Abstract
Sediment samples were collected from six springs draining the karst aquifer at Fort Campbell, Kentucky/Tennessee. These were analyzed by ICP-MS following an extraction procedure that separates the metals into exchangeable, carbonate, oxide, organic and residual fractions. Aluminum and iron are primarily present in the residual fraction while manganese may be dominantly in either the oxide or carbonate fraction. The redox-sensitive metals (Fe and Mn) have the greatest fractional distribution in the spring with the thickest sediments. Trace metals detected include Cr, Cd, Ni, Pb, and Zn in the range of a few to 200 mg/kg. In Beaver Spring, nickel is distributed between the exchangeable, carbonate and organic fractions, while in Gordon Spring, nickel is largely residual. Chromium is almost entirely associated with the organic fraction in Beaver Spring while it is largely residual in Gordon Spring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediment storage and transport are integrally linked to metal cycling in hydrologic systems. While the relationships between water flow, sediment distribution and metal chemistry have been well studied for rivers and lakes, little attention has been paid to the same relationships in karst systems.
One of the characteristics of karst aquifers is that their enhanced permeability permits high flow velocities capable of transporting suspended and bedload sediments. Calculations have shown that the transport of suspended sediments can occur in fracture openings as small as 1 cm (White 1988). The transport is episodic and occurs primarily during storm flow in the conduit system. As storm flow recedes, clastic sediments are deposited in the conduit system and at the spring mouths. The clastic material consists of either weathering residues from nearby clastic rocks flushed into the system by sinking streams or surface soils and regolith injected through sinkholes and open fractures at the base of the epikarst. Sand, silt, clays and occasionally larger rock fragments make up the bulk of most clastic cave sediments.
Karst sediments are either mobile (suspended) or sedentary (deposited) with exchange likely between the two categories. Recent studies have focused on the transport of suspended particulates particularly during storm events. Ryan and Meiman (1996) measured the turbidity of a spring in Mammoth Cave National Park over two storm events and were able to show that the suspended particulate concentration increased during storms and that it originated from an agricultural recharge area. Atteia and Kozel (1997) showed that the size of particulates discharged from a karst spring changed over the course of a storm. They found that the concentration of small particles increased slowly during a storm and remained high when the water level decreased. Similar studies in Texas (Mahler and Lynch 1999) and Pennsylvania (Prout 2001) also found changes in particle size and mineralogy during storms. Shevenell and McCarthy (2002) used turbidity in karst monitoring wells to argue that turbidity in large storms was controlled by flow dynamics whereas water chemistry played a more important role in small storms.
The flux of sediment serves to transport metals through karst aquifers attached to fine-grained suspended sediments (Vesper and White 2003). It might be expected, then, that the clastic sediment held in storage within the conduit system is also a storage site for metal contaminants. Analogous studies of river and lake sediments suggest that the degree to which metals are stored, and in what chemical form storage occurs, is a function of the physical flow system (Sandén and others 1997; Gundersen and Steinnes 2001), the geomorphology of the setting (Miller 1997; Rhoads and Cahill 1999), and the localized chemical conditions (Tessier and others 1996; Apodaca and others 2000; Stecko and Bendell-Young 2000), particularly the oxidation-reduction state (van Ryssen and others 1999).
This study compared the sediment metal chemistry from karst springs from different hydrologic and geomorphic environments. The focus of this paper is clastic sediments as a storage medium for the naturally occurring metals iron and manganese and a suite of trace contaminant heavy metals, and how flow and geologic processes influence the chemistry of the metal storage reservoir.
Study area and geology
The western Kentucky karst aquifer
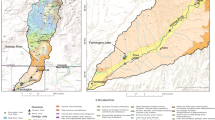
The spring sediments and soils investigated in this paper were collected on and near the Fort Campbell Army Base, along the Kentucky–Tennessee border approximately 80 km northwest of Nashville, Tennessee (Fig. 1). The developed areas of the base make up less than 20% of the 430-km2 reservation. The remainder is undeveloped and used for ranges and military training exercises. The area surrounding Fort Campbell is largely agricultural with some residential, commercial and industrial uses.
