Abstract
In the present study, the gene encoding a multicopper oxidase, more precisely a laccase from the thermoalkaliphilic aerobic bacterium Caldalkalibacillus thermarum strain TA2.A1 (CtLac), was cloned and expressed in Escherichia coli. CtLac is a monomeric protein with a molecular weight of 57 kDa as determined by native polyacrylamide gel electrophoresis. The optimum pH and temperature for 2,6-dimethoxyphenol (2,6-DMP) oxidation were 8.0 and 70 °C, respectively. The kinetic constants Km and kcat for 2,6-DMP were of 200 μM and 23 s−1, respectively. The enzyme was highly thermostable at 80 °C and retained more than 80% of its activity after 24 h preincubation under thermoalkaliphilic conditions. Remarkably, it showed a half-life of about 12 h at 90 °C. The enzyme activity was significantly enhanced by Cu2+ and Mn2+ and was not affected in the presence of most of the other metal ions. CtLac activity was stimulated in the presence of halides, organic solvents, and surfactants. Furthermore, the activity of CtLac on a dimeric lignin model compound, guaiacylglycerol-β-guaiacyl ether (GGGE) was investigated. Liquid chromatography-mass spectrometry analysis indicated that CtLac catalyzes dimerization of GGGE to form a C5-C5 biphenyl tetramer. The stability and activity of CtLac characterized herein under thermoalkaliphilic conditions make it a highly suitable biocatalyst for various biotechnological and industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignin is the second largest organic polymer on the earth, composed of a highly heterogeneous and complex polymeric structure. The oxidative coupling of p-coumaryl, coniferyl, and sinapyl alcohols with β-O-4 aryl ether, β–β, β–5, 5–5, and 5-O-4 linkages lies behind the complexity and recalcitrance of the lignin structure (Boerjan et al. 2003). Various lignolytic microorganisms such as white-rot fungi and bacteria have developed a rich collection of extracellular oxidative enzymes such as lignin peroxidases (LiP), manganese peroxidases (MnP), versatile peroxidases (VP), and dye-decolorizing peroxidases (DyPs) (Gonzalo et al. 2016; Tuor et al. 1995).
Laccases are “green” catalysts characterized by four redox-active copper ions organized into three spectrally distinct sites, namely T1, T2, and T3 (Messerschmidt and Huber 1990; Martins et al. 2002). They are able to perform single-electron oxidation of various phenolic compounds to the corresponding radical species which can undergo further hydration, oxidation, polymerization, or depolymerization reactions (Riva 2006; Munk et al. 2015). Thus laccases are widely used as a biocatalyst in diverse industrial applications such as bleaching of pulp, stabilization of beverages, bioremediation of natural and synthetic dyes, oxidation of phenol-containing biopolymers, i.e., delignification (Telke et al. 2011; Kudanga and Le Roes-Hill 2014; Mate and Alcalde 2016). Most investigated laccases are from fungal origin and are often active at acidic pH and low ionic strengths, but lack thermostability (Baldrian 2006; Freixo et al. 2008; Torres-Salas et al. 2013). Industrial processes are often best employed at extreme pH, temperature, or ionic strength. For the industrial and biotechnological larger-scale applications, there is a need for novel and more efficient laccases with improved biochemical properties, such as alkaline pH optima, and thermostability (Couto and Herrera 2006; Santhanam et al. 2011). A general tendency of the bacterial laccases in comparison to fungal laccases is stability and optimum activity at alkaline pH and high temperature (Claus 2003; Brander et al. 2014). Hence, it is of great importance to study laccases originated from the thermoalkaliphilic environment. Laccases originated from genus Bacillus such as from Bacillus subtilis subsp. subtilis str. 168 (Martins et al. 2002), Bacillus licheniformis (Koschorreck et al. 2008), Bacillus pumilus (Reiss et al. 2011), Bacillus clausii KSM-K16 (Brander et al. 2014), and Bacillus tequilensis (Sondhi et al. 2014) have been characterized for their activity on various synthetic substrates such as 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS), syringaldazine (SGZ), 2,6-dimethoxyphenol (2,6-DMP), etc. Recently, laccase from B. licheniformis was investigated for its dimerization activity on various phenolic and non-phenolic acids (Koschorreck et al. 2008). However, none of the above enzymes have been investigated for their activity on a dimeric lignin model compound such as guaiacylglycerol-β-guaiacyl ether (GGGE), composed of β-O-4 aryl ether linkage which accounts for approximately 50% of the linkages present in the native lignin structure (Masai et al. 2007; Munk et al. 2015).
