Abstract
Heavy metal pollution in agricultural soils has become a widespread serious problem with the rapid industrialization and urbanization in the past two decades. Cadmium (Cd2+) is of the most concern in soils due to its high toxicity. It is necessary to develop remediation strategies to remove or neutralize its toxic effects in Cd-contaminated soil. Microbial bioremediation is a promising technology to treat heavy metal-contaminated soils. In this study, Cd-resistant bacterium, isolated from heavy metal-polluted soil in Southern China, was characterized as Raoultella sp. strain X13 on the basis of its biochemical profile and 16S rRNA. We investigated the characterization of Cd2+ distribution in different cellular compartments after Cd2+ uptake. Cd2+ uptake by strain X13 was mainly by ion exchange and chelation binding tightly to the cell wall. In addition, X13 plant growth-promoting characteristics suggested that X13 could solubilize phosphate and produce indole acetic acid. Pot experiments for the remediation of Cd-contaminated soil in situ by X13 inoculation demonstrated that X13 application to Cd-contaminated soils significantly promoted pak choi growth and improved production. We also found that X13 substantially reduced the Cd2+ bioavailability for pak choi. Therefore, strain X13 is an effective treatment for potential application in Cd2+ remediation as well as for sustainable agronomic production programs in Cd-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in Chinese agricultural soils has become a serious problem as a result of the rapid industrialization and urbanization of the last two decades (Wei and Yang 2010; Zhang et al. 2015). Heavy metal soil pollution is mainly the result of fertilizer and pesticide use, wastewater irrigation, and mining activities (Ok et al. 2011; Fellet et al. 2014; Waqas et al. 2014). Zhang et al. reviewed 465 studies on heavy metal pollution rates in Chinese farmland soils; the results showed that about 10.18% of arable soil was polluted by heavy metals, and cadmium (Cd2+) had the highest pollution rate at 7.75% (Zhang et al. 2015). Cd2+ in soil can threaten human health via the soil-crop system and the food chain (Nogawa and Kido 1996). Studies have found that Cd2+ causes bone lesions, renal disturbances, and lung insufficiency in humans (Sharma 1995; Chakravarty et al. 2010). Therefore, the remediation of soil cadmium pollution has attracted wide attention (Kumar 2012). Unlike organic pollutants, microorganisms or chemical methods cannot degrade Cd2+ ions. Thus, it is necessary to develop remediation strategies to remove or neutralize the toxic effects of Cd-contaminated soil (Abdalla et al. 2012; Deng et al. 2014). In situ metal stabilization is a very useful method put into practice in Cd-contaminated fields (Beesley et al. 2013; Marques et al. 2013; Ahmad et al. 2014).
Immobilization reagents, such as poultry manure compost (Chen et al. 2010), lime (Bolan and Duraisamy 2003), organic materials (Mohamed et al. 2010), and natural zeolite (Shi et al. 2009), have been used to reduce Cd2+ concentrations in Cd-contaminated soil. However, it is impossible to achieve effective cadmium immobilization by applying these agents once, and the treatment must be renewed periodically (Antoniadis and Alloway 2001). Thus, microbial remediation is a good approach to immobilize Cd2+ ions.
Many studies have demonstrated that microorganisms have the ability to immobilize heavy metals from contaminated soils. Some microorganisms, such as Bacillus megaterium SR28C, Neorhizobium huautlense T1-17, and Penicillium chrysogenum XJ-1, have been used to decrease metal availability in soils and reduce plant Cd uptake (Rajkumar et al. 2013; Xu et al. 2015; Li et al. 2017; Wang et al. 2016). Inoculation of Lasiodiplodia sp. MXSF31 fungus increased the biomass of rape (Brassica napus L.), reducing rape Cd2+ uptake in Cd2+- and lead (Pb2+)-contaminated soils (Deng et al. 2014). Applying Pseudomonas aeruginosa KUCd1 in Cd-treated soil reduced Cd2+ uptake and stimulated growth in pumpkin plants. Furthermore, Alcaligenes spp., Bacillus spp., Rhodococcus spp., Mycobacterium spp., and some others have demonstrated roles in heavy metal bioremediation (Girma 2015). However, application feasibility of other bacterial species, such as Raoultella sp. strain X13, for in situ heavy metal stabilization is seldom studied.
In this study, we isolated a Cd-resistant bacterium, identified and named as Raoultella sp. strain X13, and characterized the mechanisms of Cd2+ uptake by living and nonliving X13. Furthermore, plant growth-promoting traits of strain X13 were investigated. A pot experiment was used for evaluating the remediation effect of test strain X13 in Cd-contaminated soils at various concentrations of Cd2+.