Fort Campbell is on the western Highland Rim of the Nashville Basin. The central basin to the east and south, and the Tennessee River valley to the west border the region. To the north the region transitions into the Kentucky Pennyroyal Plain, a well-known karst terrain. The Highland Rim is a broad flat area that tilts slightly to the west. The surface elevation is approximately 120 m above the Nashville Basin and only 90 m above the Tennessee River valley (Luther 1977). In the region of Fort Campbell, the Highland Rim surface has been highly dissected by the Cumberland River and its tributaries.
Fort Campbell and the surrounding areas are underlain by limestones of the Mississippian Ste. Genevieve and St. Louis Formations. The strata are nearly horizontal with a regional dip of 4–11 m/km to the west-northwest (Kemmerly 1980). However, locally, there may be a slight dip to the south (Klemic 1966; 1974). The St. Louis Formation is typically a calcarenite with discontinuous layers of siliceous and dolomitic calcarentite and cherty layers in the lower part of the formation. It outcrops primarily along river valleys and in topographic lows. The Ste. Genevieve Formation consists of thick-bedded-limestone with thinner beds of dolomite. A zone of chert nodules (the Lost River Chert) is often found in the lower 3–6 m of the formation. The Ste. Genevieve Formation is found at the surface only at higher elevations, particularly in the northern part of the army base.
The limestone surface, where exposed in road cuts, is deeply etched by solution into a cutter and pinnacle topography with a relief of 5–10 m. This weathered zone (the epikarst) is draped with a thick regolith (up to 30 m) that completely covers the bedrock surface. The regolith consists of an upper layer of reddish brown clays and a lower layer of gravely clay. Discontinuous bands and partings of silty materials are present. Chert nodules are commonly found at the regolith–bedrock contact. The cherty rubble provides a zone of high permeability at the base of the epikarst. Overlying the regolith is a well-drained loamy soil of the Pembroke-Crider association, a typical soil derived from the weathering of cavernous limestone.
Recharge to the carbonate aquifer is mainly by dispersed infiltration through the epikarst along with storm runoff into sinkholes. In spite of the generally thick regolith, storm recharge is very rapid and produces well-defined storm peaks on the spring hydrographs.
More than 30 perennial springs have been identified within a 250-km2 area around the Base. Locations and pictures of the springs selected for detailed investigation are shown in Fig. 2. The ground-water basins that drain to these springs were determined by extensive dye tracer studies carried out by Ewers Water Consultants as part of the Fort Campbell environmental assessment activities. The location and characteristics of the chosen springs are given in Table 1.
Methods
Sediment sampling
The samples collected include spring sediments and soils (Fig. 3). The springs were selected to represent a range of morphologies and flow types (Table 1). Three of the Fort Campbell springs – Beaver, Eagle and Millstone – were also used in water chemistry studies (Vesper and White 2003). The other two Fort Campbell springs – Gordon and Quarles – have very different morphologies and were included to determine the importance of morphology on sediment accumulations and chemistry. Nine surface soil samples and four subsurface samples were collected at Fort Campbell to provide data for suspected source areas (Table 2). Three of the subsurface samples were from a single location with a matching surface sample.
Approximate locations for Fort Campbell soil samples. Spring samples are identified as: GO Gordon Spring; BL Blue Spring; BO Boiling Spring; DN Dennis Spring; EA Eagle Spring; BE Beaver Spring; G1 Gate One Spring. Additional shallow soil samples were collected near the spring mouth of Eagle Spring (soil ED101) and Beaver Spring (soil BD101)
Extractions and analysis
The spring sediments and soil samples were analyzed for their physical characteristics, mineralogy, bulk chemistry and extractable metals. Mineralogy was determined by X-Ray powder diffraction (XRD) with grain-by-grain chemical analysis by energy dispersive X-Ray spectroscopy (EDX). Also analyzed were cation exchange capacity (CEC) and total organic carbon (TOC).
Total metal concentrations were determined by digesting dried sieved samples in hydrochloric and hydrofluoric acids using a microwave digester. The resulting solutions were analyzed for major elements by atomic emission spectroscopy and for trace metals with a Finnegan inductively coupled plasma mass spectrometer (ICP-MS). An indium spike was used as an internal standard. Nine pairs of duplicate samples were analyzed. Agreement between pairs was generally within 10%.