Pathways for the degradation of β-aryl ether lignin model compounds have been investigated in details in Sphingobium sp. SYK-6 which involve dehydrogenase-mediated oxidation to a benzylic ketone followed by β-etherase-mediated reductive C-O cleavage in the presence of glutathione as a cofactor (Masai et al. 2007). It appears that laccase and extracellular peroxidase have been involved in the breakdown of lignin by various aromatic degrading bacteria such as Pseudomonas putida mt-2 and Rhodococcus jostii RHA1 (Bugg et al. 2011). Laccase action on lignin polymer and lignin model compounds results in activation of lignin structure by abstraction of a single electron from a phenylpropanoid subunit and a possible series of events thereafter such as bond cleavage, modification, and/or coupling results in either polymerization or depolymerization of lignin. In addition, laccase can only oxidize phenolic lignin model compounds while non-phenolic model compounds need mediators either synthetic or natural, which can act as an electron carrier between enzyme and substrate to be oxidized (Munk et al. 2015). Thus, analysis of oxidized products generated by the laccase action on a dimeric lignin model compound will help in understanding the role of laccase in enzyme-based lignin polymerization and depolymerization processes.
Caldalkalibacillus thermarum strain TA2.A1 is a thermoalkaliphilic aerobic bacterium isolated from the alkaline thermal bore at Mount Te Aroha, New Zealand (Kalamorz et al. 2011). Its genome contains several genes encoding laccases and other multicopper oxidases. In the present study, a gene (GenBank accession no.MG591700.1) encoding one of the laccase (CtLac) was synthesized, cloned, and expressed in Escherichia coli. Furthermore, the biochemical properties of the purified enzyme were investigated. In addition, the reaction mechanism and dimerization activity of CtLac on a dimeric lignin model compound (GGGE) were explored using high-performance liquid chromatography-mass spectrometry (HPLC-MS). The results obtained in this study suggest that CtLac appears to be appropriate for various industrial and biorefinery applications and prefers oxidative polymerization over depolymerization reactions when tested with a dimeric lignin model compound.
Materials and methods
Chemicals, bacterial strains, and plasmid
ABTS, SGZ, 2,6-DMP, catechol, phloroglucinol, ferulic acid, and caffeic acid were obtained from Sigma-Aldrich products (St. Louis, MO). GGGE was purchased from TCI (Tokyo, Japan). All the other chemicals were of standard reagent grade and high purity (Sigma Aldrich, St. Louis, MO). E. coli DH5α and E. coli BL21 (DE3) (Invitrogen, Carlsbad, CA) were used for gene cloning and protein expression, respectively. The protein expression vector pET28a (+) was purchased from Novagen (Madison, WI).
Cloning of the laccase gene and construction of pECtLac expression vector
The gene (GenBank accession no. MG591700.1) encoding CtLac (AVD69558.1) from C. thermarum TA2.A1 was E. coli codon-optimized and synthesized by Bioneer Co., Ltd. in the pBHA vector which was used as a template during PCR amplification. PCR was performed by using AccuPower Pfu PCR PreMix (Bioneer, South Korea) and a pair of primer as Lac-F 5′ACGTACATATGAAACGGATTTTAACACTAGTTCTTC3′ and Lac-R 5′GATACCTCGAGTTATTCAGGTTTGTTCGGGATG3′. Restriction sites of NdeI and XhoI are underlined and the stop codon is indicated in bold letters. The 1551 bp PCR product was inserted into NdeI and XhoI sites of pET28a (+). The recombinant plasmid was named as pECtLac. Enzymes used in the gene cloning procedure were obtained from New England Biolabs (Beverly, MA). The PCR cloning primers, plasmid preparation, and gel extraction kits were purchased from Bioneer (Daejeon, South Korea). The results of cloning were confirmed by sequence analysis performed by Macrogen Inc. (Seoul, South Korea).
