Abstract
Economic and urban development in the province of Tucumán is closely associated with its hydrological network. In spite of the regulatory efforts to preserve the quality of the water resources, the lower basins of the majority of the rivers are contaminated with organic effluents derived from sugar-mill and citrus industry. In this paper, the conditions of the Colorado River basin are described. At its headwater, the lithology and geology determine the chemical composition. Calcite and gypsum dissolution and silicate influence water composition, which is slightly perturbed a few kilometers downstream by geothermal waters discharged by a tributary. Close to the discharge into the Salí River, the Colorado River receives a high organic matter load from the highly polluted Calimayo stream, which produces an increase in the organic matter and depletion of dissolved oxygen with redox conditions that promote the reduction of sulfate to sulfide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
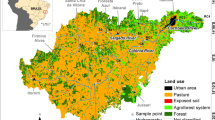
Urban and industrial development in the province of Tucumán, NW Argentina, was historically associated with its water resources. The Salí River runs in a N-S direction, along which the main cities and industries are located (Fig. 1). The Salí River has many tributaries, especially from its west margin. The catchments (headwaters) of these tributaries are along the eastern slopes of the Aconquija Range, which runs parallel to the western border of the province.
The foothills of the Aconquija Range comprise areas of industrial development (citrus, paper mill, candy factories).
The central and lower basins of the Salí River (Galindo and others 2001) as well as the lower basin of its tributaries are polluted by urban sewage and by the effluents from by industrial and agricultural activities.
The assessment of the chemistry and quality of surface and ground waters of the province has been underway since the last decade as part of a systematic study (regional project) (Padilla Torres and others 1996; Perondi and others 1999; García and others 2001). The hydrological network was subdivided into small basins that were monitored yearly (Vece and others 1997).
This paper presents the results of the chemical survey of the Colorado River basin, between September 95 and November 96. The lithology and geological characteristics control the perturbation in the water composition produced by the discharge of a tributary close to the headwaters. In contrast, the discharge of the highly polluted Calimayo Stream produces profound changes in the water composition, determined by anthropic perturbations.
The site
The Colorado River basin extends from 26°50’ to 27°08’ latitude S and 65°17’ to 65°36’ longitude W. Sampling was performed at four stations along the Colorado River, and at two stations on two tributaries (Fig. 1); the Calimayo Stream (T2), carries severely polluted waters. The study area together with the position of the sampling points is shown in Fig. 1.
The headwater of the Colorado River is on the eastern slopes of the Aconquija Range. In its course, it collects an important number of streams, mainly from the west, which transverse Tertiary sediments. In the hilly region of its upper course, the Colorado River runs in a NW-SE direction, and transverses Tertiary sediments of moderate permeability, composed of lime and clays with gypsum and calcite levels. The slope of the river falls from 2 to 0.5% as it penetrates the flatlands of its lower course. In this region, it meanders in a N-S direction, collecting the water from tributaries, mainly the Calimayo Stream and the Famaillá River, to reach its confluence with the Salí River. In the foothill region and in the plain the river transverses Quaternary sediments of alluvial origin (Navas 1988).
The vegetation in the Colorado’s catchment is rainforest, the “yunga”, which has been claimed as important (Brown and others 1995). Presently this yunga is highly perturbed, because of the intensive and uncontrolled felling of trees, which produces an increase in erosion and transport of sediments. The yunga develops between 500 and 1,500 m.a.s.l. (meters above sea level), where annual precipitation is about 1,500 mm.
Agriculture is the main land use in the rest of the basin. Citrus occupies the foothill area, where small vegetable farms also exist. In the plain, land is mainly farmed for sugar cane.
In the central basin, sand is exploited from quarries. A paper mill and a candy factory are the main industrial activities in the lower basin of the Colorado River. Environmental management of the effluents has been vaguely associated with water pollution (Fernández 2001).
Methods
Single samples, not 24 hrs-compensated, were collected monthly. In the field, conductivity and pH were measured with a Methrom E587 conductimeter and a Methrom 704 pHmeter; alkalinity and carbon dioxide were determined by titration. Samples were treated in situ with MnSO4 and KI+KOH solutions for subsequent dissolved oxygen measurement in the laboratory (Winkler method).
Samples were analyzed in the laboratory for sodium and potassium (Flame photometry, APHA 1992), calcium and magnesium (EDTA titration, APHA 1992), chloride (Mohr method, APHA 1992), sulfate (nephelometric method, Rodier 1989), dissolved oxygen (Winkler method, Rodier 1989) and organic matter (oxygen consumed by organic matter, Rodier 1989). The determinations were carried out within 48 hours after collection.
