elements: chemistry, environmental impacts and health effects. Pergamon Press, Oxford
Govil PK (1985) X-ray fluorescence analysis of major, minor and selected trace elements in new IWG reference rock samples. J Geol Soc India 26:38–42
Govil PK (1993) Reference rock standards: preparation and evaluation. NGRI-D. Applications of analytical instruments. Allied Publishers Limited, pp 140–145
Govil PK, Narayana BL (1999) New reference material of dunite rock: NGRI-UMR: Preparation and evaluation. Geostandards Newsletter. J Geostand Geoanal 23:77–85
Govil PK, Rao TG, Krishna AK (1998) Arsenic contamination in Patancheru industrial area, Medak District Andhra Pradesh. J Environ Geochem 1:5–9
Govil PK, Reddy GLN, Gnaneswara Rao T (1999) Environmental pollution due to heavy metals and radiogenic elements in Nacharam Lake India. J Environ Health 61:23–28
Govil PK, Reddy GLN, Krishna AK (2001) Contamination of soil due to heavy metals in Patancheru industrial development area, Andhra Pradesh, India. Environ Geol 41:461–469
Govindaraju K (1994) Compilation of working values and sample description for 272 Geostandards. Geostand Newsletter 18(special issue): 158 p
Huisman DJ, Vermeulen FJH, Baker J, Veldkamp A, Kroonenberg SB, Klaver GT (1997) A geological interpretation of heavy metal concentrations in soils and sediments in the Southern Netherlands. J Geochem Explon 59:163–174
Krzyztof L, Danutta W, Irena K (2004) Metal contamination of farming soils affected by industry. Environ Int 30(2):159–165
Manjunatha BR, Balakrishna K, Shankar R, Mahalingam TR (2001) Geochemistry and assessment of metal pollution in soils and river components of monsoon-dominated environment near karwar, southwest coast of India. Environ Geol 40:1462–1470
Moon JW, Moon HS, Woon NC, Hahn JS, Won JS, Song Y, Lin X, Zhao Y (2000) Evaluation of heavy metal contamination and implication of multiple sources from Hunchun basin, northeastern China. Environ. Geol 39:1039–1052
Proctor J, Baker AJM (1994) The importance of nickel for plant growth in ultramafic (serpentine) soils. In: Ross SM (ed) Toxic metals in soil-plant system. Wiley, New York, pp 417–432
Rao TG, Govil PK (1995) Merits of using barium as a heavy absorber in major element analysis of rock samples by XRF: new data on ASK-1 and ASK-2 reference samples. Analyst 120:1279–1282
Rawlins BG, Lister TR, Mackenzie AC (2002) Trace metal pollution of soils in Northern England. Environ. Geol 42:612–620
Romic M, Romic D (2003) Heavy metal distribution in agricultural topsoils in urban area. Environ Geology 43:795–805
Romic M, Romic D, Kraljickovic J (2001) Copper in Vinyard soils. In: Proceedings 9th congress of croation society of soil science, Land Management and Soil protection for future generations, 3–7 July, Brijumi, Croatia
Shankar V, Martin M, Bhatt A, Erkman S (1994) Draft on status of implementation of the hazardous waste Rules 1989. Ministry of Environment and Forests, NeDelhi
Thuy HTT, Tobschall HJ, An PV (2000) Distribution of heavy metals in Urban soils-case study of Danag-Hoian area (Vietnam). Environ Geol 39:603–610
Webster R, Oliver M (2001) Geostatistics for environmental scientists. Wiley, New York, pp 11–35
World Health Organization (WHO) Guidelines for drinking-water quality (1996) Geneva
Xiangdong Li, Siu-Lan Lee, Sze-Chung Wong, Wenzhong, Iain Thornton (2004) The study of metal contamination in urban soils of Hong-Kong using a GIS-based approach. Environ Pollut 129(1):113–124
Zhang CS, Selinus O (1998) Statistics and GIS in environmental geochemistry- some problems and solutions. J Geochem Explor 64(1–3):339–354
Zhang H, Ma D, Xie Q, Chen X (1999) An approach to studying heavy metal pollution caused by modern city development in Nanjinga; China. Environ Geol 38:223–228
Acknowledgements
The authors are thankful to Dr. V.P. Dimri, Director, National Geophysical Research Institute, Hyderabad, for his support and permission to publish this paper. Thanks are also due to the Ministry of Environment and Forests, New Delhi, for partially funding this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna, A.K., Govil, P.K. Heavy metal distribution and contamination in soils of Thane–Belapur industrial development area, Mumbai, Western India. Environ Geol 47, 1054–1061 (2005). https://doi.org/10.1007/s00254-005-1238-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-005-1238-x