The selective extraction procedure was modified from the European Community Bureau of Reference (BCR) three-step protocol (Quevauviller and others 1993, 1994). Modifications were implemented to separate the exchangeable and carbonate fractions (given the likelihood of carbonate phases) and to include analysis of the residual material. The results are operationally defined by the extracting solution but generally represent the five fractions listed in Table 3. While selective extraction procedures are considered problematic due to various issues including sample heterogeneity, selectivity of reactants, redistribution of metals during extraction, re-adsorption, and pre-treatment handling (Tessier and others 1979; Rendell and others 1980; Rapin and others 1986; Rauret and others 1989; Xiao-Quan and Bin 1993; Morabito 1995), they provide a means of comparing metal distributions between locations when the procedure is consistent.
To evaluate the data quality, replicate samples were included for one sediment (Quarles Spring) and one soil (BS-A) location. These replicates were collected from adjacent locations (within 1 m). Procedural and equipment blanks were also incorporated and each extraction was conducted in duplicate or triplicate. All results are reported as average concentrations. Based on these data, Cu was excluded from the final results due to its presence in the process blanks and poor reproducibility in the replicate samples. Overall, however, the difference between extracted masses between fractions is significantly higher than the error within each fractional mass and thus it is acceptable to compare concentrations between fractions. This is less true for metals with concentrations that split between several fractions and, in those cases, the conclusion can only be that multiple fractions are important and the quantification of the distribution is not exact. All extraction data are reported as dry weight.
Results
Characteristics of spring sediments and upland soils
The distribution of spring sediment and soil particle sizes is shown in Fig. 4. According to particle size alone, the spring sediments contain between 10 and 30% clay. The Gordon Spring sediment has the highest sand content of any of the springs. The subsurface soil samples have the highest percent clay.
Grain size distribution for spring sediments and surface soils. Sizes are based on U.S. Department of Agriculture classification (Brady and Weil 1996). Clay=<0.002 mm; silt=0.002 to 0.05 mm; and sand=0.05 to 2 mm
X-Ray diffraction data indicate quartz is the dominant mineral in all samples. Minor minerals identified include feldspar, muscovite, and kaolinite. Calcite and dolomite occur very sparsely in only a few samples showing that soils and spring sediments are well leached. In an effort to identify clay minerals, random mounts, packed slides, preferred-orientation slides, and slides of separated fine-grained materials were examined. The diffraction patterns did not depend on slide preparation and except for minor kaolinite, the quantity of clay identified in the X-Ray diffraction pattern did not account for the clay-sized particles found in the particle-size analysis.
The EDX chemical analyses (Fig. 5) generally complimented the XRD data. The two samples with calcite peaks in their XRD patterns also had high Ca by EDX. As expected from the dominant quartz in the XRD data, 70–80 elemental percent is Si in most samples. Al is in the range of 10%. Al should be a proxy for clay, along with the minor feldspar. On a mole basis, a matching amount of Si is required for the clay structures, for example, kaolinite, Al4Si4O10(OH)8. Thus, a substantial amount of clay appears to be present in all samples in spite of its sparse occurrence in the X-Ray diffraction patterns. Another element with a ubiquitous presence in the EDX spectra, which does not appear in the XRD patterns, is Fe, also typically present in the range of 10–15%. Fe in the form of hydroxides and oxyhydroxides often occurs as coatings on other mineral grains. The electron beam in the scanning electron microscope has a relatively low penetrating power so that chemical analyses are obtained mainly from the surfaces of the mineral grains. A significant concentration of Fe determined by EDX in the absence of Fe-containing minerals by X-Ray is entirely consistent with Fe-oxyhydroxide coatings on mineral grains.
Comparisons of the composition of shallow surface soils, deep soils, and spring sediments (Fig. 5) did not reveal any dramatic differences. The subsurface soils tend to have higher Al concentrations as would be expected from their higher content of clay-sized particles.
Bulk metallic elements
Chemical analyses obtained from complete digestion of the sediment samples revealed Si, Al, Fe and some Ca as major constituents, K, Na, Mg, and Mn as minor constituents, and Zn, Cr, Ni, Pb and Cd as trace metals. Complete numerical data for all samples may be found in the thesis from which this paper is extracted (Vesper 2002).
