Abstract
The methylotrophic bacterium Methylorubrum extorquens AM1 holds a great potential of a microbial cell factory in producing high value chemicals with methanol as the sole carbon and energy source. However, many gene functions remain unknown, hampering further rewiring of metabolic networks. Clustered regularly interspaced short palindromic repeat interference (CRISPRi) has been demonstrated to be a robust tool for gene knockdown in diverse organisms. In this study, we developed an efficient CRISPRi system through optimizing the promoter strength of Streptococcus pyogenes–derived deactivated cas9 (dcas9). When the dcas9 and sgRNA were respectively controlled by medium PR/tetO and strong PmxaF-g promoters, dynamic repression efficacy of cell growth through disturbing a central metabolism gene glyA was achieved from 41.9 to 96.6% dependent on the sgRNA targeting sites. Furthermore, the optimized CRISPRi system was shown to effectively decrease the abundance of exogenous fluorescent protein gene mCherry over 50% and to reduce the expression of phytoene desaturase gene crtI by 97.7%. We then used CRISPRi technology combined with 26 sgRNAs pool to rapidly discover a new phytoene desaturase gene META1_3670 from 2470 recombinant mutants. The gene function was further verified through gene deletion and complementation as well as phylogenetic tree analysis. In addition, we applied CRISPRi to repress the transcriptional level of squalene-hopene cyclase gene shc involved in hopanoid biosynthesis by 64.9%, which resulted in enhancing 1.9-fold higher of carotenoid production without defective cell growth. Thus, the CRISPRi system developed here provides a useful tool in mining functional gene of M. extorquens as well as in biotechnology for producing high-valued chemicals from methanol.
Key points
-
Developing an efficient CRISPRi to knockdown gene expression in C1-utilizing bacteria
-
CRISPRi combined with sgRNAs pool to rapidly discover a new phytoene desaturase gene
-
Improvement of carotenoid production by repressing a competitive pathway
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylorubrum extorquens AM1 (formerly Methylobacterium extorquens AM1), a pink-pigment and facultative methylotroph α-proteobacterium, is one representative of methylotrophs that are capable of growing on one-carbon compounds such as methanol or methylamine as the sole carbon and energy source (Peel and Quayle 1961). The genome of the M. extorquens AM1 contains one chromosome, one megaplasmid and three plasmids, harboring a total of nearly 6800 genes (Vuilleumier et al. 2009). Recently, much effort has been devoted to elucidating the gene functions, metabolic networks as well as its regulatory mechanisms (Nayak et al. 2016; Ochsner et al. 2017; Ueoka et al. 2018). For one-carbon metabolism, Nayak et al. disclosed the metabolic routes and regulatory network for methylamine oxidation of M. extorquens AM1 in different concentrations of methylamine (Nayak et al. 2016), and Ochsner et al. identified almost 100 new methylotrophic genes and elucidated an important regulatory mechanism of the serine cycle using TnSeq method (Ochsner et al. 2017). For secondary metabolism, Ueoka et al. discovered a new trans-acyltransferase polyketide biosynthetic gene cluster for synthesizing the antibiotic toblerol by genome mining (Ueoka et al. 2018). Currently, gene knockout through sucrose counter-selection is still the most used approach to investigate the gene function in M. extorquens AM1 (Marx 2008); however, for rapidly investigating unknown genes, it is still laborious and time-consuming. Transposon mutation has been also developed to screen for gene-phenotype associations or genetic interactions across different environments (Ochsner et al. 2017; Van Dien et al. 2003). One limitation for this technology is that transposon elements insert into the chromosome randomly, making this inappropriate for investigating the targeted gene from the pooled putative genes. Therefore, it is urgent to develop more efficient genetic tools to identify the unknown genes associated with cell phenotypes.
The CRISPR interference (CRISPRi) system has been developed for gene repression in diverse organisms by creating a nuclease-null Streptococcus pyogenes Cas9 (deactivated Cas9, or dCas9) in which two inactive mutations of D10A and H840A have been introduced into its RuvC1 and HNH nuclease domains (Larson et al. 2013; Qi et al. 2013; Du et al. 2017; Choudhary et al. 2015; Bruder et al. 2016; Huang et al. 2016; Wang et al. 2019; Hogan et al. 2019; Zhan et al. 2019; Caro et al. 2019). This technology can also be applied as an efficient tool in systematically manipulating transcription of endogenous genes, enabling rapid discovery of new functional genes using pooled sgRNAs (Gilbert et al. 2014; Wang et al. 2018; Lee et al. 2019). Moreover, since the intermediate metabolites serving as the main precursors for biosynthetic pathways are also key metabolites for cell growth, deletion of the genes responsible for core metabolism usually significantly impacts the physiological biochemistry of cells. Therefore, CRISPRi has been applied to fine-tune expression of essential genes with fewer effects of cell phenotype, making it a more favorable tool for manipulating metabolic flux distribution to the desired pathway (Cleto et al. 2016; Lv et al. 2015; Park et al. 2018; Westbrook et al. 2018; Woolston et al. 2018; Tian et al. 2019). For example, Cleto et al. interfered with the expression of pck or pyk by CRISPRi to achieve a 2- to 3-fold higher titer of L-glutamate, exceeding that in pck and pyk deletion strains, with the entire process only requiring 3 days (Cleto et al. 2016). In M. extorquens, three interlocked metabolic cycles are important for one-carbon assimilation: the serine cycle, the ethylmalonyl-CoA (EMC) pathway, and the poly-3-hydroxybutyrate (PHB) cycle (Zhang et al. 2019). It has been shown that metabolic flux goes through the serine cycle and EMC pathway when cells are grown on methanol, generating a supply of pyruvate and acetoacetyl-CoA, which could be employed as the precursors for production of value-added terpenoids (Van Dien et al. 2003; Zhang et al. 2019). Moreover, previous reports demonstrated to utilize acetyl-CoA and crotonyl-CoA as the precursors to produce mevalonate (Liang et al. 2017), 3-hydroxypropionic acid (Yang et al. 2017), 1-butanol as well as butadiene (Hu et al. 2016; Yang et al. 2018), all of which must be carefully balanced between growth maintenance and product biosynthesis in engineered M. extorquens. However, the lack of efficient tools of gene knockdown impedes its further development as a chemicals-production platform.
Just recently the CRISPR-Cas9 genome editing system was developed in methanotrophic bacteria Methylococcus capsulatus Bath (Tapscott et al. 2019), and the CRISPRi system was established in the methylotrophic thermophile Bacillus methanolicus MGA3 (Schultenkämper et al. 2019). However, considering the specificity and difference of genetic elements (e.g., promoter strength and initial replicon of expression plasmids), a CRISPRi system tailored to the strain is required to modify gene expression in the model methylotroph M. extorquens. Herein, we developed the CRISPRi system to effectively knockdown exogenous and endogenous genes in M. extorquens, and further application of CRISPRi system led us to identify a new phytoene desaturase involved in carotenoid biosynthesis. In addition, we also validated the usefulness of CRISPRi system in modulating the production of carotenoid via an interrupting competing pathway. The developed CRISPRi system provides a convenient tool in rapidly mining gene functions as well as pathway engineering for production of high value chemicals in M. extorquens.