Expression and purification of CtLac
E. coli BL21(DE3) harboring pECtLac was grown overnight in LB broth supplemented with 50 μg/ml kanamycin at 37 °C under shaking condition (200 rpm). One percent of the overnight culture was used as an inoculum in LB medium and the culture was grown to OD 0.6 at 180 rpm at 30 °C followed by induction with 100 μM of isopropyl β-d-thiogalactopyranoside (IPTG) and 250 μM of CuCl2. Incubation was extended at 120 rpm and 20 °C for 4 h followed by 16 h of static incubation at 20 °C. The cells were harvested by centrifugation at 10,000×g for 20 min, and lysis was carried out using BugBuster protein extraction reagent (Novagen, Billerica, MA) following manufacturer’s instructions. The lysate was cleared by centrifugation or filtration through 0.20 μm filter and applied on a 5 ml His-Trap HP column (GE Healthcare, Piscataway, NJ) and eluted with a linear gradient (100 ml) of 0 to 0.25 M imidazole buffered with 20 mM Tris-HCl (pH 8.0). Fractions containing the laccase were collected and dialyzed against 1× PBS (phosphate-buffered saline) (pH 7.4). The proteins were dialyzed against 20 mM Tris-HCl (pH 7.9) before being applied to a Superdex G 200 10/300 column (GE Healthcare, Piscataway, NJ). The proteins were eluted with 20 mM Tris-HCl (pH 8.0) and dialyzed twice with 20 mM Tris-HCl (pH 8.0) containing CuCl2 to give four copper atoms per active site of an enzyme and then stored at.
− 80 °C.
Determination of protein concentration and enzyme activities
The protein concentration was determined by Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) with purified bovine serum albumin (BSA) as a standard. Laccase activity was measured using ABTS, SGZ, and 2,6-DMP as substrates. One unit of enzyme activity was defined as the amount of enzyme required to oxidize 1 μmol of the substrate per minute. Oxidations of ABTS (200 μM), SGZ (50 μM), and 2,6-DMP (0.5 mM) were monitored at 420 nm (ε = 36,000 M−1 cm−1), 525 nm (ε = 65,000 M−1 cm−1), and 468 nm (ε = 49,600 M−1 cm−1), respectively (Kalamorz et al. 2011). In addition, the substrate specificity of CtLac was investigated spectrophotometrically by testing the ability to oxidize various lignin-derived phenolics such as catechol, ferulic acid, caffeic acid, and phloroglucinol. All assays were performed in triplicate.
Biochemical characterization of CtLac
The molecular weight of the purified CtLac was determined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 12% w/v polyacrylamide) and zymogram analysis on both SDS-PAGE and native-PAGE. The enzyme band was visualized by staining the gel in 50 mM citrate phosphate buffer (pH 8.0) containing 0.5 mM of 2,6-DMP as a substrate at 37 °C.
The effects of pH on CtLac activity were tested at pH 3–8 in 50 mM citrate-phosphate or 50 mM Tris-HCl (pH 8–9) buffer using 2,6-DMP and SGZ as a substrate. The laccase activity toward 2,6-DMP as a substrate was examined at 20–90 °C at the optimal pH to determine its optimum temperature. The thermostability of the laccase was investigated at 60–90 °C by preincubating the enzyme solution in 50 mM citrate/phosphate buffer, containing 1 mM CuCl2 at pH 8.0 and 9.0, and residual activity was determined using the 2,6-DMP assay. At high pH values, slight auto-oxidation was observed in enzyme-free controls for 2,6-DMP. These auto-oxidation values were subtracted from the experiments with CtLac before calculating the actual relative activities as described previously (Ihssen et al. 2015). Kinetic parameters of the CtLac were determined using different concentrations of 2,6-DMP (50–2000 μM). The data was analyzed using SigmaPlot (Systat Software Inc., Erkrath, Germany) with Michaelis–Menten equation by non-linear regression.
The effects of halides (0–1 M NaCl or KCl), metal ions, commonly used water missile organic solvents % (v/v), various surfactants (0–10 mM), and inhibitors on the activity of CtLac were determined in 1 ml of assay mixture containing an appropriate amount of enzyme, citrate/phosphate buffer, at pH 8.0 with 1 mM CuCl2 and 0.5 mM of 2,6-DMP as a substrate. The activity without supplementation of any additives was defined as 100% and the relative activity was then analyzed as per the standard assay conditions. All the experiments were carried out in triplicates and data was expressed as a mean ± standard deviation.