Linear correlations exist between the concentrations of certain constituents. Matrix correlations for the spring sediment samples indicate that statistically consistent correlations exist for the parameters CEC-Al-K-Fe. The Si concentration is closely correlated with Al and Fe in suspended sediment samples (Vesper and White 2003) but was negatively correlated in the spring sediment samples. Similar patterns exist in the surface soil data.
Because the analytical data suggest that the spring sediments and the soils do not represent distinct chemical populations, they can be combined into a larger data set in which more correlations are statistically significant at α=0.01 (Table 4). While the combined data set has more statistically significant correlations, many of the correlation coefficients are low, especially for the trace metals. As was found for the individual data sets, Si does not correlate with Al and Fe although it does correlate with Mn.
The lack of correlation between Si and Al reflects the high concentration of quartz in both sets of sediments and the variability of clay content. While Mn correlations with Si and Fe are not strong (R2 values of 0.49 and –0.60, respectively), they suggest that Mn occurs predominantly as coatings on quartz particles. Mn coatings are known to deposit preferentially on sandstone pebbles or on chert nodules in cave streams.
Selective extraction of major components and trace metals
In general, the major metal distributions identified by selective extraction fell into two groups: those that fell primarily into the residual fraction and those that were more widely distributed between multiple fractions.
The Fe was primarily present in the residual fraction (Fig. 6a) suggesting that Fe-coatings on mineral grains are not extracted by the hydroxylamine hydrochloride or that separate Fe-phases exist in addition to the Fe-coatings. Only samples from Quarles Spring (QD200 and QD201) contained substantial concentrations of Fe in the carbonate and oxide fractions.
Metal fractionation by concentration (+) and percent distribution (bar charts) for: a iron and b manganese. Sample IDs in order from left to right on all graphs, are sediments: BD200, ED200, GD200, MD200, QD200, QD201, surface soils: BD101, BS-A, BS-A(dup), BS-B, BS-C, BS-D, BS-E, ES-0-2, and subsurface soils: ES-9–11, ES-39–41, PX01
The distribution of Mn between fractions was the most variable of the major metals (Fig. 6b). In the surface soil Mn is predominantly in the oxide fraction. In the sediments the dominant fraction is residual (Eagle Spring), oxide (Gordon and Millstone Springs), or carbonate (Quarles Spring). Mn is one of the few metals investigated where its distribution in the soils (mostly oxide) is observably different from the spring sediments (split between oxide, carbonate and residual). Mn in surface sample PX01, collected from a deep conduit, was distributed similarly to Mn in the Beaver and Quarles Spring sediments.
Cd is a very minor constituent in most of the sediments, present at less than 10 mg/kg in all samples and typically less than 5 mg/kg (Fig. 7a). Cd is distributed throughout the fractions. The residual fraction accounts for 32 to 95%. While either the carbonate or oxide fraction may be the second most common, it should be noticed that Cd is one of the few metals with considerable mass in the carbonate fraction (up to 31% of the Cd is in the carbonate fraction of the spring sediments).
Metal fractionation by concentration (+) and percent distribution (bar charts) for: a cadmium and b chromium. Sample IDs in order from left to right on all graphs, are sediments: BD200, ED200, GD200, MD200, QD200, QD201, surface soils: BD101, BS-A, BS-A(dup), BS-B, BS-C, BS-D, BS-E, ES-0–2, and subsurface soils: ES-9–11, ES-39–41, PX01
Cr is present in the range of 5–140 mg/kg (Fig. 7b). Most of the Cr is concentrated in the residual fraction with the second most common fraction being the organically bound (up to 10%). The valence state of Cr is not known. If present as Cr3+, it will be highly insoluble. Under these circumstances it is not surprising to find Cr in the residual fraction.
Ni occurred in the concentration range of 20 to almost 200 mg/kg (Fig. 8a). Ni was primarily in the residual fraction although some samples contained observable organic or oxide-bound concentrations.