Materials and methods
Strains, media, and culture conditions
The plasmids and strains used in this study are listed in Table 1. All Escherichia coli strains were cultured in Luria-Bertani (LB) agar or liquid medium at 37 °C supplemented with antibiotics where necessary at a final concentration of 20 μg/ml tetracycline (Tet) or 25 μg/ml kanamycin (Km). M. extorquens AM1 ATCC 14718 and the derivative strains were routinely cultured in a minimal medium at 30 °C as described previously (Yang et al. 2017). Substrates and antibiotics were supplied at the following concentrations: methanol (125 mM) or succinate (15 mM) as the sole carbon source. All chemicals used in media were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Construction of CRISPRi plasmids and recombinant M. extorquens strains
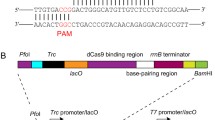
The primers used in this study are listed in Table 2 and Table S1. The codons for the S. pyogenes dcas9 were optimized according to M. extorquens AM1 codon preferences (Yang et al. 2017), and the sequences for dcas9 (GenBank accession number is MN896903) were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China). The sgRNAs were designed by using Python software package (Guo et al. 2018). Each sgRNA biobrick contained three parts: a 20-bp DNA region complementing the gene sequence of interest called the based-pairing region (BPR), a 42-bp hairpin region for dCas9 protein binding termed dCas9 handle (DH), and a 40-bp terminator rrnB. The sequences containing PmxaF-g-sgRNA-dcas9 handle-rrnB were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China). The pCM80 plasmid was used as the parent plasmid for developing the CRISPRi system. We amplified PmxaF-g and sgRNA by primers of PmxaF-g-1F and PmxaF-g-1R to replace the original PmxaF on pCM80 to obtain the plasmid pgRNA. After dcas9 amplified by primers of PmxaF-dCas9-F and dCas9-R as well as promoter PmxaF amplified by primers of PmxaF-F and PmxaF-R, the cassette “PmxaF-dcas9” was amplified by PCR using primers of PmxaF-F and dCas9-R with dcas9 fragment and promoter PmxaF as template. The PmxaF-dcas9 cassette was cloned into plasmid pgRNA digested with restriction enzymes SacI and BamHI by using ClonExpress®II One Step Cloning Kit (Vazyme Biotech Co. Ltd., Nanjing, China) and transformed into E. coli DH5α to confer the plasmid pAIS. The promoters of PcoxB and PfumC amplified from M. extorquens AM1, Plac amplified from pCM80, and PR/tetO amplified from pLC291 were respectively used to construct the plasmids pAIM, pAIW, pAIL, and pAIO as described above.
By using reverse PCR technology with the primers containing the 20-bp DNA region complementing the targeting gene sequence as reported (Van Dien et al. 2003), three plasmids pAIM-glyA-NT41, pAIM-glyA-NT571, and pAIM-glyA-NT691 that contained the sgRNAs targeting different regions of the non-template strand of glyA gene were constructed. The constructed plasmids were introduced into M. extorquens AM1 by electroporation, and then, the recombinant strains were cultured in the medium containing succinate as the sole carbon and energy source, leading to afford YMZA-2, YMZA-3, and YMZA-4, respectively. Similar to the strains of YMZA-2 to YMZA-4, the strains of YMZA-5 to YMZA-8, YMZA-9 to YMZA-12, YMZA-13 to YMZA-16, and YMZA-17 to YMZA-20 were obtained as described above.
To interfere the phytoene desaturase gene crtI (META1_3665) and squalene-hopene cyclase gene shc, three sgRNAs targeting different regions of the non-template strand of coding sequence were designed for each gene. By using reverse PCR technology with plasmid pAIO as template, six plasmids (pAIO-crtI-NT6, pAIO-crtI-NT212 and pAIO-crtI-NT1168 for targeting crtI, pAIO-shc-NT14, pAIO-shc-NT1017 and pAIO-shc-NT1956 for targeting shc) were constructed, which were introduced into M. extorquens AM1 to create the strains YMZA-21 to YMZA-23 and YMZA-27 to YMZA-29, respectively.
Interference of red fluorescence protein gene mCherry
The red fluorescence protein gene mCherry was inserted into the chromosome of M. extorquens AM1 between glmS and META1_4547 using the method described previously (Marx 2008). Briefly, after amplification of genes glmS and META1_4547 from M. extorquens AM1 and PmxaF-mCherry cassette from plasmid pSWU-mCherry (constructed by our Lab), then, the three fragments were overlapped by PCR to generate glmS-PmxaF-mCherry-META1_4547, which was further digested by BglII and SacI and cloned into pCM433 to afford plasmid pAIF. The pAIF was then electroporated into the wild-type M. extorquens AM1. M. extorquens AM1 bearing pAIF was firstly selected by using tetracycline resistance phenotype, and double-crossover mutants occurred by growing on the plates containing 5% sucrose (w/v). The mutant strain with successful allele swapping was confirmed by PCR with primers of genome-glmS-F and genome-1199-R, and the PCR fragment with expected size was further sequenced. The mutant strain with correct PmxaF-mCherry cassette was termed as YAIM.
Five sgRNAs targeting the different region of the non-template strand of mCherry were designed. The plasmids pAIO-NT0, pAIO-mCherry-NT32, pAIO-mCherry-NT77, pAIO-mCherry-NT483, and pAIO-mCherry-NT641 for interfering with mCherry were constructed by using reverse PCR with plasmid pAIO-NT0 as template, which were then introduced into the YAIM strain to generate the strains of YAIM-1 to YAIM-5. The interference efficacy toward mCherry was evaluated by determining the fluorescent value. The strains of YAIM-1, YAIM-2, YAIM-3, YAIM-4, and YAIM-5 were inoculated into the tubes and grown to OD600 with about 0.8. Then, 1.2 ml cells were transferred into 250 ml flasks with 50 ml fresh medium to obtain the initial OD600 with 0.02. The cells were cultured on rotary shaker at 200 rpm at 30 °C. When the OD600 reached about 1.0, 200 μl cells were transferred into a 96-well plate and the fluorescence intensity were measured by Cytation1 imaging reader (BioTek, Winooski, VT, USA) under the instruction of the protocol. Relative fluorescence values reported here are as follows:
\( \mathrm{Relative}\ \mathrm{fluorescence}\ \left(\mathrm{A}.\mathrm{U}.\right)=\frac{\mathrm{RFU}}{{\mathrm{OD}}_{600}}\times {10}^{-3} \), A.U. means arbitrary units, and RFU means red fluorescence unit.
Data were expressed as means ± standard deviations of results from three biological replicates.
Measurement of growth of M. extorquens and recombinant strains
M. extorquens and its recombinant strains were initially inoculated into the tubes containing 3 ml medium with 15 mM succinate as the sole carbon source. For each strain, three biological parallels were prepared. When the strains were grown to the middle exponential phase with OD600 value of about 0.8, the cells were harvested, and the supernatants were discarded. Then, the pellets were resuspended, and 1.2 ml cell culture was transferred into 250-ml flask with 50-ml minimal medium to obtain the initial OD600 of 0.02 and then grown on rotary shaker at 200 rpm at 30 °C for further monitoring growth curve. For each time point, a 0.5-ml sample was taken for OD600 measurement by using UV-visible spectrophotometer (Genesys10S, CA, USA). The growth rates presented here represent the mean plus standard deviations calculated from triplicate biological replicates. The specific growth rates were determined by fitting an exponential growth model using Curve Fitter software (Harcombe et al. 2016; http://www.evolvedmicrobe.com/Software.html).
RNA isolation and RT-qPCR analysis
When M. extorquens AM1 and the recombinants were grown to the exponential phase with OD600 value of about 0.8 in a minimal medium using methanol as the sole carbon source, 1 ml aliquots of each culture were taken and centrifuged (10,000×g, 1 min). The pellets were immediately frozen in liquid nitrogen and were stored at − 80 °C until further RNA isolation. The cells were lysed by resuspension of pellets in 1 ml TozolUP buffer (TransGen Biotech, China), 200 μl chloroform, and bead beating for 4 × 90 s with intermittent cooling on ice in Bioprep-6 biological sample homogenizer (All For Life Science Co. Ltd., Hangzhou, China). The RNase-free steel beads used here were 0.3 mm in diameter. The total RNA was extracted by using TransZol Up RNAspin Mini Kit (TransGen Biotech, Beijing, China) according to the instruction of protocol. The residual DNA in the extracted RNA sample was digested by DNaseI (Vazyme Biotech Co. Ltd., Nanjing, China). For each strain, the RNA was isolated from three biological replicates. The RNA degradation and contamination were checked on 1% agarose gels. Quality and quantity of RNA were determined by using spectrophotometer Nano Drop 1000 (Thermo Fisher Scientific, MA, USA).