Materials and methods
Isolation and identification of cadmium-resistant bacteria
Cadmium-resistant bacteria used in this study were isolated from soil collected from Guangzhou Province (23° 7′12.00″N 113°15′0.00″E), Southern China, using slightly modified methods (Yang et al. 2015).The main physico-chemical properties of soils are shown in Table S1 (Supplementary Material). Firstly, 10 g of soil was dissolved in 90 mL sterile deionized water and shaken on a rotatory shaker at 28 °C, 180 rpm for 30 min, then set aside for half an hour. Then using the previously described methods (Yang et al. 2015), we obtained colonies that could grow on LB (10 g L−1 tryptone, 5 g L−1 yeast extract, 10 g L−1 NaCl) agar containing 200 g L−1 Cd2+ at 28 °C.
Molecular identification of purified colonies was determined by 16S rRNA gene sequencing. Gene 16S rRNA was amplified using universal primer 27f (5′-AGAGTTTGATCCTGGCTCAG3′) and 1492r (50ACGGHTACCTTGTTTACGACTT-30) as previously reported (Aksu 2005). Then PCR products were purified using a PCR Cleanup Kit (Sangon Biotech Co. Ltd. Shanghai, China) and sequenced at Sangon Biotech Co. Ltd. (Shanghai, China). Nucleotide sequences were aligned on NCBI GenBank.
Determination of minimum inhibitory concentration (MIC)
Raoultella sp. X13 was cultured (28 °C, 180 rpm) overnight in LB medium and diluted to OD600 = 0.5 as an inoculum, followed by inoculating into fresh LB liquid medium supplemented with different concentrations of CdSO4 (0.5–10 mM), CuSO4·5H2O (0.5–10 mM), ZnSO4 (0.5–12 mM), Pb(NO3)2 (0.5–8 mM), CoCl3 (0.5–8 mM), MnCl2·4H2O (0.5–16 mM), and K2Cr2O7 (0.5–2 mM), respectively. After 3 days of culturing at 28 °C, the metal MIC values were evaluated according to the lowest concentrations at which no single clone grew after being inoculated onto LB agar plates.
Determination of X13 plant growth-promoting (PGP) characteristics
Determination of phosphate solubilization
The ability of the isolate to solubilize phosphate was evaluated by growing the strain in modified Pikovskaya’s medium (Zaidi et al. 2006) (per 1000 mL: 10 g glucose; 10 g Ca3(PO4)2; 0.5 g (NH4)2SO4; 0.3 g NaCl; 0.2 g KCl; 0.3 g MgSO4 7H2O; 0.03 g MnSO4·4H2O; 0.03 g FeSO4·7H2O; pH 7.0 ± 0.02) with 1.0% of tricalcium phosphate (TCP) at 28 °C, 180 rpm. Then 6 mL of culture obtained from bacterial culture was equally divided into three parts. Two parts were used to determine pH and optical density at 600 nm, respectively. And the other was centrifuged at 8000 rpm for 10 min, then the soluble phosphate in the culture supernatant was estimated according to the ammonium molybdate spectrophotometric method. The control was produced using the same operation with no bacterial inoculation. All experiments were conducted in triplicate.
Production of indole acetic acid (IAA)
IAA production was detected using the methods described by Glickmann and Dessaux with slight modifications (Glickmann and Dessaux 1995). The isolate was cultured overnight in LB medium and was diluted to OD600 = 0.5 as inoculum. The inoculation solution was inoculated (v/v = 1:100) in Erlenmeyer flasks containing 100 mL LB medium and then cultured at 28 °C, 180 rpm, in the dark. Two milliliters of culture was removed at different time points (0, 4, 6, 8, 12, 24, 36, and 48 h), then centrifuged (10 min at 8000 rpm) to obtain 1 mL of supernatant, followed by mixing with 1 mL Salkowski’s reagent (37.50 mL H2SO4, 1.88 mL 0.5 M FeCl3·6H2O, and 60.62 mL distilled H2O). The mixture was allowed to stand for 30 min in the dark and OD530 was measured. Non-inoculated LB medium acted as the control and all experiments were conducted in triplicate. The IAA concentration in the culture was determined using an adjusted calibration curve of pure IAA (Glickmann and Dessaux 1995).
Production of siderophores and utilization of ACC
Production of siderophores of strain X13 was measured using the Chrome Azural S (CAS) method in the liquid medium as described by Schwyn and Neilands (1987). The concentration of siderophores in the culture supernatant was evaluated according to previously reported method (Venkat et al. 2017). The ability to use ACC as nitrogen source was also determined. Strain X13 was inoculated into minimal medium containing ACC (3 g L−1) at 28 °C, 180 rpm for 24 h, and then α-ketobutyrate in the culture supernatant was evaluated (OD540) and quantified using a standard curve of α-ketobutyrate (Das et al. 2014).