Metal fractionation by concentration (+) and percent distribution (bar charts) for: a nickel and b lead. Sample IDs in order from left to right on all graphs, are sediments: BD200, ED200, GD200, MD200, QD200, QD201, surface soils: BD101, BS-A, BS-A(dup), BS-B, BS-C, BS-D, BS-E, ES-0–2, and subsurface soils: ES-9–11, ES-39–41, PX01
Pb is present in all sediment samples in the range of 20–90 mg/kg (Fig. 8b). In the spring sediments, Pb was in the residual and oxide fractions. However, in the surface soils the Pb mass was distributed between the residual, oxide and organic fractions. Although Pb carbonates might be expected in these karst waters, Pb in the carbonate fraction was negligible.
Total Zn concentrations ranged from approximately 80 to 280 mg/kg (Fig. 9). Zn is primarily in the residual phase (greater than 50%) with the remaining metal typically split between carbonate, oxide and organic fractions.
Metal fractionation by concentration (+) and percent distribution (bar charts) for zinc. Sample IDs in order from left to right on all graphs, are sediments: BD200, ED200, GD200, MD200, QD200, QD201, surface soils: BD101, BS-A, BS-A(dup), BS-B, BS-C, BS-D, BS-E, ES-0–2, and subsurface soils: ES-9–11, ES-39–41, PX01
Comparison of bed sediments with water and suspended sediment compositions
In the parallel investigation to this study (Vesper and White 2003), it was shown that the metal flux in suspended sediments increased during storm events. It was shown that the primary transport mechanism for metals was by adsorption onto small-suspended particulates in the storm water. Comparison of the total concentration of each metal in the spring mouth bed sediments (C sed) and the mean maximum concentration in digested storm water (C dig-water) gives a power law fit (Fig. 10) of
where C sed is calculated as the sum of the extracted sediment fractions and C dig-water is the concentration of the metal in water plus the suspended sediments. This is probably a direct proportion to within the fit of the data, R 2=0.95. A similar relation is obtained if the metal concentrations in surface soils are plotted against the concentrations in the spring water. Whether the bed or suspended sediment concentration is the dependent variable depends on whether the suspended sediments contribute to the bed sediments (a depositional process within the conduit system) or the other way around (an erosional process within the conduit system). It is likely that both processes occur depending on storm intensity. This would provide for considerable sediment mixing and homogenization of the metal concentrations.
It is also possible to compare metal concentrations in the spring mouth sediments with the concentrations of metals actually dissolved in the spring waters (Fig. 11). The metal concentrations in the water were obtained by analyzing water samples that had passed through a 0.45 μm filter (C filt-water). These data can be also fitted with a power law fit of
for this fit, R 2=0.93. There is a relation between metal concentrations in bed sediments and concentrations of dissolved metals in the water but it is not linear.
An attempt was made to compare the metal concentrations from the individual fractions with the concentrations in the water. Reasonable plots similar to Figs. 10 and 11 were obtained for the residual fraction and the organic fraction. Data for the other fractions had too much scatter to be useful. In part, this may have been due to the very low concentrations in these fractions.
Discussion and conclusions
Metal distribution in bed sediments
Comparison of metal speciation between different studies is problematic due to the use of different extraction procedures and the limitations of any given procedure. However, the identification of the dominant species, or lack thereof, can generally be compared between studies. A study of sediments from multiple ecosystems (Stecko and Bendell-Young 2000) suggests that some metals are controlled by location while others behave similarly in a range of ecosystems.
The fractional distribution of metals in the spring sediments generally agrees with studies done in river and lake sediments. For example, Fe is typically found in the residual form while Mn is highly variable. Chakrapani and Subramanian (1993) found similar distributions for Fe and Mn in sediments from the Mahanadi River Basin, India, and van Ryssen and others (1999) had similar results in both oxygenated and suboxic settings from two rivers in Belgium. Bendell-Young and others (2002) compared Fe and Mn speciation over a range of ecosystems including lakes, peatlands, wetlands and esturary/intertidal zones and found that Fe was largely residual in all systems while the Mn distribution varied more by ecosystem type and between locations within a given ecosystem type. It should be noted, however, the Fe in Quarles Spring was less than 70% residual and that the carbonate-fraction accounted for 18 and 27% of total Fe. The different distribution in Quarles Spring may be because the geomorphology permits the accumulation of organic sediments thereby creating reducing conditions. The percent of Mn in the residual fraction was also lowest at Quarles Spring.