One thousand nanograms of RNA was reversely transcribed to cDNA by using HiScript® IIQRT SuperMix kit for qPCR (Vazyme Biotech Co. Ltd., Nanjing, China). The real-time RT-qPCR was performed with AceQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co. Ltd., Nanjing, China) by using an ABI 7500 (QuantStudio 5, Thermo Fisher Scientific, MA, USA) instrument equipped with step two real-time system (Applied Biosystems, MA, USA). The gene rpsB encoding for 30S ribosomal protein S2 was employed as a reference gene (Chou et al. 2009). RT-qPCR was performed in accordance with the manufacturer’s instructions. Each reaction was conducted in three replicates. The relative quantification for RT-qPCR experiments was analyzed by 2−ΔΔCt method (Chou et al. 2009; Chou and Marx 2012). The Ct values obtained were used as the original data to calculate the relative transcriptional expression level of the candidate genes normalized against that of 30S ribosomal protein S2 gene.
Mining unknown genes involved in biosynthesis of carotenoid
Blastp analysis of M. extorquens AM1 proteome by using identified phytoene desaturase (crtI, META1_3665) and annotated phytoene synthase (crtB, META1_3220) revealed homologous phytoene desaturase genes (META1_2923, META1_3219, META1_3670, and META1_3679) and terpene/phytoene synthase (META1_4618, META1_1815, META1_1816, and META1_3220) in M. extorquens AM1 genome. For each gene, three sgRNAs targeting the front, middle, and terminal region of coding sequences were designed. The primers targeting phytoene desaturase genes (META1_2923, META1_3219, META1_3670, META1_3679, META1_3665) were mixed in equal concentration, and the primers targeting terpene/phytoene synthase (META1_4618, META1_1815, META1_1816, and META1_3220) were also mixed in equal concentration. By using reverse PCR with plasmid pAIO-NT0 as template and mixed primers, followed by DpnI digestion template, the PCR fragments were recovered from gel and introduced into E. coli DH5α competent cells to afford two pools that the plasmids carried all related sgRNAs. All the transformants on the plate were washed into flasks for overnight culture, and the plasmids were extracted and introduced into M. extorquens AM1 by electroporation. By this method, two M. extorquens AM1 recombinant libraries carrying different plasmids for CRIPSRi were developed. For the recombinants carrying plasmids targeting phytoene desaturase genes, 50 faint pink or colorless recombinant strains were randomly picked up, and the sgRNA regions of the plasmids were amplified by PCR with the primers of upstream-F and downstream-R for further sequencing.
For verifying the gene functions, the phytoene desaturase gene META1_3670 and phytoene synthase gene META1_3220 were respectively deleted by using the similar approach as the gene mCherry integration described above. For the mutant strain YAIP lacking META1_3670, the complementary experiment was conducted. The META1_3670 was amplified and cloned into pCM80 under promoter PmxaF to afford the plasmid pACR, which was then electroporated into the strain YAIP to perform the YACR strain.
Extraction and analysis of carotenoid
The carotenoid was extracted from M. extorquens AM1 as described previously (Van Dien et al. 2003; Tian et al. 2019). The extracts from 50 ml of cell culture was dissolved in 0.1 ml of DMSO, and 30 μl was subject to HPLC analysis by using Waters HPLC 1260 (Waters, MA, USA) equipped with a Waters Spherisorb 5.0 μm ODS2 (4.6 mm × 250 mm, 5 μm) column. The mobile phases were consisting of acetonitrile-water (solvent A, 9:1, V/V) and methanol-isopropanol (solvent B, 3:2, V/V). The elution program was set as follows: 100% A to 5% A in 0–10 min, 5% A retained from 10 to 20 min, and 5% A to 100% A in 20 to 25 min. The flow rate was 1.0 ml/min, and the UV absorbance of the peaks was collected from 200 to 600 nm using a photodiode array detector and monitored at 460 nm. Absorbance was converted to total carotenoid concentration by comparison to a carotenoid standard calibration curve and normalization to cell dry weight.
Statistical analysis
T test was applied to analyze all quantitative data. For each quantitative experiment, all data represent the averages of at least 3 parallels. p < 0.05 was considered as significant, *p < 0.05; **p < 0.01; ***p < 0.001.
Results
Construction of a CRISPRi system in M. extorquens AM1
The serine hydroxymethyl transferase catalyzing the conversion from glycine plus methylene H4F to serine is a rate-limited enzyme in the serine cycle, which is required for carbon assimilation from methanol in M. extorquens AM1 (Smejkalová et al. 2010). Consequently, its encoding gene glyA was chosen as the target for constructing and optimizing the CRISPRi system in this study. We placed sgRNA under a natural strong promoter PmxaF derived from methanol dehydrogenase of M. extorquens AM1, as insufficient sgRNA expression has been reported to cause low repression behavior (Schada et al. 2015). Since the mismatch sequence of sgRNAs in the 5′-terminus has demonstrated to critically impact the interference efficiency (Larson et al. 2013), the sequence of PmxaF was then carefully truncated to 190-bp (PmxaF-g) based on the putative transcriptional start site by BPROM software (Fig. S1) (Schada et al. 2015). The GC content of dcas9 gene derived from S. pyogenes (35%) is much lower than that of M. extorquens AM1 (68%) (Vuilleumier et al. 2009); thus, we optimized the coding sequences of S. pyogenes dcas9 according to M. extorquens codon preferences (Yang et al. 2017). In addition, because the promoter regions in M. extorquens AM1 have not been well characterized yet and a number of reports have shown success with the sgRNA targeting non-template strand of coding sequences in bacteria, yeast, or human cells (Qi et al. 2013; Du et al. 2017; Choudhary et al. 2015; Bruder et al. 2016; Huang et al. 2016; Wang et al. 2019), the non-template strand of glyA was selected for further interference. Three sgRNAs were designed to target different coding regions of glyA as well as one control sgRNA that did not target the genome of M. extorquens AM1. The CRISPRi elements are shown in Fig. 1a, and backbone plasmid pCM80, derived plasmids, and primers for constructing CRISPRi are shown in Tables 1 and 2. To establish an initial test of CRISPRi-mediated glyA repression, the plasmids pAIS-glyA-NT0, pAIS-glyA-NT41, pAIS-glyA-NT571, and pAIS-glyA-NT691 with PmxaF driving dcas9 were introduced into M. extorquens AM1 by electroporation. The growth curves of the resulting recombinant strains were measured when grown on methanol. Unexpectedly, compared to the control sgRNA (the YMZA-1 strain), the sgRNAs targeting three sites of glyA (the strains of YMZA-2, YMZA-3, and YMZA-4) showed no decreased growth rates at all (Fig. 1b). Previous studies have reported the recombinant M. extorquens expressing the heterologous genes from a promoter of intermediate strength produced the higher product titer (Rohde et al. 2017), which made us consider that the decrease of transcript levels of dcas9 might help to generate the effective interference.