Preparation of biomass
The X13 strain was grown in LB medium at 28 °C, 180 rpm, for 2–3 days (stationary stage). Living cells were collected by centrifugation (8000 rpm, 15 min) at 4 °C, while dead cells were harvested same way after autoclaving at 121 °C for 20 min (Huang et al. 2013). The pellets were triply washed with distilled deionized water then stored at 4 °C. The biosorbent content were quantified by grams (dry weight) per liter after drying to a constant weight at 80 °C.
Cd2+ uptake by biomass
There are several mechanisms to bind metal ions on bacterial cell adsorbents, which can be quantitatively assessed using the appropriate eluent. For this purpose, ddH2O, 0.1 mol L−1 EDTA-Na, and 1.0 mol L−1 of NH4NO3 were used as eluent (Li et al. 2010; Fang et al. 2011; Bai et al. 2014). One gram per liter biosorbent (live or dead cells) in 50 mL setup solution with a pH of 7 initially containing 1 mM Cd2+ concentration was shaken at 28 °C, 180 rpm, for 2 h. Then cell pellet was collected by centrifugation (6000 rpm, 4 °C, 8 min) and resuspended in 10 mL deionized water for 10 min. After further centrifugation, Cd2+ content was determined; this fraction is adsorbed by physical entrapment which is bound weakly to the cell wall mesh structure (Bai et al. 2014). Water-eluted cell pellets were resuspended in 10 mL of NH4NO3 for 10 min and centrifuged. Cd2+ was determined as the ion-exchangeable fraction (Li et al. 2010; Bai et al. 2014). The same operation was performed with EDTA-Na, and this partially eluted Cd2+ is tightly bound to the functional group. Each eluate was eluted three times and the procedure was repeated in triplicate. Finally, the pellets were digested with concentrated H2SO4 and concentrated HCLO4 at 180 °C for 4 h as previously reported (Xu et al. 2015). The digested fraction was considered to be accumulated inside the cell (Gadd 1990; Xu et al. 2015; Zhu et al. 2016). By filtering using 0.22 mm membrane filters, Cd2+ in the obtained solution was determined by atomic adsorption spectroscopy (AAS, F-240, VARIAN, Palo Alto, CA, USA).
Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM-EDS) analysis
FTIR (Nicolet 5700, Thermo) was used to identify the main chemical functional groups of the X13 strain under Cd2+ untreated and Cd2+ stressed conditions. The dried powder was mixed with a KBr, and was mixed and thoroughly ground in an agate mortar. The translucent tablet was analyzed in the range of 400–4000 cm−1 with 8 cm−1 resolution. The morphological structure and elemental analysis were observed and recorded by scanning electron microscope (SEM, JEOL JSM-6390LV, Tokyo, Japan) in combination with an energy dispersive X-ray spectrometer (EDX, OXFORD INCA, Abingdon, Oxfordshire, UK).
Pot experiment
Soil was collected from the university farm (0–15 cm depth) at Huazhong Agriculture University, Hubei Province, China. The sample was air-dried and sieved, and the main soil parameters were detected according to previously described methods (Bolan and Duraisamy 2003; Chen et al. 2010). Main physico-chemical parameters of soil are shown in Table S2 (Supplementary Material). Pak choi (Brassica chinensis L.) is widely grown in China and was selected as a test plant because it grows quickly and can be harvested in 40 to 65 days. In addition, pak choi responds significantly to bioavailable Cd2+ and is a commonly used plant for assessing the environmental risk of heavy metal-polluted soils (Chen et al. 2002; Chen et al. 2010).
A total of 42 plastic pots, each containing 2 kg air-dried soil, were used for the pot experiments. Soil samples were treated with three levels of CdNO3 (0, 0.5, and 1.5 mg Cd kg−1 soil; marked as Cd 0, Cd 0.5, and Cd 1.5, respectively) and maintained for at least 48 h to balance the water in the soil. The pots for each level of Cd(NO3)2 were equally divided into two groups: one group was inoculated with Raoultella sp. strain X13, the other was not inoculated to act as the control. Raoultella sp. X13 strain used for inoculation was harvested by centrifugation (8000 rpm, 15 min) at 4 °C after growing in LB medium for 2.5 days. The pellet was washed three times with sterile distilled water, re-centrifuged, and re-suspended. Cell density was about 108 CFU g−1 soil dry weight. Control pots were sprayed with an equal volume of sterile distilled water. Cadmium-sensitive Escherichia coli DH5α was also implemented as a negative control at Cd levels of 0 and 0.5 mg kg−1 soil. In addition, the autoclaved soil treatments at Cd levels of 0 and 0.5 mg kg−1 soil inoculated with/without X13 were also carried out. Seven replicates were set up for each treatment.