In the karst sediments Cd was widely distributed between fractions. Other studies have found either broadly distributed Cd or that the Cd fractionation was highly variable with location. Van Ryssen and others (1999) found Cd was in the residual and more available fractions in the suboxic muddy sediments and in all fractions except the residual in the oxic sandy sediments. While Bendall-Young and others (2002) found that the distribution of Cd in sediments depended on the ecosystem studied, Cd in lake sediments was largely residual regardless of whether the lakes were acidified or circumneutral. In the spring study the concentration of Cd in most fractions was typically less than 0.5 mg/kg and therefore the error associated with the percent distribution is higher than for the other metals present at higher concentrations.
Pb has been found in the residual, organic and oxide fractions in both this study and the suboxic and oxic sediments from Belgium (van Ryssen and others 1999). However, in the Fraser River estuary study, Stecko and Bendell-Young (2000) found no Pb associated with the organic fractions. In the Fraser River study, sediment samples were collected on a monthly basis and a distinct change occurred in the dominant Pb fractions over time. During October 1994 through January 1995, Pb was distributed approximately evenly between the residual and easily reducible fractions (Mn oxides and amorphous Fe oxides). However, during March through August the sediment Pb was primarily in the reducible fraction (Fe oxides) with up to approximately 50% of residual metal (Stecko and Bendell-Young 2000). Stecko and Bendell-Young attributed the seasonality of the metal distribution to changes in temperature, which other authors have linked to sorption affinities (Byrne and others 1988; Johnson 1990). Stecko and Bendell-Young (2000) also noted that the distribution of Pb in suspended sediments in the estuary had a similar seasonality as did the deposited sediments. The extraction method used by Stecko and Bendell-Young does not incorporate a carbonate-specific step and is therefore not exactly translatable to the karst spring data. However, the seasonality of their results suggests that the Fort Campbell data – and data from other studies – may only provide a snapshot of the chemical setting for sediments and that longer-term studies are necessary to fully understand metals in sediments.
Numerous researchers have looked at distributions of Zn in sediments via selective extractions. In the karst springs, Zn was largely residual which agrees with data from the Mahanadi River (Chakrapani and Subramanian 1993). Van Ryssen and others (1999), however, found that the Zn distribution varied between the oxic and suboxic river sediments. In the suboxic river sediments Zn was split between the residual and oxide fractions while in the oxic river sediments Zn was primarily in the exchangeable and oxide fractions. Stecko and Bendell-Young’s (2000) estuary data from Fraser River, suggests that Zn is distributed between oxide, organic and residual fractions with no obvious seasonality in the distribution (their extraction procedure did not explicitly include a carbonate fraction).
The similarities between the metal fractionation in the karst spring sediments, surface soils and subsurface soils indicates that the bed sediment chemistry in the springs is controlled by physical transport of the source materials. One exception to this is Pb, which is more likely to be in the organic fraction in soils than in sediments and thus changes its chemical distribution between the source and spring sediments. This cannot be accounted for by the concentration of total organic carbon (TOC). The average TOC concentration was 1.2 mg/kg for spring sediments and surface soils. Furthermore, there is no significant correlation between the Pb and TOC concentrations. The rapid response of the springs to rain events (Vesper and White 2003) and the corresponding high turbidity of the spring water indicates that at least some of the spring sediment is transported from the source areas in the spring basins.
Relationship between suspended sediments and bed sediments
Stecko and Bendell-Young (2000) compared the speciation of various metals in suspended particulates with the speciation in the deposited sediments and found that concentrations for Cu, Pb and Zn in the suspended sediments were typically higher than those found in the deposited sediments. In contrast, the metal concentrations in the suspended sediments in this study (represented by the digested water samples) were lower than the concentrations in the bed sediments for the 1999 and 2000 data. The comparison of the two sediment types is problematic given the difference in grain sizes in the two populations. Suspended sediments are finer grained and more likely to bond to metals via surface reaction or sorption and thereby are more likely to have metals fractionated in the most available forms. In support, data from the Fraser River estuary (Stecko and Bendell-Young 2000) indicates there is typically more readily available (Mn and Fe oxide bound) metal on the suspended sediments than on the deposited sediments.