Construction of the CRISPRi system in M. extorquens AM1. a The developed CRISPRi elements. The biobrick of sgRNAs contained three parts: a 20-bp sequence complementary to non-template strand of coding sequence region of targeted gene, a 42-bp dCas9 handle, and a 40-bp terminator, which were located under the transcription start site of the promoter PmxaF-g. The dcas9 derived from S. pyogenes was under control of PmxaF, Plac, PfumC, PcoxB, or PR/tetO. The primer binding sites for inverse PCR are highlighted by yellow (upper panel). b Comparison of growth curves and specific growth rate of the recombinant strains of YMZA-1 to 4 carrying the CRISPRi plasmids for interfering with the different regions of glyA, in which, the dcas9 were under control of PmxaF. Data represent means and standard deviations calculated from three biological replicates. Significance was assessed using t test, *p < 0.05; **p < 0.01; ***p < 0.001
Four relatively low activities of promoters (constitutive promoters of PcoxB, PfumC, and Plac as well as the inducible promoter PR/tetO) were explored to analyze dcas9 expression (Schada et al. 2015; Chubiz et al. 2013). For the constitutive promoters, previous research has demonstrated that the relative activities are followed by the order of PfumC (5–15%) < PcoxB (40–50%) < Ptuf (70–100%) ≈ PmxaF (100%) in M. extorquens AM1 (Schada et al. 2015). For the inducible promoter, as the maximal inducible level of PR/tetO achieves about 33% of PmxaF activity (Chubiz et al. 2013), at the beginning, this promoter was introduced into pCM80 without its regulatory protein TetR. We then extracted the RNA and evaluated the transcriptional levels of dcas9 by RT-qPCR (Fig. S2; Fig. 2). As shown in Fig. 2, the mRNA level of dcas9 driven by PcoxB was about 40% lower than that of PmxaF and 3.5-fold and 4.0-fold higher than that of PfumC and Plac. The mRNA level obtained by PR/tetO without TetR was slightly lower than that of PcoxB. These results were basically in line with the previously reported activities of promoters (Schada et al. 2015; Chubiz et al. 2013).
Comparison of the transcriptional levels acquired by real-time RT-qPCR among the recombinant strains with the CRISPRi plasmids carrying dcas9 drove by different promoters. For calculating relative expression, the mRNA abundance of dcas9 was firstly normalized to rpsB in each corresponding strain, and then relative expression was the ratio of dcas9 mRNA between the strains of YMZA-5, YMZA-9, YMZA-13, and YMZA-17 (dcas9 drove by Plac, PfumC, PcoxB, or PR/tetO) to the strain YMZA-1 (dcas9 drove by PmxaF) respectively. Data represent means and standard deviations calculated from three biological replicates
Furthermore, we examined the interference efficacy for each recombinant strain. As shown in Fig. 3 a and b, Plac driving dcas9 did not result in any significant growth repression for three targeting sites NT41, NT571, and NT691, but PfumC could obtain 21.7% of repression efficacy for the site NT691 in the recombinant strain YMZA-12. Moreover, we found that PcoxB could reduce the growth rate of the strains of YMZA-14 and YMZA-16 with even higher repression efficacy of 29.3% and 63.5% for the targeting sites NT571 and NT691 (Fig. 3c). Notably for the promoter PR/tetO, we were surprised to find that the recombinant strains YMZA-18 to YMZA-20 had very significantly reduced growth rates of 71.1%, 41.9%, and 96.6% for all three targeting sites (Fig. 3d). For more details, after cultured in methanol for about 40 h, the OD600 value of the YMZA-18 to YMZA-20 strains were attenuated to 0.11, 0.076, and 0.034 with the specific growth rate of 0.035/h, 0.068/h, and 0.004/h (Fig. 3d). In contrast, the control strain YMZA-17 had already entered stationary phase with the OD600 value over 1.60 in the same time period. To further explore if we could obtain a more dynamic range of interference, the regulatory protein TetR was introduced along with PR/tetO to create pAIR. Unfortunately, the highest repression efficacy at different concentrations of inducer anhydrotetracycline (ATc) (10, 20, and 50 ng/ml were added when the cells were inoculated in the cultural medium) was not as effective as that of PR/tetO only on plasmid (data not shown), suggesting that PR/tetO itself could perform as a constitutive promoter transcribing dcas9 to efficiently interfere with gene expression. Taken together, our results demonstrated that PR/tetO without TetR could achieve dynamic ranges of interference dependent on the targeting sites.
Optimization of the CRISPRi system in M. extorquens AM1. a–d Comparison of growth curves and specific growth rate of the recombinant strains of YMZA-5 to YMZA-20 carrying the CRISPRi plasmids for interfering with the different regions of glyA, in which, the dcas9 were under control of Plac, PfumC, PcoxB, or PR/tetO. Data represent means and standard deviations calculated from three biological replicates. Significance was assessed using t test, *p < 0.05; **p < 0.01; ***p < 0.001
Exploring the feasibility of CRISPRi system in repressing other exogenous and endogenous genes
To explore the feasibility of CRISPRi system developed here, the exogenous gene mCherry encoding for the red fluorescent protein and endogenous gene crtI involved in carotenoid biosynthetic pathway was interfered respectively. The plasmids with dcas9 gene driven by PR/tetO without TetR and sgRNAs under control of PmxaF-g were constructed as shown in Table 1. The mCherry cassette under control of promoter PmxaF was firstly integrated into the chromosome between glmS and META1_4547 of M. extorquens AM1 through homologous recombination [35]. We imaged recombinant strains and subjected the images to automated analysis to determine the average fluorescence. Compared to the control strain YAIM-1, the YAIM-3 strain showed the highest repression effect with the fluorescence value reduced by 51.3% (Fig. 4), and the following repression efficacy for the strains of YAIM-2, YAIM-4, and YAIM-5 was 37.9%, 25.2%, and 31.3%, respectively. Then, we evaluated the transcriptional level of mCherry in the strain of YAIM-3, in which the mRNA of mcherry was decreased by 88.0% accordingly (Fig. 4a).
The repression of mCherry by CRISPRi system. The strains of YAIM-2 to 5 carried the sgRNAs targeting different regions of mCherry sequence. The inserted panel was the relative expression of mCherry between the recombinant strain YAIM-3 and the control strain YAIM-1. The red fluorescence was visualized and quantified by Cytation1 imaging reader. Data represent means and standard deviations calculated from three biological replicates. Significance was assessed using t test, *p < 0.05; **p < 0.01; ***p < 0.001
To interfere crtI (META1_3665) encoding a phytoene desaturase involved in secondary metabolism of pink-pigment carotenoid biosynthesis [7], three plasmids pAIO-crtI-NT6, pAIO-crtI-NT212, and pAIO-crtI-NT1168 targeting different sites of the non-template strand were introduced into M. extorquens AM1. Comparing with the control strain YMZA-17, the production of carotenoid was decreased by 89.5%, 25.3%, and 97.7% in the recombinant strains (Fig. 5 a and b). The transcriptional levels of crtI were also significantly decreased to 14.3%, 62.5%, and 3.1% of that in native mRNA expression, respectively (Fig. 5b). These results clearly demonstrated that our CRISPRi system was also very efficient for the genes participating in secondary metabolism.