Results and discussion
Table 1 shows major ions and silica concentrations measured in November 1995, March, May and September 1996 at each sampling station. The letter T identifies sampling points at the tributaries. On the basis of the average conductivity values, calculated from the monthly data, the water of the main course may be classified as mineralized (French Regulations, Rodier 1989), and the mineralization of both tributaries is excessive. The Piper Langellier diagram shown in Fig. 2 illustrates the different composition of water from both tributaries as compared with the main Colorado River. “Average composition” in rivers of highly seasonal volumes should be viewed with caution.
Until the confluence of the Calimayo tributary, sulfate is the main anion contributing to the conductivity, but in sampling station 4, there is an increase in alkalinity and chloride, and a sharp decrease of sulfate due to the effect of Calimayo and/or Famaillá water (see Table 1). In both T1 and T2 sodium and chloride predominate, but the origin and the impact on the Colorado River is diverse. Water in the recharge area is essentially meteoric, and the interaction with the sediments defines the composition; an important contribution of the dissolution of calcium sulfate to the salinity is apparent from the data in sampling stations 1 to 3. The stream water sampled at T1 is highly enriched in sodium chloride (see Table 1), even though the distance between sampling stations 1 and T1 is only 1 km. Hydrothermal waters from springs (Mon and Mansilla 1998) contribute to water volume in T1. The higher levels of sodium, chloride and dissolved silica observed in T1 are associated with the hydrothermal water (Hidalgo and others 1997). On the other hand the high sodium chloride level in the Calimayo Stream (T2, see Table 1) is due to human activities associated with the discharge of industrial effluents without treatment (Perondi and others 1997). The most important factories in this region are shown in Fig. 1.
Figure 3 shows the spatial trends in the composition of Colorado River waters. The impact of the hydrothermal water of the first stream on the Colorado is small, because of the low volume of this tributary, whereas the ingress of the Calimayo waters produces a severe chemical perturbation of the Colorado River (sampling point 4, Tables 1 and 2). In the latter sampling point, both [SO4 2−] and [Ca2+] decrease, whereas [Alk] increases substantially. In all sampling stations the conservative ions Na+ and Cl− vary in a correlated way; in T1 the [Na+]/[Cl−] ratio is almost constant, 1.11±0.05. The [Na+] and [Cl−] and the ratios [Na+]/[Cl−] in Station 2 can be attributed to the mixture of V1 and VT water volume, each carrying Na1 and NaT (or Cl1 and ClT) concentration. Mass balance equations require large seasonal fluctuations in the VT/V1 ratios. These ratios follow the trends expected from the seasonal changes in V1, with approximately constant VT values. The hydrothermal origin and small volume of the affluent supports this interpretation.
The same procedure cannot be applied to describe water from sampling point 4, because in this case ΔNa+, the increase in Na+ concentration, is not equal to ΔCl−, the increase in Cl− concentration. The dilution caused by the Famaillá River is influenced by seasonal factors and further complicates the analysis.
The composition of water sampled at Station 4 is influenced by the inflow from the Famaillá River (Fig. 1). The water chemistry of this river in August 1995 and December 1996, in Table 3, indicates a composition with low salt concentration and adequate levels of dissolved oxygen and organic matter. Thus, the Famaillá River produces a dilution effect in the hydrological balance of the Colorado River.
The ratio [SO4 2−]/[Ca2+] is appreciably lower than one only in T1, T2 and 4 (see Table 1). In these cases [Alk] is high, indicating a contribution of the dissolution of calcite and/or andesine [Eqs. (1) and (2)], further to the dissolution of gypsum [Eq. (3)].
This contribution accounts for the ratios [Ca2+]/[SO4 2−] observed in T1 and T2. In sampling point 4 however, the ratio is much higher, and is associated with the reduction of sulfate to sulfide [see Eq. (4)] produced after the discharge of the Calimayo into the Colorado River.
This reaction, due to the pollution in the tributary, may also affect the ratio in T2. The contamination is severe enough to give rise to anaerobic conditions in the confluence with the Colorado River, permitting the reduction of sulfate to sulfide, which increases dramatically downstream.
Figure 4 shows the spatial variations of pH (a), organic matter (b), and dissolved oxygen (c). There is a sharp increase in organic matter and a drastic drop in dissolved oxygen in sampling point 4. These features result from the merging of the Calimayo Stream waters, and are associated with an increase in [Na+], [K+], [Alk] and [Cl−].
Table 2 shows the degrees of saturation of gypsum and calcite (ΩG and ΩC), expressed as the ratio between the ionic activity product, IAP, and the equilibrium constant, K. Adequate corrections were made to take into account the influence of ionic strength on the solubility product constant. In all cases the water is undersaturated in gypsum and, except for T2 and for all sampling points in March 1996, is supersaturated in calcite.
The decrease in [Ca2+] in sampling station 4 is therefore not indicative of removal by gypsum reprecipitation; a more likely explanation is a simple dilution effect. The increase in the concentration of other ions due to the pollution of the Calimayo is therefore underestimated by the data in sampling point 4.