About 15 pak choi seeds were sown in each pot. During plant germination, soil moisture was kept at approximately 60%. Three plants per pot were maintained by pulling out additional seedlings. Soil moisture content was maintained at 30% during the growing phase. Pak choi was harvested by uprooting 55 days after sowing. Residual soil on the roots was removed using distilled water. Plant yields were examined and plant heights were measured. After drying to constant weight at 70 °C, the dry weight of plants was measured and the shoots and roots were divided. The shoots and roots were ground with a grind bowl for subsequent analysis. The content of total soluble sugar was determined using the anthrone method (John et al. 1950).
Cd analysis
Cadmium content was analyzed by AAS. Digestion using H2SO4-HCLO4 was required before measuring Cd content in the shoots and roots. The soil Cd in each pot was fractionated according to previously reported methods (Sposito et al. 1982). Four types of extracted solution were attained and measured by AAS.
Statistical analysis
All data analyses were performed using an SPSS 19.0 software package. All plots were made using OriginPro 8.0 software. Data were evaluated by ANOVA and Tukey’s test with significance set at p < 0.05.
Accession number
Strain Raoultella sp. X13 has been deposited at the China Center for Type Culture Collection under accession number CCTCC no. M2016662. The 16S rRNA sequence of Raoultella sp. X13 has been deposited at the NCBI GenBank under accession no. MH645797.
Results
Isolation and identification of Cd-resistant bacteria
Through domestication, screening, and re-screening, cadmium-resistant strain X13 was obtained from heavy metal-contaminated soil in Guangdong Province, China. The obtained 16S rRNA X13 gene sequences were aligned with sequences published in NCBI database by BLAST. X13 showed the closest match to Raoultella sp. (99% similarity), and according to morphological (Table S3) and phylogenetic (Fig. 1) analyses, strain X13 is most closely related to Raoultella sp.
Heavy metal MIC
The heavy metal MICs against Raoultella sp. X13 are shown in Table 1. The results show that Raoultella sp. X13 possesses a high tolerance to a variety of heavy metals. The X13 strain MIC of Cd2+, Cu2+, Mn2+, Zn2+, Pb2+, Co2+, and Cr6+ was 8, 6, 14, 12, 6, 6, and 1 mM, respectively. The toxicity of the heavy metals to strain X13 is as follows: Cr6+ > Co2+, Pb2+, Cu2+ > Cd2+ > Zn2+ > Mn2+.
PGP potential of strain X13
In natural environments, bacteria with PGP characteristics play an important role in plant growth and increasing crop production. To identify potential plant growth promotion (PGP) of X13, phosphorus solubilization ability, IAA, siderophore production, and utilization of ACC were determined. From the Fig. 2a, we found that the X13 strain was able to solubilize phosphate, the amount of soluble phosphate was determined to be 84.67 μg mL−1 at 48 h, and the amount of soluble phosphate released in liquid medium had positive correlation with the strain biomass. Furthermore, the phosphate content exhibited inverse relationship with the pH of the liquid medium (Fig. S1), which illustrated that the pH of the medium and the phosphate solubilization ability were closely related. Figure 2b demonstrates the production of IAA during growing in LB medium. The trend of IAA content in the culture medium is similar to bacteria growth curve during the 48-h incubation process, and IAA content reached the maximum, up to 8.66 μg mL−1 measured at 36 h. The results showed that X13 could slightly produce siderophore (the value was 12.64 ± 1.80% at 48 h), but did not produce ACC-deaminase activity.
Cd localization in cells after Cd2+ uptake
Table 2 showed Cd distribution in different cell sites and the Cd2+ biosorption ability of living and nonliving biomass treated with 1 mM Cd2+. For the live biomass, Cd2+ bound by ion exchange was 34.28 ± 0.47 mg g−1 dry weight (72.89%), and Cd2+ bound by physical entrapment, functional groups, and intracellular absorption were 4.31 ± 0.01 mg g−1 dry weight (9.17%), 6.26 ± 0.01 mg g−1 dry weight (13.32%), and 2.17 ± 0.14 mg g−1 dry weight (4.62%), respectively. Table 2 also showed that Cd2+ bound in nonliving cells by physical entrapment, ion exchange, functional groups, and intracellular absorption were 5.13 ± 0.30 mg g−1 dry weight (27.04%), 11.86 ± 0.31 mg g−1 dry weight (62.49%), 1.88 ± 0.07 mg g−1 dry weight (9.90%), and 0.11 ± 0.03 mg g−1 dry weight (0.57%), respectively.