The repression of crtI by CRISPRi system. a Comparison of the change of colony color among the recombinant strains carrying different CRISPRi plasmids. b The relative expression of crtI and production of carotenoids between the recombinant strains (YMZA-21, YMZA-22, and YMZA-23) and the control strain YMZA-17. RT-qPCR analysis and production measurement are performed in three biological replicates. Significance was assessed using t test, *p < 0.05; **p < 0.01; ***p < 0.001
Application of CRISPRi to mine unknown enzymes involved in carotenoid biosynthesis
The essential genes such as crtB and crtI responsible for carotenoid biosynthesis are usually clustered in the chromosome of bacteria (Fukaya et al. 2018; Sedkova et al. 2005); however, our bioinformatics analysis and previous research revealed that the phytoene desaturase gene crtI (META1_3665) was not flanked by the possible phytoene synthase gene crtB in M. extorquens AM1 (Fig. 6a) (Van Dien et al. 2003). Thus, we tried to employ the developed CRISPRi system to rapidly identify other unknown genes possibly participating in synthesizing the carotenoid (Fig. 6b). We mined eight potential genes encoding for four putative phytoene synthases (META1_2923, META1_3219, META1_3670, and META1_3679), one terpene synthase (META1_4618), and three putative phytoene synthases (META1_1815, META1_1816, META1_3220) (Fig. 6a). We then constructed two CRISPRi libraries, in which Library 1 included 14 sgRNAs interfering with four putative desaturase genes and crtI (META1_3665) and Library 2 included 12 sgRNAs interfering with four putative synthase genes. All 26 sgRNAs were covered by 2470 recombinant strains of M. extorquens AM1. For Library 1 with 1320 strains, about 20 to 45% of strains appeared faint pink on each plate as shown an example in Fig. 6c, suggesting that they likely produced less pink carotenoid than that of the wild-type strain. Resultant PCR fragments of sgRNAs from 50 faint pink recombinant strains were re-sequenced, and the distribution of sgRNAs is listed in Table 3. All the sgRNAs from faint pink strains were found to target the gene META1_3670 encoding putative phytoene desaturase and the control crtI. RT-qPCR analysis demonstrated that the mRNA levels of META1_3670 in the strains of YMZA-24, YMZA-25, and YMZA-26 were reduced by 61.5 to 83.5% (Fig. 6d). Subsequently, to verify the function of META1_3670, it was deleted from the chromosome and the mutant strain YAIP was shown colorless on the plate (Fig. 6e). When the mutant strain was complemented with overexpressing META1_3670, the complementary strain YACR could restore the pink color and produce about 69% carotenoid of the wild-type strain (Fig. 6 e and f; Fig. S3A). All these results clearly demonstrated the gene META1_3670 was involved in the carotenoid biosynthetic pathway in M. extorquens AM1. Moreover, phylogenetic analysis revealed that the sequences of phytoene desaturase respectively encoded by crtI (META1_3665) and META1_3670 were diverged from each other (Fig. S4). META1_3670 encoding a desaturase and its homologous proteins from other species of Methylobacteria indicated a close evolutionary relationship to ζ-carotene desaturase CrtQa from Nostoc sp. PCC 7120 and CrtIb from Myxococcus xanthus (Fig. S4). CrtQa has been demonstrated to possess the function of Z to E isomerase and CrtIb has been validated to dehydrogenate carotenes in the trans-conformation (Breitenbach et al. 2013; Iniesta et al. 2007; Botella et al. 1995). Thus, we hypothesized that the enzymes encoded by META1_3670 and crtI might be responsible for dehydrogenating carotenes with different conformation and that cooperation of two carotene desaturases could result in completing the four dehydrogenation steps in M. extorquens AM1.
Mining the putative enzymes involved in carotenoid biosynthesis by CRISPRi with pooled sgRNAs. a The putative phytoene synthases in orange and phytoene desaturases in red participating in carotenoid synthesis. b The schematic chart for interference of putative phytoene synthases and desaturases. c The color of colonies was changed due to the repression of putative phytoene desaturases. d The relative expression of phytoene desaturase gene META1_3670 in the recombinant strains carrying different CRISPRi plasmids. e Functional analysis of META1_3670 by deletion and gene complementation. f Comparison of carotenoid production in the recombinant strains. The YMZA-17 strain carried the plasmid with the control sgRNA, and the strains of YMZA-24 to YMZA-26 carried the plasmids targeting different regions of the non-template strand of META1_3670. YAIP was the strain with deleted META1_3670. YACR was the strain with META1_3670 reintroduced into the YAIP strain. RT-qPCR analysis and production measurement are performed in three biological replicates. Significance was assessed using t test, *p < 0.05; **p < 0.01; ***p < 0.001
However, for Library 2 with 1150 strains, 12 sgRNAs targeting three predicted synthase genes did not result in any change of colony color on the plate (Fig. S5A). We then knocked out the previously annotated crtB (META1_3220) and did not observe the changed color of colonies either in the mutant strain of YAIB (Fig. S3B and Fig. S5B). Our current results proposed that other unknown genes are likely responsible for this reaction. It also did not rule out the redundant function of these three predicted genes, meaning that single gene knockdown or deletion was not enough to significantly change the cell phenotype.
Application of CRISPRi to enhance the production of carotenoid by knocking down a competitive pathway
In recent years, M. extorquens has attracted the attention to be developed as a single cell protein for aquaculture (Tlusty et al. 2017). One particular beneficial trait is that M. extorquens produces a suite of natural carotenoid pigments that have been associated with both imparting color and enhancing immunity of shrimps and fishes (Tlusty et al. 2017). In order to improve the production of carotenoid, one plausible strategy is to increase the supply of precursor intermediates. In M. extorquens AM1, the biosynthesis of carotenoid and hopanoid shares the same precursor isoprene. And the squalene-hopene cyclase encoded by gene shc is an essential enzyme for the hopanoid biosynthesis (Bradley et al. 2017). Because the hopanoid is important for membrane fluidity and lipid packing, the direct deletion of shc can cause the mutant strain to alter physiological properties and suppress growth (Bradley et al. 2017; Sáenz et al. 2015). Here, we evaluated whether we could enhance the production of carotenoid by knocking down the expression of shc through CRISPRi without disturbing the growth phenotype. Three CRISPRi plasmids were constructed to target different sites of shc. By comparison of the control strain YMZA-17, the production of carotenoid was not significantly improved in the strains of YMZA-27 and YMZA-28 but was increased by 1.9-fold in the recombinant strain YMZA-29 (Fig. 7a). Meanwhile, the growth rate and biomass yield of the strain YMZA-29 did not change significantly (Fig. 7b; Table S2). RT-qPCR analysis demonstrated that the mRNA levels of shc in the strains YMZA-27 to YMZA-29 dropped to 42.2%, 75.7%, and 35.1% of that of the wild-type strain, respectively (Fig. 7a). Therefore, with designing the sgRNAs targeting different sites, the developed CRISPRi could be applied to enhance the production of targeted metabolites through downregulating the expression of genes involved in the competitive pathway with the essential function in physiological process.
Improvement of the carotenoid production in M. extorquens AM1 through interfering the expression of shc. a The relative expression of shc and relative production of carotenoid in the recombinant strains. b Comparison of growth curve of the recombinant strains. RT-qPCR analysis, production measurement, and growth analysis are performed in three biological replicates. Significance was assessed using t test, *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
M. extorquens AM1 is capable of growing on C1 compounds as the sole carbon and energy source, which makes it a promising cell factory for biotechnology in a methanol- and formate-based bioeconomy. Its central carbon metabolism pathways such as the serine cycle and EMC pathway contain C2 to C5 intermediates that are all interesting precursors for producing value-added chemicals such as mevalonate, 3-hydroxypropionate, and 1-butanol (Schada et al. 2018). Thus, the relevant genes in these pathways cannot be simply deleted for pushing the flux through the certain intermediate because the knockouts usually result in a lethal growth phenotype on C1 compounds. In view of this, CRISPRi provides a useful tool to fine-tune transcriptional level of essential genes and channel flux through biosynthetic pathways of targeted products. In this study, a CRISPRi system was developed to effectively knock down the gene expression in M. extorquens AM1. The highest interference efficacy was found to be dcas9 under control of PR/tetO without the TetR repressor and sgRNA under control of strong promoter PmxaF-g. In general, the highest repression efficacy can be achieved in which both dcas9 and sgRNA were droved by strong promoters (Fontana et al. 2018); however, for M. extorquens AM1, no significant repression efficacy was achieved when both dcas9 and sgRNA were droved by strong PmxaF. We propose that an appropriate balance of expression level of dcas9 and sgRNA was critically important to determine the efficiency of CRISPRi in M. extorquens AM1. Similar phenomena have also been observed in other bacteria such as E. coli, in which expression of dcas9 by a moderate level promoter and the sgRNA by strong promoter achieved the optimal production of isopentenol (Tian et al. 2019). Inducible promoters are often applied to control the dynamic expression of dcas9 to achieve dynamic repression of target gene with different concentrations of inducers (Wang et al. 2019; Tan et al. 2018). In M. extorquens AM1, the inducible promoters confer weak or leaky expression under control of repressor (Marx and Lidstrom 2001), and in this study, we tried to use inducible promoter PR/tetO along with its repressor TetR to dynamically regulate the repression of gene expression, whereas it did not make the improvement of repression efficacy.