Stecko and Bendell-Young (2000) also observed that not only do the concentrations of the suspended sediments change on a seasonal basis, but the distribution of the suspended-sediment metals within the extracted fractions changes seasonally. While annual data are not available for the karst springs, two storms were sampled (1999 and 2000) and provide a range of suspended sediment concentrations for comparison. Although the 1999 metal concentrations are higher than those detected in 2000, a similar relationship exists between the suspended and deposited sediment concentrations in both years (a power law relationship with a slope between 0.80 and 0.95). The different suspended sediment concentrations in 1999 and 2000 can be attributed to different sizes of storms suggesting that short-term storm variability must also be considered as well as the seasonal variability observed by Stecko and Bendell-Young (2000).
Conceptual model for metal-sediment storage and transport
The results of this investigation can be summarized in a conceptual model (Fig. 12) and requires that physical and chemical processes be incorporated. Physical processes dictate sediment fluxes and the potential for sediment accumulation. Chemical processes within the sediments control metal fractionation and availability.
It appears that surface soils are the primary source of spring and conduit sediments in the hydrogeologic setting investigated. They are injected into the aquifer during storm events and are stored within the aquifer and discharged through the springs. Storm flow provides a means of winnowing the metal concentrations by flushing out the fine-grained materials. The strong major-trace metal correlations in the suspended sediments are not present in the stored bed sediments.
In addition to transport of sediments from the surface and through the aquifer, spring sediment is introduced via slumpage at the spring mouth. Back-flood deposits from adjacent surface waters may also introduce considerable sediments in some locations. Sediments stored in the conduits and the springs interact with the water chemically; the interaction may be limited by the degree to which the water flows through the sediments. Springs in which the water discharges in a wide area through the sediments have considerable sediment/water interaction while other springs have limited interaction because of low diffusion capacities in the sediments and a localized water source.
Geomorphology at the spring may be the most important control on the bed sediment chemistry. Springs that decant water from rise pools and have a low-gradient spring run are more likely to accumulate fine-grained sediment than are springs with highly variable discharges and a high-gradient spring run. Accordingly, spring sediments that are characterized by erosional processes are less likely to accumulate and chemically alter sediments than are springs characterized by depositional processes. The presence of organic compounds, regardless of whether their source is contamination or detrital organic matter, can catalyze microbial induced reducing conditions and thereby alter the spring sediment significantly.
In summary, deposited sediments in karst springs can act as storage locations for major and trace metals although the means of their storage varies by metal and by location. The deposited sediments are closely linked to the source materials and suspended sediments by physical processes and predictable patterns of metal concentrations. Ultimately, the nature of the stored metals and their bioavailability may be a function of the spring’s ability to accumulate sediments, which is linked to the spring hydrology and geomorphology.
References
Apodaca LE, Driver NE, Bails JB (2000) Occurrence, transport, and fate of trace elements, Blue River Basin, Summit County, Colorado: an integrated approach. Environ Geol 39:901–913
Atteia O, Kozel R (1997) Particle size distributions in water from a karstic aquifer: from particles to colloids. J Hydrol 201:102–119
Bendell-Young LI, Thomas CA, Stecko JRP (2002) Contrasting the geochemistry of oxide sediments across ecosystems: a synthesis. Appl Geochem 17:1563–1582
Brady NC, Weil RR (1996) The nature and properties of soils. Prentice Hall, Englewood Cliffs, NJ, 559 pp
Byrne RH, Kump LR, Cantrell KJ (1988) The influence of temperature and pH on trace metal speciation in seawater. Mar Chem 25:163–181
Chakrapani GJ, Subramanian V (1993) Heavy metals distribution and fractionation in sediments of the Mahanadi River basin, India. Environ Geol 22:80–87
Gundersen P, Steinnes E (2001) Influence of temporal variations in river discharge, pH, alkalinity and Ca on the speciation and concentration of heavy metals in some mining polluted rivers. Aquat Geochem 7:173–193