FTIR and SEM-EDS analysis
The visual morphologies of both biosorbents before and after Cd2+ adsorption were observed by SEM. Live cell surfaces appeared extremely rough, while dead cells seemed to be smoother because of the autoclave treatment (Fig. 3a, c). An alteration in bacterial morphology after Cd2+ adsorption was observed (Fig. 3b, d). After Cd2+ adsorption, live cell morphologies could be observed a certain degree of change but no obvious cell breakage. We only found some cells had depressions on the surface. These results suggested that 1 mM Cd2+ did not cause severe damage to living cells. As shown in Fig. 3d, dead cells appeared to be plump and smooth after the biosorption process. These results indicate that changes in cell morphology might be due to Cd2+ fixation to the cells after Cd2+ adsorption; this theory is supported by the EDS spectra. The characteristic peak of Cd2+ was detected after Cd2+ adsorption, compared to the biosorbents detected before Cd2+ adsorption.
The FTIR analysis was implemented to understand the functional groups interacting with Cd2+. Characteristic peaks were identified according to previous reports (Yee et al. 2004; Mukhopadhyay 2008; Ozdemir et al. 2009; Xu et al. 2012; Elsayed 2015; Xia et al. 2015; Khraisheh et al. 2016) and listed in Table S4. Our results showed that the similar functional groups were present in the living and dead cells, but their transmission peaks were significantly different. After Cd2+ treatment, the absorption bands changed for both live and dead biomass. For living biomass, peaks around 1073.52 cm−1 were significantly reduced to 1069.80 cm−1, suggesting the involvement of amide (C-N) groups in Cd2+ biosorption. Moreover, the peaks at 3315.05, 2960.77, and 2340.99 cm−1 were slightly reduced, involving -OH, -NH, and -CH2, and protonated amino (NH2+ and NH+) functional groups, while 1242.86 cm−1 (-SO3) and 668.76 cm−1 (phosphate or sulfate functional groups) were slightly increased. Those peaks indicated that groups such as -OH and -NH, -CH2 and protonated amino (-NH2+, -NH+) functional groups, and -SO3 groups and fingerprint zone groups (phosphate or sulfate functional groups) might also participate in the Cd2+ biosorption process for living biosorbent. For nonliving biomass, the peaks of biosorbent loaded with Cd2+ at 3298.79, 2960.98, 2929.15, 1658.10, 1529.99, 1234.38, 1070.01, and 2669.14 cm−1 presented a slight shift in comparison with the dead raw biosorbent. The shifts in the detected peaks showed that -OH or -NH groups, -CH3 groups, C=O and C–N groups, -SO3 groups, and phosphate or sulfate functional groups might participate in Cd2+ biosorption.
Effects of strain X13 on biomass and Cd2+ accumulation in pak choi
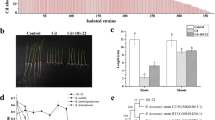
We determined the yield and sugar content of pak choi under experimental conditions (Fig. 5). In Cd-amended soil conditions, the plant yield of pak choi, fresh weight, and dry weight were obviously (p < 0.05) inhibited (Fig. 5a, b). Plant biomass decreased considerably in the presence of Cd (0.5 and 1.5 mg kg−1)-contaminated soil, with a 15.13 and 24.5% reduction in wet weight along with a 4.51 and 8.32% reduction in dry weight, respectively (Fig. 5a, b). Application of microbial agent X13 to the soil significantly increased the pak choi biomass (Fig. 5a, b). Plant fresh weight was increased by 20.96% (Cd 0), 14.36% (Cd 0.5), and 19.38% (Cd 1.5), and the dry weight was increased by 5.93% (Cd 0), 3.29% (Cd 0.5), and 3.87% (Cd 1.5), respectively. Soil Cd pollution can significantly affect the sugar content of pak choi. In the presence of Cd2+ (Cd 0.5 and Cd 1.5), the soluble sugar content of pak choi considerably decreased by 22.2 and 32.9% (Fig. 5c), respectively. Application of strain X13 increased soluble sugar accumulation by 14.2% (Cd 0), 9.9% (Cd 0.5), and 16.9% (Cd 1.5), respectively.
Cd concentration in pak choi
The presence of cadmium or inoculant X13 affects the enrichment of cadmium ions in roots and shoots (Fig. 6a, b). For soils contaminated with 0.5 and 1.5 mg kg−1 Cd level treatments, the Cd concentration in pak choi shoots under X13 treatment was decreased by 54.6 and 44.4%, respectively, and in plant roots by 13.9 and 39.6%, respectively.
Distribution of Cd in soil
The percentage of different cadmium fractions under different treatment conditions is shown in Fig. 6c and Table S4 (Supplementary Material). Inoculant X13 redistributed cadmium among fractions at different Cd levels. When the concentration of Cd ions increased from Cd 0 to Cd 1.5, the soluble/exchangeable Cd increased from 14.5 to 42.5%. Compared to the non-inoculated group in all Cd treatments, inoculation agent X13 reduced soluble/exchangeable Cd by 8–36.4% and enhanced inorganic-bound Cd by 8.2–25.0%.