CtLac action on GGGE
GGGE is a dimeric lignin model compound considered as a close resemblance of native lignin and diversely used for studying the activity of various lignolytic enzymes (Gonzalo et al. 2016; Ramalingam et al. 2017). CtLac (50 μg/ml) was incubated with 0.5 mM of GGGE (0.1 M stock solution in dimethylformamide) in citrate phosphate buffer (pH 8.0) containing 1 mM of CuCl2 at 70 °C in a rotary shaker (150 rpm) for 12 h. Reaction mixtures were centrifuged and supernatant was extracted with three volumes of ethyl acetate. Ethyl acetate extract was evaporated in vacuo and the residue was dissolved in an appropriate volume of methanol and filtered with a polyvinylidene fluoride (PVDF) syringe filter. The oxidized products produced from GGGE after CtLac activity were analyzed using HPLC-MS. The control tube without CtLac addition was also run simultaneously and processed in the same manner.
HPLC and LC-MS/MS analysis of oxidized products derived from GGGE
Analytical HPLC was performed using an Agilent HPLC 1260 series equipped with a photodiode array detector (PDA) and a reverse-phase C18 column (Zorbax Eclipse Plus C18, 3.5 μm in particle size, 4.6 mm × 100 mm) (Agilent, Santa Clara, CA, USA). The mobile phase consisted of 10 mM ammonium formate in water (A) and 10 mM ammonium formate in 80% acetonitrile (B). Ammonium formate was used as a mobile phase additive to enhance the ionization of negative ion mode. The separation gradient was made by changing the composition of buffer B as follows: 0–55% B for 0–30 min, 55–100% B for 30–35 min, 100% B for 35–50 min, with subsequent equilibrium at 0% B for 20 min. The flow rate was 0.5 ml/min with 10 μl injection volume and the products derived from GGGE were monitored at 280 and 310 nm. To identify the oxidized reaction products of GGGE, the mass spectrometric analysis was performed using an Agilent 6520 quadrupole time-of-flight (Q-TOF) mass spectrometer (Agilent, Santa Clara, CA) equipped with electrospray ionization (ESI) source. The elution program followed the same method as employed for analytical HPLC. MS source parameters were as follows: fragmentor voltage 100 V, skimmer voltage 65 V, spray voltage 4000 V, drying gas (N2) flow 10 L/min, drying gas temperature 350 °C, nebulizer gas (N2) flow 35 psig, and source temperature 350 °C. The collision energy was at 20 and 40 V for collision-induced dissociation (CID) and the acquisition rate for both MS and MS/MS scans was 2 spectra per min. The negative ionization mode was applied and the resulting deprotonated ions ([M–H]−) were analyzed over m/z range of 120–1500 for MS and 30–1500 for MS/MS. The top 5 ions from the MS survey scan were subjected to MS/MS analysis. The absolute threshold for the precursor ion selection was 200 counts and the relative threshold was 0.01% of the base peak.
Results
Putative laccase from C. thermarum strain TA2.A1
Searching for a novel thermoalkaliphilic laccase from a wide variety of thermoalkaliphilic bacteria, the gene encoding a protein WP_007502678.1 was identified as a two-domain multicopper oxidase from the genome of C. thermarum TA2.A1 (Kalamorz et al. 2011). The protein is composed of 516 amino acids and the regions corresponding to typical tri-nuclear Cu binding sites found in laccases. BlastP analysis showed that CtLac has the highest amino acid sequence identity of 64% and similarity of 76% to a multicopper oxidase from Bacillus halodurans (WP_010898240.1). Furthermore, multiple sequence alignment of the CtLac with other enzymes was performed using Clustal-omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) (Supplementary Fig. S1). It showed 27% of amino acid identity to Cyathus bulleri laccase (ABW75771.2) while it had only ~ 20% identity to laccases from B. licheniformis (YP_077905.1) (Koschorreck et al. 2008), B. subtilis subsp. subtilis str. 168 (NP_388511.1) (Martins et al. 2002), B. pumilus (ZP_03054403.1) (Reiss et al. 2011), and B. clausii KSM-K16 (BAD65184.1) (Brander et al. 2014), and copper oxidase from Bacillus coagulans 36D1 (WP_014097300.1). However, the amino acids involved in the copper binding sites of type 1 (M492, H429, C482, H487), type 2 (H432, H113), and type 3 (H115, H155, H157, H434, H481, H483) from CtLac were highly conserved when compared with laccases from various bacilli (Martins et al. 2002; Reiss et al. 2011).