Previous studies have demonstrated that variant sgRNAs targeting different regions show important relationship with repression efficacy, which was also observed in our study by the repression activity of glyA, mCherry, crtI, META1_3670, and shc. When sgRNA targeting the beginning region of the transcript or − 10 and − 35 regions of promoter, it is indicated to result in higher repression (Larson et al. 2013; Qi et al. 2013; Du et al. 2017; Huang et al. 2016). However, for interfering the coding region, it is difficult to accurately predict which locus region on the non-template strand would be more suitable for achieving high interference efficacy. Many reports have revealed that the sgRNA targeting the coding region of non-template strand with small distance to transcriptional start site can achieve better repression efficacy (Zhan et al. 2019; Li et al. 2017; Hogan et al. 2019), but sometimes, the repression efficacy of sgRNA is not well correlated to the distance (Tao et al. 2017; McInally et al. 2019). For M. extorquens AM1, as shown from the repression efficacy interfering glyA, crtI, META1_3670, and shc, it was more likely that interfering initial and terminal coding region of non-template strands both showed good repression efficacy. By this developed CRISPRi technology, we successfully knocked down the transcriptional level of squalene-hopene cyclase gene shc without influencing cell growth and introduced more flux into carotene biosynthesis resulting in an about 2-fold improvement in carotenoid production.
CRISPRi has been demonstrated to be very useful in identifying targeted gene from a series of candidate genes in traditional industrial strains such as E. coli and Corynebacterium glutamicum and in the human pathogen Vibrio cholerae (Wang et al. 2018; Lee et al., 2018; Caro et al. 2019). For instance, by using CRISPRi technology, Lee et al. rapidly identify an unknown carboxyl esterase for degradation of methyl acetate in C. glutamicum (Lee et al., 2018). In this study, by application of the CRISPRi system with a small pool of sgRNAs, we rapidly screened out a new phytoene desaturase encoded by META1_3670 responsible for carotenoid biosynthesis. Whereas Van Dien et al. identified a phytoene desaturase CrtI (META1_3665) participating in carotenoid biosynthesis from approximately 7000 screened visually through transposon mutagenesis technology (Van Dien et al. 2003), CRISPRi developed here presented to be a high throughput genetic tool to achieve precise interference of a number of candidate genes and reduce insertion bias on the chromosome. Up to now, many bacterial carotenoid biosynthetic genes are reported to form a cluster pattern (Tian and Hua 2010), and only a few characterized examples, such as in Gemmatimonas aurantiaca, Deinococcus radiodurans, and Salinispora tropica, have been found to either be not colocalized or be loosely clustered and, thus, not co-transcribed (Tian and Hua 2010; Takaichi et al. 2010; Richter et al. 2015). Moreover, one single phytoene desaturase has been demonstrated to be enough to catalyze four desaturation steps to produce lycopene in bacteria (Tian and Hua 2010; Fischbach and Voigt 2010; Paniagua et al. 2012). So far, there is only one example found in Myxococcus xanthus that two carotenoid desaturases CrtIa and CrtIb are required to complete four desaturation steps to convert phytoene into lycopene (Iniesta et al. 2007). In addition, in cyanobacteria and plants, phytoene desaturase Pds and ζ-carotene desaturase Zds have been also described to catalyze the sequential conversion from phytoene to ζ-carotene, and then, to lycopene (Nupur et al. 2016; Hirschberg 2001; Nisar et al. 2015). Our study provides the second example in bacteria that two desaturases encoding by META1_3665 and META1_3670 are involved in carotenoid biosynthesis. For the chemical structures of carotenoids in methylotrophic bacteria, currently, it has not been well demonstrated except for a few species (Sato et al. 1982; Ayako et al. 2015). For instance, four carotenoids with an attached sugar moiety have been isolated from six Methylobacterium strains (i.e., Methylobacterium sp. 189, Methylobacterium sp. 32, Methylobacterium sp. F15, Methylobacterium sp. 680, M. populi BJ00118 and M. radiotolerans JCM2831) (Ayako et al. 2015). The structures of carotenoids produced by M. extorquens AM1 were proposed to be similar with that produced by Methylobacterium strains (Ayako et al.2015) according to their accurate mass and UV absorption (data not shown), which could be the C30-carotenoids with a β-glucose and a fatty acid chain. Considering the exact structures of carotenoids in M. extorquens AM1 are still unclear at this moment, it is difficult to postulate the clear role that each desaturase played. The characterization of the chemical structures and enzymatic function are subjects of ongoing research.
A number of genes are poorly identified in the model methylotroph of M. extorquens AM1, leading to a large challenge of engineering this type of bacterium as a powerful chassis host. Our work herein demonstrates that the developed CRISPRi system can efficiently and gradually repress the expression of diverse targeted genes in M. extorquens AM1, providing an important basis for further uncovering unknown gene functions with pooled sgRNA libraries in a high-throughput phenotypic screening approach. Additionally, fine-tuning gene expression by CRISPRi provides a valuable strategy for gene knockdown of competing pathways to channel more flux into the desired pathway.
References
Ayako O, Kaseya Y, Koue N, Schrader J, Knief C, Vorholt JA, Sandmann G, Shindo K (2015) 4-[2-O-11Z-Octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopene-4,4′-dioic acid and 4-[2-O-9Z-hexadecenoyl-β-glucopyranosyl]-4,4′-diapolycopene-4,4′-dioic acid: new C30-carotenoids produced by Methylobacterium. Tetrahedron Lett 56:2791–2794. https://doi.org/10.1016/j.tetlet.2015.04.042
Botella JA, Murillo FJ, Ruiz-Vázquez R (1995) A cluster of structural and regulatory genes for light-induced carotenogenesis in Myxococcus xanthus. Eur J Biochem 233:238–248. https://doi.org/10.1111/j.1432-1033.1995.238_1.x
Bradley AS, Swanson PK, Muller EE, Bringel F, Caroll SM, Pearson A, Vuilleumier S, Marx CJ (2017) Hopanoid-free Methylobacterium extorquens DM4 overproduces carotenoids and has widespread growth impairment. PLoS One 12:e0173323. https://doi.org/10.1371/journal.pone.0173323
Breitenbach J, Bruns M, Sandmann G (2013) Contribution of two ζ-carotene desaturases to the poly-cis desaturation pathway in the cyanobacterium Nostoc PCC 7120. Arch Microbiol 195:491–498. https://doi.org/10.1007/s00203-013-0899-1
Bruder MR, Pyne ME, Moo-Young M, Chung DA, Chou CP (2016) Extending CRISPR-Cas9 technology from genome editing to transcriptional engineering in the genus Clostridium. Appl Environ Microbiol 82:6109–6119. https://doi.org/10.1128/AEM.02128-16
Caro F, Place NM, Mekalanos JJ (2019) Analysis of lipoprotein transport depletion in Vibrio cholerae using CRISPRi. Proc Natl Acad Sci U S A 116:17013–17022. https://doi.org/10.1073/pnas.1906158116
Chou HH, Marx CJ (2012) Optimization of gene expression through divergent mutational paths. Cell Rep 1:133–140. https://doi.org/10.1016/j.celrep.2011.12.003