Discussion
Soil cadmium contamination has resulted in severe crop yield reduction (Zhang et al. 2015). In situ metal stabilization by microorganism application is a useful method that is put into practice in Cd-contaminated fields (Xu et al. 2015; Deng et al. 2014; Girma 2015). However, application of novel cadmium-resistant bacterium, Raoultella sp. strain X13, for in situ heavy metal stabilization is seldom studied.
In this study, Cd-resistant bacterial Raoultella sp. strain X13 was obtained from heavy metal-contaminated soil. X13 possesses innate PGP traits (Fig. 2), has exhibited resistance up to 8 mM Cd in LB medium, and has demonstrated Cd2+ biosorption and bioaccumulation abilities. The biosorption capacity of live and dead cells was 50.30 and 25.13 mg g−1 dry weight biomass, respectively, at the initial cadmium concentration (1 mM) (Table 2). Therefore, strain X13 can be used as a potential immobilizing agent for Cd2+ in polluted soil. It can promote both plant growth and Cd2+ fixation. Previous reports have demonstrated that metal-resistant PGP bacteria can promote plant growth, enhance heavy metal stabilization, and increase plant tolerance to heavy metals in the case of Cd2+ contamination (Das et al. 2014).
Results from cellular Cd localization after Cd2+ uptake indicated Cd2+ removal was principally precipitated by ion exchange and functional groups binding tightly to cell wall fractions for both biosorbents (Table 2). A similar result was reported by Fang et al. (2011). The data from FITR showed that functional groups (-OH or -NH groups, -CH3 groups, C=O and C-N groups, -SO3 groups, and phosphate or sulfate functional groups) might participate in Cd2+ biosorption (Fig. 4). In addition, intracellular absorption of Cd2+ is significantly different between living and nonliving biomass. The intracellular-bound Cd in living cells was 4.62%, far greater than that for the nonliving biomass, which indicated living biomass could possibly transport metal ions into cells through metabolic activity.
In order to prove that strain X13 can enhance crop yield and effectively neutralize toxic Cd2+ effects in Cd-contaminated soil, we conducted a pot experiment. Results showed that X13 application to Cd-contaminated soils significantly promoted pak choi growth and improved production and quality (Fig. 5). The similar result was also found in autoclaved soil treatment (Fig. S2). However, inoculation of cadmium-sensitive strain E. coli DH5α to Cd-contaminated soils did not promote pak choi growth (Fig. S3). These results suggested that the strain X13 with PGP characteristics may play an important role in plant growth. Cd concentration in pak choi demonstrated that inoculation of microbial agent X13 to Cd-contaminated soil significantly depressed Cd2+ uptake by pak choi (Fig. 5). Cd distribution in soil implied that the X13 inoculation effectively transferred bioavailable Cd2+ to another steady state (mainly inorganic-bound fraction) that are difficult to uptake by plants. This result differs from previous reports. Xu et al. reported that the inoculation of Penicillium chrysogenum XJ-1 mainly converted the bioavailable Cd2+ to an organic-bound fraction in Cd-contaminated soils (Xu et al. 2015). Although our results suggested that ion exchange, functional groups, and intracellular absorption might participate in Cd2+ biosorption, these did not play a major role in in situ heavy metal stabilization in our pot experiment. The data showed X13 inoculation mainly transferred the bioavailable Cd to the inorganic-bound fraction in Cd-contaminated soils (Fig. 6c and Table S5). This may be mainly due to the ability of strain X13 to biosynthesize CdS (Fig. S4). Microorganism-mediated biosynthesis of metal chalcogenides served as a powerful tool to transform toxic effluents into functional nanomaterials and less harmful compounds (Vena et al. 2016). However, this study did not involve related research. Ongoing work is focusing on biosynthesis of CdS by strain X13.
In summary, the Cd-resistant Raoultella sp. strain X13 isolated from heavy metal-contaminated soil can adsorb Cd mainly by ion exchange and chelation binding tightly to cell walls and possessed innate PGP traits that enable the production of IAA and siderophores, and the ability to solubilize phosphates. Our results also found that X13 could reduce Cd bioavailability and promote pak choi growth in Cd-stressed soils. Moreover, bioavailable Cd was mainly transferred to the inorganic-bound fraction by inoculating Cd-contaminated soils with strain X13. Therefore, our findings indicated potential applications of strain X13 for Cd immobilization as well as sustainable agronomic production programs in Cd-contaminated soils.