CtLac production and purification
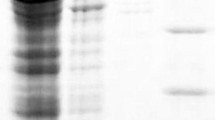
The recombinant laccase was obtained by expressing a plasmid pECtLac in E. coli BL21 DE3. Several parameters such as the combination of growth and induction at low temperature, addition of Cu2+, and use of static conditions after initial 4 h of shaking incubation during induction were employed in order to obtain maximal amount of active form of laccase fully loaded with copper as described previously (Durao et al. 2008; Koschorreck et al. 2008). Further, CtLac was purified to homogeneity using the His-trap affinity chromatography and the gel filtration chromatography. CtLac showed a single band on SDS-PAGE at a relatively higher molecular weight of about 120 kDa than expected, which is also confirmed by the zymogram analysis on SDS-PAGE. However, the native-PAGE zymogram analysis using 2,6-DMP indicated a protein band at the expected molecular weight of about 57 kDa when compared with BSA as a standard (Fig. 1).
SDS-PAGE and zymogram activity analysis of CtLac a SDS-PAGE and SDS-PAGE zymogram analysis. Lanes represent (1) molecular weight marker, (2) soluble proteins, (3) His-tag purified CtLac, (4) gel filtration chromatography fraction of CtLac, (5) SDS-PAGE zymogram of His-tag purified CtLac, and (6) SDS-PAGE zymogram of Gel filtration chromatography fraction of CtLac. b Native-PAGE zymogram analysis. Lanes represent (1) standard bovine serum albumin fraction, (2) native-PAGE analysis of CtLac, and (3) native-PAGE zymogram analysis of CtLac using 2,6-dimethoxyphenol (2,6-DMP) as substrate. Arrows indicate purified CtLac
Characterization of the purified CtLac
Various biochemical properties were investigated using purified CtLac. 2,6-DMP was the most preferred and efficiently oxidized by CtLac among all the substrates tested (Table 1). The optimum pH value for the oxidation of SGZ and 2.6-DMP was pH 8.0 (Fig. 2a). Interestingly, ABTS was not oxidized by CtLac. The highest activity (537 U/mg) for the oxidation of 2,6-DMP was observed at 70 °C (Fig. 2b). The enzyme showed about 70% of its activity at 90 °C. The thermostability of the laccase was investigated within the temperature range of 60–90 °C and pH 8.0–9.0. The enzyme was highly stable at 80 °C and retained 83% of its activity after 24 h of preincubation at pH 8.0 (Fig. 2c). The half-life of CtLac at 90 °C was measured to be 12 h at pH 8.0 and about 6 h at pH 9.0 (Fig. 2d). CtLac exhibited Km and kcat of 200 μM and 23 s−1, respectively for 2,6-DMP. The effects of various concentrations of halides and metal ions on the laccase activity were investigated. The 2,6-DMP oxidation activity of CtLac was stimulated to 250% at 400 mM of NaCl as well as KCl and the enzyme is highly active even in the presence of 1 M of NaCl (Fig. 3a). Cu2+ and Mn2+ enhanced the CtLac activity to 280 and 160%, respectively at 1.0 mM concentration while in the presence of other metal ions such as Ca2+, Mg2+, Li2+, Ni 2+, and Al3+, the enzyme retained 75–100% activity (Fig. 3b). In contrast, Zn2+ inhibited the CtLac activity to 25% while ethylenediaminetetraacetic acid (EDTA) appears to chelate the Cu2+ ions from the copper center and completely inhibits the enzyme activity. The enzyme activity was completely inhibited when the reducing agents such as dithiothreitol (DTT), cysteine, and β-mercaptoethanol were added to the final concentration of 0.1 mM in the reaction mixture. Sodium azide was observed as the strongest inhibitor of the laccase. In addition, the effect of organic solvents and various surfactants on laccase activity was investigated. The laccase was fairly stable and retained more than 70% of its activity in 30% (v/v) of the tested organic solvents except for acetone (Table 2). 2,6-DMP oxidation was stimulated significantly in the presence of 10% (v/v) of methanol, ethanol, and dimethyl sulfoxide (DMSO). Moreover, CtLac was highly active in the presence of high concentration of various surfactants showing more than 80% of its activity (Table 3). The enzyme activity was stimulated by 40% with 1–10 mM of Tween 80.