Johnson BB (1990) Effects of pH, temperature, and concentrations on the adsorption of cadmium on goethite. Environ Sci Technol 24:112–118
Kemmerly PR (1980) Sinkhole collapse in Montgomery County, Tennessee, State of Tennessee Division of Geology. Environ Geol Series No. 6, 37 pp
Klemic H (1966) Geologic map of the Oak Grove Quadrangle, Kentucky. U.S. Geological Survey
Klemic H (1974) Geologic map of the New Providence Quadrangle, Kentucky. U.S. Geological Survey
Luther ET (1977) Our restless earth. The geologic regions of Tennessee. University of Tennessee Press, Knoxville, TN
Mahler BJ, Lynch FL (1999) Muddy waters: temporal variation in sediment discharging from a karst spring. J Hydrol 214:165–178
Miller JR (1997) The role of fluvial geomorphic processes in the dispersal of heavy metals from mine sites. J Geochem Explor 58:101–118
Morabito R (1995) Extraction techniques in speciation analysis of environmental samples. Fresenius J Anal Chem 351:378–385
Prout EE (2001) Temporal variation of sediment distribution in a karst aquifer, Easton, Pennsylvania. Master of Arts, Temple University, PN, 102 pp
Quevauviller P, Rauret G, Muntau H, Ure AM, Rubio R, López-Sánchez JF, Fiedler HD, Griepink B (1994) Evaluation of a sequential extraction procedure for the determination of extractable trace metal contents in sediments. Fresenius J Anal Chem 349:808–814
Quevauviller P, Ure A, Muntau H, Griepink B (1993) Improvement of analytical measurements within the BCR-programme: single and sequential extraction procedures applied to soil and sediment analysis. Intern J Environ Anal Chem 51:129–134
Rapin F, Tessier A, Campbell PGC, Carignan R (1986) Potential artifacts in the determination of metal partitioning in sediments by a sequential extraction procedure. Environ Sci Technol 8:836–840
Rauret G, Rubio R, López-Sánchez JF, Casassas E (1989) Specific procedure for metal solid speciation in heavily polluted river sediments. Intern J Environ Anal Chem 35:89–100
Rendell PS, Batley GE, Cameron AJ (1980) Adsorption as a control of metal concentrations in sediment extracts. Environ Sci Technol 14:314–318
Rhoads BL, Cahill RA (1999) Geomorphological assessment of sediment contamination in an urban stream system. Appl Geochem 14:459–483
Ryan M, Meiman J (1996) An examination of short-term variations in water quality in a karst spring in Kentucky. Ground Water 34:23–30
Sandén P, Karlsson S, Düker A, Ledin A, Lundman L (1997) Variations in hydrochemistry, trace metal concentration and transport during a rain storm event in a small catchment. J Geochem Explor 58:145–155
Shevenell L, McCarthy JF (2002) Effects of precipitation events on colloids in a karst aquifer. J Hydrol 255:50–68
Stecko JRP, Bendell-Young LI (2000) Contrasting the geochemistry of suspended particulate matter and deposited sediments within an estuary. Appl Geochem 15:753–775
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Tessier A, Fortin D, Belzile N, DeVitre RR, Leppard GG (1996) Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: narrowing the gap between field and laboratory measurements. Geochim Cosmochim Ac 60:387–404
van Ryssen R, Leermakers M, Baeyens W (1999) The mobilisation potential of trace metals in aquatic sediments as a tool for sediment quality classification. Environ Sci Pol 2:75–86
Vesper DJ (2002) Transport and storage of trace metals in a karst aquifer: an example from Fort Campbell, Kentucky. PhD Dissertation, Pennsylvania State University, University Park, PA, 276 pp
Vesper DJ, White WB (2003) Metal transport to karst springs during storm flow: an example from Fort Campbell, Kentucky/Tennessee, U.S.A. J Hydrol 276:20–36
White WB (1988) Geomorphology and hydrology of karst terrains. Oxford University Press, New York
Xiao-Quan S, Bin C (1993) Evaluation of sequential extraction for speciation of trace metals in model soil containing natural minerals and humic acid. Anal Chem 65:802–807
Acknowledgements
The work was supported by the Army Research Office under Grant Number DAAD19–01–1-0707; Sigma Xi, the Scientific Research Society; the Geological Society of America; and the Krynine Fund of the Pennsylvania State University Department of Geosciences. The authors would like to thank the Fort Campbell Environmental Restoration Office and the Clark and Hunt families for spring access.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vesper, D.J., White, W.B. Spring and conduit sediments as storage reservoirs for heavy metals in karst aquifers. Env Geol 45, 481–493 (2004). https://doi.org/10.1007/s00254-003-0899-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0899-6