References
Abdalla MH, Morsy FM, Elenany AWE, Ohyama T (2012) Isolation and characterization of a heavy-metal-resistant isolate of Rhizobium leguminosarum bv. viciae potentially applicable for biosorption of Cd2+ and Co2+. Int Biodeterior Biodegrad 67(2):48–55
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99(3): 19-33
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40(3):997–1026
Antoniadis V, Alloway BJ (2001) Availability of Cd, Ni and Zn to Ryegrass in Sewage Sludge-Treated Soils at Different Temperatures. Water Air Soil Pollut 132(3-4):201–214
Bai J, Yang XH, Du RY, Chen YM, Wang SZ, Qiu RL (2014) Biosorption mechanisms involved in immobilization of soil Pb by Bacillus subtilis DBM in a multi-metal-contaminated soil. J Environ Sci 26(10):2056–2064
Beesley L, Marmiroli M, Pagano L, Pigoni V, Fellet G, Fresno T, Vamerali T, Bandiera M, Marmiroli N (2013) Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci Total Environ 454-455(3):598–603
Bolan NS, Duraisamy VP (2003) Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: a review involving specific case studies. Soil Res 41(3):533–555
Chakravarty P, Sarma NS, Sarma HP (2010) Biosorption of cadmium(II) from aqueous solution using heartwood powder of Areca catechu. Chem Eng J 162(3):949–955
Chen HS, Huang QY, Liu LN, Cai P, Liang W, Ming LI (2010) Poultry manure compost alleviates the phytotoxicity of soil cadmium: influence on growth of pakchoi (Brassica chinensis L.). Pedosphere 20(1):63–70
Chen YX, Zhu GW, Tian GM, Zhou GD, Luo YM, Wu SC (2002) Phytotoxicity of dredged sediment from urban canal as land application. Environ Pollut 117(2):233–241
Das S, Jean JS, Kar S, Chou ML, Chen CY (2014) Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from agricultural soil and their potential implication for arsenic bioremediation. J Hazard Mater 272(10):112–120
Deng Z, Zhang R, Shi Y, Hu L, Tan H, Cao L (2014) Characterization of Cd-, Pb-, Zn-resistant endophytic Lasiodiplodia sp. MXSF31 from metal accumulating Portulaca oleracea and its potential in promoting the growth of rape in metal-contaminated soils. Environ Sci Pollut Res Int 21(3):2346–2357
Elsayed MT (2015) An investigation on tolerance and biosorption potential of Aspergillus awamori ZU JQ 965830.1 TO Cd(II). Ann Microbiol 65(1):69–83
Fang L, Chen Z, Peng C, Chen W, Rong X, Ke D, Wei L, Gu JD, Huang Q (2011) Binding characteristics of copper and cadmium by cyanobacterium Spirulina platensis. J Hazard Mater 190(1-3):810–815
Fellet G, Marmiroli M, Marchiol L (2014) Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci Total Environ 468-469:598–608
Gadd GM (1990) Heavy metal accumulation by bacteria and other microorganisms. Cell Mol Life Sci 46(8):834–840
Girma G (2015) Microbial bioremediation of some heavy metals in soils: an updated review. Ind J Sci Res 6:147–161
Glickmann E, Dessaux YA (1995) Critical Examination of the Specificity of the Salkowski Reagent for Indolic Compounds Produced by Phytopathogenic Bacteria. Appl Environ Microbiol 61(1):793–796
Huang F, Dang Z, Guo CL, Lu GN, Gu RR, Liu HJ, Zhang H (2013) Biosorption of Cd(II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloids Surf B: Biointerfaces 107:11–18
John A, Barnett G, Miller TB (1950) The determination of soluble carbohydrate in dried samples of grass silage by the anthrone method. J Sci Food Agric 1(11):336–339
Khraisheh MAM, Al-Degs YS, Allen SJ, Ahmed MN (2016) Elucidation of controlling steps of reactive dye adsorption on activated carbon. Ind Eng Chem Res 41(6):1651–1657
Kumar A (2012) Role of plant-growth-promoting rhizobacteria in the management of cadmium-contaminated soil. Springer Vienna pp: 163-178
Li Y, Pang HD, He LY, Wang Q, Sheng XF (2017) Cd immobilization and reduced tissue Cd accumulation of rice (Oryza sativa wuyun-23) in the presence of heavy metal-resistant bacteria. Ecotoxicol Environ Saf 138:56–63
Li Y, Yue Q, Gao B (2010) Adsorption kinetics and desorption of Cu(II) and Zn(II) from aqueous solution onto humic acid. J Hazard Mater 178(1):455–461
Marques AP, Moreira H, Franco AR, Rangel AO, Castro PM (2013) Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria-effects on phytoremediation strategies. Chemosphere 92(1):74–83