Biochemical characterization of CtLac. a Effect of pH on CtLac activity using 2,6-DMP (filled triangles) and syringaldazine (SGZ) (filled diamonds) as a substrate. b Effect of temperature on CtLac activity at optimum pH. The highest activity was defined as 100% (537 U/mg) and results expressed as relative activity in (%). c Thermostability of CtLac at pH 8.0 and d at pH 9.0, respectively. The activity without preincubation step was defined as 100%. Values represent the means ± standard deviation of the triplicate experiments
Effect of halides and metal ions on CtLac activity. a Activity of CtLac as a function of different concentrations (0–1 M) of NaCl and KCl at pH 8.0. The activity with no addition of salts was defined as 100% (537 U/mg). b Effects of metal ions on CtLac activity. Metal chloride solution of various metal ions was added at a final concentration of 1 mM in the reaction mixture. The activity of CtLac with no supplementation of metal ion was defined as 100% (190 U/mg) and results expressed in terms of relative activity (%). Values represent the means ± standard deviation of the triplicate experiments
Identification of GGGE oxidized products using HPLC and LC-MS/MS analysis
The HPLC chromatogram of GGGE treated with CtLac indicated a significant decrease of GGGE with a retention time of 22.5 min and an increase of the peak at 27.5 min compared to the control sample without CtLac treatment (Fig. 4). The peak at 27.5 min of the CtLac-treated sample showed maximum absorbance at 310 nm which seemed to correspond to the oxidized product of GGGE after CtLac treatment. To identify this unknown product, Q-TOF MS/MS analysis was performed using an authentic standard of GGGE. The MS spectra of the standard GGGE and oxidized form of GGGE showed m/z values at 319.13 and 637.25 of the deprotonated ion in negative ionization mode [M–H]− (Fig. 5a, b), respectively. Further structural information of this ion (m/z 637.25) was obtained using a CID experiment. Fourteen product ions were produced from the deprotonated precursor ion, [M–H]− at a collision energy of 20 and 40 V (Fig. 5c, d). Considering the difference of m/z between the precursor and product ions, there were typical neutral losses of 18 Da (H2O, water), 30 Da (CH2O, formaldehyde), and 48 Da (H2O + CH2O), which are the MS/MS fragmentation characteristic of a β-aryl ether linkage (Morreel et al. 2010). The major fragmentation products deduced from [M–H]− were [M–H–H2O–CH2O]− (m/z 589.2280), [M–H–guaiacol]− (m/z 513.1945), and [M–H–2guaiacol–CH2O]− (m/z 329.1150) (Supplementary Table S1). The CID-MS/MS experiment indicated the compound produced from GGGE has a biphenyl tetramer structure and the possible candidates could be narrowed down to two compounds: 5-O-4 biphenyl ether tetramer and C5-C5 biphenyl tetramer (Fig. 6).
Discussion
Laccases are versatile biocatalysts composed of four copper ions in the catalytic site. Type I copper acts as a primary electron acceptor and is involved in the oxidation of reducing substrate. Type 2 and type 3 copper sites form a tri-nuclear copper cluster where transferred electrons reduce molecular oxygen to water (Jones and Solomon 2015). In the present study, we described the cloning, expression, purification, and characterization of a thermoalkaliphilic laccase from C. thermarum strain TA2.A1. In addition, the major focus of the work was to investigate CtLac action on a dimeric lignin model compound GGGE. CtLac is a monomeric protein of 57 kDa; however, its migration on SDS-PAGE showed the phenomenon of “gel shifting” as the observed molecular weight of protein does not correlate with the formula molecular weight (Rath et al. 2009). This finding indicates that CtLac is highly resistant to the thermal denaturation by SDS treatment and exhibits a strong binding affinity toward SDS, which might lead to impaired mobility on the SDS-PAGE gel (Rath et al. 2009). It also suggests the tolerance of CtLac toward various surfactants as discussed further.