Chou HH, Berthet J, Marx CJ (2009) Fast growth increases the selective advantage of a mutation arising recurrently during evolution under metal limitation. PLoS Genet 5:e1000652. https://doi.org/10.1371/journal.pgen.1000652
Choudhary E, Thakur P, Pareek M, Agarwal N (2015) Gene silencing by CRISPR interference in mycobacteria. Nat Commun 6:6267. https://doi.org/10.1038/ncomms7267
Chubiz LM, Purswani J, Carroll SM, Marx CJ (2013) A novel pair of inducible expression vectors for use in Methylobacterium extorquens. BMC Res Notes 6:183. https://doi.org/10.1186/1756-0500-6-183
Cleto S, Jensen JV, Wendisch VF, Lu TK (2016) Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi). ACS Synth Biol 5:375–385. https://doi.org/10.1021/acssynbio.5b00216
Du D, Roguev A, Gordon DE, Chen M, Chen SH, Shales M, Shen JP, Ideker T, Mali P, Qi LS, Krogan NJ (2017) Genetic interaction mapping in mammalian cells using CRISPR interference. Nat Methods 14(6):577–580. https://doi.org/10.1038/nmeth.4286
Fischbach M, Voigt CA (2010) Prokaryotic gene clusters: a rich toolbox for synthetic biology. Biotechnol J 5:1277–1296. https://doi.org/10.1002/biot.201000181
Fontana J, Dong C, Ham JY, Zalatan JG, Carothers JM (2018) Regulated Expression of sgRNAs Tunes CRISPRi in E. coli. J Bacteriol. 13(9):e1800069. https://doi.org/10.1002/biot.201800069
Fukaya Y, Takemura M, Koyanagi T, Maoka T, Shindo K, Misawa N (2018) Structural and functional analysis of the carotenoid biosynthesis genes of a Pseudomonas strain isolated from the excrement of autumn darter. Biosci Biotechnol Biochem 82:1043–1052. https://doi.org/10.1080/09168451.2017.1398069
Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS (2014) Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661. https://doi.org/10.1016/j.cell.2014.09.029
Guo J, Wang T, Guan C, Liu B, Luo C, Xie Z, Zhang C, Xing XH (2018) Improved sgRNA design in bacteria via genome-wide activity profiling. Nucleic Acids Res 46:7052–7069. https://doi.org/10.1093/nar/gky572
Harcombe WR, Betts A, Shapiro JW, Marx CJ (2016) Adding biotic complexity alters the metabolic benefits of mutualism. Evolution 70(8):1871–1881. https://doi.org/10.1111/evo.12973
Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218. https://doi.org/10.1016/S1369-5266(00)00163-1
Hogan AM, Rahman ASMZ, Lightly TJ, Cardona ST (2019) A broad-host-range CRISPRi toolkit for silencing gene expression in Burkholderia. ACS Synth Biol 8:2372–2384. https://doi.org/10.1021/acssynbio.9b00232
Hu B, Yang YM, Beck DA, Wang QW, Chen WJ, Yang J, Lidstrom ME, Yang S (2016) Comprehensive molecular characterization of Methylobacterium extorquens AM1 adapted for 1-butanol tolerance. Biotechnol Biofuels 9:84. https://doi.org/10.1186/s13068-016-0497-y
Huang CH, Shen CR, Li H, Sung LY, Wu MY, Hu YC (2016) CRISPR interference (CRISPRi) for gene regulation and succinate production in cyanobacterium S. elongatus PCC 7942. Microb Cell Factories 15:196. https://doi.org/10.1186/s12934-016-0595-3
Iniesta AA, Cervantes M, Murillo FJ (2007) Cooperation of two carotene desaturases in the production of lycopene in Myxococcus xanthus. FEBS J 274:4306–4314. https://doi.org/10.1111/j.1742-4658.2007.05960.x
Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8:2180–2196. https://doi.org/10.1038/nprot.2013.132
Lee SS, Shin H, Jo S, Lee SM, Um Y, Woo HM (2018) Rapid identification of unknown carboxyl esterase activity in Corynebacterium glutamicum using RNA-guided CRISPR interference. Enzym Microb Technol 114:63–68. https://doi.org/10.1016/j.enzmictec.2018.04.004
Lee HH, Ostrov N, Wong BG, Gold MA, Khalil AS, Church GM (2019) Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat Microbiol 4:1105–1113. https://doi.org/10.1038/s41564-019-0423-8
Li D, Lv L, Chen JC, Chen GQ (2017) Controlling microbial PHB synthesis via CRISPRi. Appl Microbiol Biotechnol 101:5861–5867. https://doi.org/10.1007/s00253-017-8374-6
Liang WF, Cui LY, Cui JY, Yu KW, Yang S, Wang TM, Guan CG, Zhang C, Xing XH (2017) Biosensor-assisted transcriptional regulator engineering for Methylobacterium extorquens AM1 to improve mevalonate. Metab Eng 39:159–168. https://doi.org/10.1016/j.ymben.2016.11.010
Lv L, Ren YL, Chen JC, Wu Q, Chen GQ (2015) Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study: controllable P(3HB-co-4HB) biosynthesis. Metab Eng 29:160–168. https://doi.org/10.1016/j.ymben.2015.03.013
Marx CJ (2008) Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res Notes 1:1. https://doi.org/10.1186/1756-0500-1-1
Marx CJ, Lidstrom ME (2001) Development of improved versatile broad-host-range vectors for use in methylotrophs and other gram-negative bacteria. Microbiology 147:2065–2075. https://doi.org/10.1099/00221287-147-8-2065
McInally SG, Hagen KD, Nosala C, Williams J, Nguyen K, Booker J, Jones K, Dawson SC (2019) Robust and stable transcriptional repression in Giardia using CRISPRi. Mol Biol Cell 30:119–130. https://doi.org/10.1091/mbc.E18-09-0605
Nayak DD, Agashe D, Lee MC, Marx CJ (2016) Selection maintains apparently degenerate metabolic pathways due to tradeoffs in using methylamine for carbon versus nitrogen. Curr Biol 26:1416–1426. https://doi.org/10.1016/j.cub.2016.04.029
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82. https://doi.org/10.1016/j.molp.2014.12.007
Nunn DN, Lidstrom ME (1986) Phenotypic characterization of 10 methanol oxidation mutant classes in Methylobacterium sp. strain AM1. J Bacteriol 166(2):591–597. https://doi.org/10.1128/jb.166.2.591-597.1986
Nupur LN, Vats A, Dhanda SK, Raghava GP, Pinnaka AK, Kumar A (2016) ProCarDB: a database of bacterial carotenoids. BMC Microbiol 16:96–98. https://doi.org/10.1186/s12866-016-0715-6
Ochsner AM, Christen M, Hemmerle L, Peyraud R, Christen B, Vorholt JA (2017) Transposon sequencing uncovers an essential regulatory function of phosphoribulokinase for methylotrophy. Curr Biol 27:2579–2588. https://doi.org/10.1016/j.cub.2017.07.025
Paniagua-Michel J, Olmos-Soto J, Ruiz MA (2012) Pathways of carotenoid biosynthesis in bacteria and microalgae. Methods Mol Biol 892:1–12. https://doi.org/10.1007/978-1-61779-879-5_1
Park J, Shin H, Lee SM, Um Y, Woo HM (2018) RNA-guided single/double gene repressions in Corynebacterium glutamicum using an efficient CRISPR interference and its application to industrial strain. Microb Cell Factories 17:4. https://doi.org/10.1186/s12934-017-0843-1
Peel D, Quayle JR (1961) Microbial growth on C1 compounds. 1. Isolation and characterization of Pseudomonas AM1. Biochem J 81:465–469. https://doi.org/10.1042/bj0810465
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. https://doi.org/10.1016/j.cell.2013.02.022
Richter TK, Hughes CC, Moore BS (2015) Sioxanthin, a novel glycosylated carotenoid, reveals an unusual subclustered biosynthetic pathway. Environ Microbiol 17:2158–2171. https://doi.org/10.1111/1462-2920.12669
Rohde MT, Tischer S, Harms H, Rohwerder T (2017) Production of 2-hydroxyisobutyric acid from methanol by Methylobacterium extorquens AM1 expressing (R)-3-hydroxybutyryl coenzyme a-isomerizing enzymes. Appl Environ Microbiol 83:e02622–e02616. https://doi.org/10.1128/AEM.02622-16