Mohamed I, Ahamadou B, Li M, Gong CX, Cai P, Liang W, Huang QY (2010) Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soils Sediments 10(6):973–982
Mukhopadhyay M (2008) Role of surface properties during biosorption of copper by pretreated Aspergillus niger biomass. Colloids Surf A: Physicochem Eng Aspects 329(1-2):95–99
Nogawa K, Kido T (1996) Itai-Itai disease and health effects of cadmium. In: Toxicology of Metals. Ed. L W Chang. CRC Lewis Publishers, NY, pp 353–370
Ok YS, Usman AR, Lee SS, Abd ElAzeem SA, Choi B, Hashimoto Y, Yang JE (2011) Effects of rapeseed residue on lead and cadmium availability and uptake by rice plants in heavy metal contaminated paddy soil. Chemosphere 85(4):677–682
Ozdemir S, Kilinc E, Poli A, Nicolaus B, Guven K (2009) Biosorption of Cd, Cu, Ni, Mn and Zn from aqueous solutions by thermophilic bacteria, Geobacillus toebii sub.sp. decanicus and Geobacillus thermoleovorans sub.sp. stromboliensis: equilibrium, kinetic and thermodynamic studies. Chem Eng J 152(1):195–206
Rajkumar M, Ma Y, Freitas H (2013) Improvement of Ni phytostabilization by inoculation of Ni resistant Bacillus megaterium SR28C. J Environ Manag 128:973–980
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Sharma YC (1995) Economic treatment of Cadmium(II)-Rich hazardous waste by indigenous material. J Colloid Interface Sci 173(1):66–70
Shi WY, Shao HB, Li H, Shao MA, Du S (2009) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170(1):1–6
Sposito G, Lund LJ, Chang AC (1982) Trace metal chemistry in arid-zone field soils amended with sewage sludge: I. Fractionation of Ni, Cu, Zn, Cd and Pb in solid phases. Soil Sci Soc Am J 46(2):260
Vena MP, Jobbágy M, Bilmes SA (2016) Microorganism mediated biosynthesis of metal chalcogenides; a powerful tool to transform toxic effluents into functional nanomaterials. Sci Total Environ 565:804–810
Venkat KS, Menon S, Agarwal H, Gopalakrishnan D (2017) Characterization and optimization of bacterium isolated from soil samples for the production of siderophores. Resour Eff Technol 3(4)
Wang Q, Chen L, He LY, Sheng XF (2016) Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1-17 and biochar. Agric Ecosyst Environ 228:9–18
Waqas M, Khan S, Huang Q, Reid BJ, Cai C (2014) The effects of sewage sludge and sewage sludge biochar on PAHs and potentially toxic element bioaccumulation in Cucumis sativa L. Chemosphere 105(3):53–61
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107
Xia L, Xu XJ, Zhu W, Huang QY, Chen WL (2015) A comparative study on the biosorption of Cd2+ onto Paecilomyces lilacinus XLA and Mucoromycote sp. XLC. Int J Mol Sci 16(7):15670–15687
Xu X, Xia L, Huang Q, Gu J, Chen W (2012) Biosorption of cadmium by a metal-resistant filamentous fungus isolated from chicken manure compost. Environ Technol 33(13-15):1661–1670
Xu X, Xia L, Zhu W, Zhang Z, Huang Q, Chen W (2015) Role of Penicillium chrysogenum XJ-1 in the detoxification and bioremediation of cadmium. Front Microbiol 6:1422
Yang Z, Lu L, Berard VF, He Q, Kiely C, Berger BW, Mcintosh S (2015) Biomanufacturing of CdS quantum dots. Green Chem 17:3775–3782
Yee N, Benning LG, Phoenix VR, Ferris FG (2004) Characterization of metal-cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environ Sci Technol 38(3):775–782
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64(6):991–997
Zhang X, Zhong T, Liu L, Ouyang X (2015) Impact of soil heavy metal pollution on food safety in China. PLoS One 10(8):e0135182
Zhu W, Xu X, Xia L, Huang Q, Chen W (2016) Comparative analysis of mechanisms of Cd2+ and Ni2+ biosorption by living and nonliving Mucoromycote sp. XLC. Geomicrobiol J 33(3-4):274–282
Funding
This study was funded by The National Key Research and Development Program of China (2017YFA0605001 and 2016YFD0800206), and the Technical Innovation Major Projects of Hubei Province (2018ABA092).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 362 kb)
Rights and permissions
About this article
Cite this article
Xu, S., Xing, Y., Liu, S. et al. Role of novel bacterial Raoultella sp. strain X13 in plant growth promotion and cadmium bioremediation in soil. Appl Microbiol Biotechnol 103, 3887–3897 (2019). https://doi.org/10.1007/s00253-019-09700-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09700-7