Calcite precipitation/dissolution may also play a role in controlling the chemistry; the effect is modulated by the changes in pH and dilution effects due to precipitation and ingress of Famaillá water.
Pollution in the Calimayo Stream
Table 4 shows the values of pH, conductivity and dissolved oxygen measured in T2 over a more extended period of time (August 1992–December 2002). The results illustrate the drastic effect of paper mill effluents. The sampling between May 1993 and December 1994 coincides with a period of low or inexistent industrial activity; and high dissolved oxygen and low conductivity values are observed, together with a rather stable pH value in the range 6.5–7.6.
When the industrial effluents are discharged, dissolved oxygen drops drastically, conductivity increases sharply, and pH fluctuates within wide limits. This last observation reflects deficiencies in the conditioning with acid of the effluent before it is discharged.
Conclusions
Among the main rivers of the Province of Tucumán, the Colorado River presents the highest salinity. This results from interaction between water and the Tertiary sediments in the headwater area and probable hydrothermal occurrence.
The Calimayo Stream, a tributary of Colorado River, receives industrial sewage without treatment during the whole year, and the composition of Colorado River water is dramatically altered below the Calimayo Stream discharge. Most evident changes are organic matter increase, dissolved oxygen decrease and sulfate concentrations decrease. The last one is related to reduction processes developed as a consequence of anaerobic conditions present in the lower river course.
References
APHA (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, New York
Brown AD, Grau HR (eds) (1995) Investigación, conservación y desarrollo en selvas subtropicales de montaña. Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Tucumán, 265 pp
Fernández DS (2001) Caracterización de las alteraciones ambientales producidas por la explotación de áridos en la provincia de Tucumán. In: Actas de la III Reunión Nacional de Geología Ambiental y Ordenación del Territorio y I Reunión de Geología Ambiental y Ordenación del Territorio del área del Mercosur, 10 pp (digital format)
Galindo MC, Vece MB, Perondi ME, Monserrat Aráoz M, García G, Hidalgo M del V, Apella MC, Blesa MA (2001) Chemical behavior of the Salí River, Province of Tucumán, Argentina. Environ Geol 40:847–852
García MG, Hidalgo M del V, Blesa MA (2001) Geochemistry of groundwaters in the alluvial Plain of Tucumán, Argentina. Hydrogeol J 9:597–610
Hidalgo M del V, Apella MC, Mena R (1997) Posible hidrotermalismo en la vertiente oriental de las cumbres del Aconquija, Tucumán, Argentina. In: Dpto de Cs Geol, Fac de Ing y Cs Geológicas, Univ Católica del Norte (eds) Actas VIII Congreso Geológico Chileno, Antofagasta, Chile, vol I, Sección Temática 4, pp 745–749
Mon R, Mansilla N (1998) Geology of Tucumán 2ª Ed. Gianfrancisco M, Puchulu ME, Durango de Cabrera J, Aceñolaza GF (eds) Graduate College in Geological Science of Tucumán, Tucumán, pp 147–154
Navas BF (1988) Geología e hidrología de la cuenca del Río Colorado, provincia de Tucumán. MSc Thesis, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán
Padilla Torres SI, Apella MC, Hidalgo M del V (1996) Ríos de la provincia de Tucumán, conductividad y clasificación. Serie Monográfica y Didáctica 32. Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, pp 1–15
Perondi ME, Vece MB, Mena R (1997) Factores litológicos y antropogénicos en la hidroquímica del río Colorado, Tucumán, Argentina. In: Secretaría de Extensión Universitaria y Medio Ambiente, UNT (eds) VI Congreso El NOA y su Medio Ambiente, Tucumán, 139 pp
Perondi ME, Vece MB, Galindo MC, Hidalgo M del V, Apella MC (1999) Materia orgánica y calidad de aguas superficiales. Tucumán, Argentina. Rev Bol de Ecol 6:211–216
Rodier J (1989) Water analysis, 6th edn. Omega, Barcelona, 1059 pp
Vece MB, Perondi ME, Galindo MC, Apella MC, Hidalgo M del V, Blesa MA (1997) Chemical composition control in the Medina River, Tucumán, Argentina. An Asoc Quím Arg 85:141–152
Acknowledgements
The work was supported by grants from Consejo de Investigaciones de la Universidad Nacional de Tucumán (Project 26/G104), Consejo Nacional de Investigaciones Científicas y Técnicas (Grant PIP 4196/96) and Agencia Nacional de Promoción Científica y Técnica (Grant PICT 00806/97). M. C. Apella and M. A. Blesa are members of CONICET. M. G. García acknowledges a postdoctoral fellowship from CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galindo, M.C., Vece, M.B., Perondi, M.E. et al. Natural and anthropic perturbations to the chemical composition of the Colorado River (Tucumán, Argentina). Env Geol 45, 940–946 (2004). https://doi.org/10.1007/s00254-003-0939-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0939-2