Sáenz JP, Grosser D, Bradley AS, Lagny TJ, Lavrynenko O, Broda M, Simons K (2015) Hopanoids as functional analogues of cholesterol in bacterial membranes. Proc Natl Acad Sci U S A 112(38):11971–11976. https://doi.org/10.1073/pnas.1515607112
Sato K, Mizutani T, Hiraoka M, Shimizu S (1982) Carotenoid containing sugar moiety from a facultative methylotroph, Protaminobacter ruber. J Ferment Technol 60:111–115
Schada von Borzyskowski L, Remus-Emsermann M, Weishaupt R, Vorholt JA, Erb TJ (2015) A set of versatile brick vectors and promoters for the assembly, expression, and integration of synthetic operons in Methylobacterium extorquens AM1 and other alpha-proteobacteria. ACS Synth Biol 4:430–443. https://doi.org/10.1021/sb500221v
Schada von Borzyskowski L, Sonntag F, Pöschel L, Vorholt JA, Schrader J, Erb TJ1, Buchhaupt M (2018) Replacing the ethylmalonyl-CoA pathway with the glyoxylate shunt provides metabolic flexibility in the central carbon metabolism of Methylobacterium extorquens AM1. ACS Synth Biol 7:86–97. https://doi.org/10.1021/acssynbio.7b00229
Schultenkämper K, Brito LF, López MG, Brautaset T, Wendisch VF (2019) Establishment and application of CRISPR interference to affect sporulation, hydrogen peroxide detoxification, and mannitol catabolism in the methylotrophic thermophile Bacillus methanolicus. Appl Microbiol Biotechnol 103:5879–5889. https://doi.org/10.1007/s00253-019-09907-8
Sedkova N, Tao L, Rouvière PE, Cheng Q (2005) Diversity of carotenoid synthesis gene clusters from environmental Enterobacteriaceae strains. Appl Environ Microbiol 71:8141–8146. https://doi.org/10.1128/AEM.71.12.8141-8146.2005
Smejkalová H, Erb TJ, Fuchs G (2010) Methanol assimilation in Methylobacterium extorquens AM1: demonstration of all enzymes and their regulation. PLoS One 5:e13001. https://doi.org/10.1371/journal.pone.0013001
Takaichi S, Maoka T, Takasaki K, Hanada S (2010) Carotenoids of Gemmatimonas aurantiaca (Gemmatimonadetes): identification of a novel carotenoid, deoxyoscillol 2-rhamnoside, and proposed biosynthetic pathway of oscillol 2,2′-dirhamnoside. Microbiology 156:757–763. https://doi.org/10.1099/mic.0.034249-0
Tan SZ, Reisch CR, Prather KLJ (2018) A robust CRISPR interference gene repression system in Pseudomonas. J Bacteriol 200:e00575–17. https://doi.org/10.1128/JB.00575-17
Tao W, Lv L, Chen GQ (2017) Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb Cell Factories 16:48. https://doi.org/10.1186/s12934-017-0655-3
Tapscott T, Guarnieri MT, Henard CA (2019) Development of a CRISPR/Cas9 system for Methylococcus capsulatus in vivo gene editing. Appl environ Microbiol 85. Pii: e00340-e00319. doi: https://doi.org/10.1128/AEM.00340-19
Tian B, Hua Y (2010) Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria. Trends Microbiol 18:512–520. https://doi.org/10.1016/j.tim.2010.07.007
Tian T, Kang JW, Kang A, Lee TS (2019) Redirecting metabolic flux via combinatorial multiplex CRISPRi-mediated repression for isopentenol production in Escherichia coli. ACS Synth Biol 8:391–402. https://doi.org/10.1021/acssynbio.8b00429
Tlusty M, Rhyne A, Szczebak JT, Bourque B, Bowen JL, Burr G, Marx CJ, Feinberg L (2017) A transdisciplinary approach to the initial validation of a single cell protein as an alternative protein source for use in aquafeeds. Peer J 5:e3170. https://doi.org/10.7717/peerj.3170
Ueoka R, Bortfeld-Miller M, Morinaka BI, Vorholt JA, Piel J (2018) Toblerols: cyclopropanol-containing polyketide modulators of antibiosis in Methylobacteria. Angew Chem Int Ed 57:977–981. https://doi.org/10.1002/anie.201709056
Van Dien SJ, Marx CJ, O'Brien BN, Lidstrom ME (2003) Genetic characterization of the carotenoid biosynthetic pathway in Methylobacterium extorquens AM1 and isolation of a colorless mutant. Appl Environ Microbiol 69:7563–7566. https://doi.org/10.1128/AEM.69.12.7563-7566.2003
Vuilleumier S, Chistoserdova L, Lee MC, Bringel F, Lajus A, Zhou Y, Gourion B, Barbe V, Chang J, Cruveiller S, Dossat C, Gillett W, Gruffaz C, Haugen E, Hourcade E, Levy R, Mangenot S, Muller E, Nadalig T, Pagni M, Penny C, Peyraud R, Robinson DG, Roche D, Rouy Z, Saenampechek C, Salvignol G, Vallenet D, Wu Z, Marx CJ, Vorholt JA, Olson MV, Kaul R, Weissenbach J, Médigue C, Lidstrom ME (2009) Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One 4:e5584. https://doi.org/10.1371/journal.pone.0005584
Wang T, Guan C, Guo J, Liu B, Wu Y, Xie Z, Zhang C, Xing XH (2018) Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance. Nat Commun 9:2475. https://doi.org/10.1038/s41467-018-04899-x
Wang T, Wang M, Zhang Q, Cao S, Li X, Qi Z, Tan Y, You Y, Bi Y, Song Y, Yang R, Du Z (2019) Reversible gene expression control in Yersinia pestis by using an optimized CRISPR interference system. Appl Environ Microbiol 85:e00097–e00019. https://doi.org/10.1128/AEM.00097-19
Westbrook AW, Ren X, Oh J, Moo-Young M, Chou CP (2018) Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab Eng 47:401–413. https://doi.org/10.1016/j.ymben.2018.04.016
Woolston BM, Emerson DF, Currie DH, Stephanopoulos G (2018) Rediverting carbon flux in Clostridium ljungdahlii using CRISPR interference (CRISPRi). Metab Eng 48:243–253. https://doi.org/10.1016/j.ymben.2018.06.006
Yang YM, Chen WJ, Yang J, Zhou YM, Hu B, Zhang M, Zhu LP, Wang GY, Yang S (2017) Production of 3-hydroxypropionic acid in engineered Methylobacterium extorquens AM1 and its reassimilation through a reductive route. Microb Cell Factories 16:179. https://doi.org/10.1186/s12934-017-0798-2
Yang J, Zhang CT, Yuan XJ, Zhang M, Mo XH, Tan LL, Zhu LP, Chen WJ, Yao MD, Hu B, Yang S (2018) Metabolic engineering of Methylobacterium extorquens AM1 for the production of butadiene precursor. Microb Cell Factories 17:194. https://doi.org/10.1186/s12934-018-1042-4
Zhan Y, Xu Y, Zheng P, He M, Sun S, Wang D, Cai D, Ma X, Chen S (2019) Establishment and application of multiplexed CRISPR interference system in Bacillus licheniformis. Appl Microbiol Biotechnol 104:391–403. https://doi.org/10.1007/s00253-019-10230-5
Zhang M, Yuan XJ, Zhang C, Zhu LP, Mo XH, Chen WJ, Yang S (2019) Bioconversion of methanol into value-added chemicals in native and synthetic methylotrophs. Curr issues Mol biol 33:225–236. https://doi.org/10.21775/cimb.033.225
Acknowledgments
We thank Joseph D. Groom and Mary E. Lidstrom at the University of Washington for their assistance with English editing.
Funding
This work was supported by the National Key R&D Program of China (grant No. 2018YFA0901500); the National Natural Science Foundation of China (grant No. 21776149); Shandong Provincial Key Research and Development Project, China (2016GSF117026); and Shandong Provincial Natural Science Foundation, China (ZR2015CM017).
Author information
Authors and Affiliations
Contributions
HZ and XM perform the experiments. SY and XM conceived the experiments, SY, XM, TW, CZ, CZ, and XX analyzed data. XM and SY wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 425 kb)
Rights and permissions
About this article
Cite this article
Mo, XH., Zhang, H., Wang, TM. et al. Establishment of CRISPR interference in Methylorubrum extorquens and application of rapidly mining a new phytoene desaturase involved in carotenoid biosynthesis. Appl Microbiol Biotechnol 104, 4515–4532 (2020). https://doi.org/10.1007/s00253-020-10543-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10543-w