Substrate specificity study of CtLac with 2,6-DMP, ABTS, catechol, caffeic acid, ferulic acid, and phloroglucinol as a substrate indicated an efficient oxidation of phenolic compounds. CtLac oxidized o-phenols (in order of 2,6 DMP>catechol) more efficiently than m-phenols (in order of phloroglucinol>ferulic acid>caffeic acid) as observed in case of SN4LAC, which poorly oxidized m-phenols (Sondhi et al. 2014). However, ABTS as a non-phenolic substrate was not oxidized by CtLac. It was also observed that the CtLac was not soluble in the reaction buffers under acidic condition (pH 3.0–6.0), which is the most favorable condition for ABTS oxidation by laccases (Reiss et al. 2011; Lu et al. 2013; Brander et al. 2014). It seems like that CtLac prefers to oxidize phenolic compounds probably due to the structural differences in the substrate binding site of the enzyme (Xu et al. 1996). The non-phenolic nature of ABTS and inability of CtLac to be active under acidic conditions are the possible reasons behind why ABTS was not oxidized by the CtLac. In addition, the fact that laccases possesses a vast substrate specificity and the target substrate that is to be oxidized varies upon the source and origin of laccase as well as from one laccase to another (Madhavi and Lele 2009).
CtLac is a highly alkali-thermotolerant laccase. McoA, a copper-activated metallo-oxidase from the thermotolerant bacterium Aquifex aeolicus, exhibited thermostability at 90 °C with activity durable for 5 h (Fernandes et al. 2007). A thermostable laccase from Azospirillum lipoferum showed a half-life of 43 min at 70 °C, but it was completely inhibited within 2–3 min incubation at 80 °C (Diamantidis et al. 2000). B. subtilis CotA laccase has a half-life of 2 h at 80 °C while B. clausii CotA has a half-life of 20 min at 80 °C. On the other hand, CotA laccase from B. licheniformis showed only 8% of its activity after 1-h incubation at 80 °C (Martins et al. 2002; Koschorreck et al. 2008; Brander et al. 2014). In contrast, the current laccase CtLac has a much higher stability at 80 °C with a half-life of above 8 h at pH 9.0. The most thermostable laccase from Thermus thermophilus exhibited a half-life of over 14 h at 80 °C as determined by oxidation of ABTS (Miyazaki 2005).
Thermostability of CtLac can be explained by analyzing some parameters that might affect the protein thermostability such as proline content and aliphatic index (Miyazaki 2005; Kalyani et al. 2016). The values of proline content and aliphatic index were calculated using the ProtParam tool maintained by the Swiss Institute of Bioinformatics (https://web.expasy.org/protparam/). CtLac has a proline content of 5.4% and an aliphatic index of 78.29% which were lower than those of T. thermophilus laccase (proline content 10% and aliphatic index 96.39%) and Meiothermus ruber laccase (proline content 6.8% and aliphatic index 92.24%). Laccases from hyperthermophiles such as Pyrobaculum aerophilum IM2 (proline content 7.60% and aliphatic index 89.80%) and Aquifex aeolicus VF5 (proline content 4.99% and aliphatic index 79.32%) showed lowest values of proline content and aliphatic index, although experimental evidence for this in affecting thermostability is lacking. It appears that the lower proline content and comparable aliphatic index of CtLac might be responsible for its higher thermostability. Clearly, further detailed structural and crystallographic analysis is needed to reveal the mechanism of thermostability of CtLac.
The Km value of CtLac for 2,6-DMP was 3.4 and 5 times lower compared to those of B. pumilus (680 ± 27 μM) and B. clausii (1020 μM) laccases, respectively, indicating a high substrate affinity for 2,6-DMP, while the Kcat value was close to the laccases from B. subtilis and B. licheniformis and greater than of B. pumilus and B. clausii laccases (Martins et al. 2002; Koschorreck et al. 2008; Reiss et al. 2011; Brander et al. 2014).
The activity of alkaline thermophilic laccases was stimulated in the presence of halides (Ruijssenaars and Hartmans 2004; Brander et al. 2014). CtLac exhibited 2,6-DMP oxidation activity in the presence of high concentration of NaCl and KCl, which confirms the salt tolerance capability of the laccase. This activation effect with halides under alkaline conditions was observed probably due to destabilization of labile salt bridges at high ionic strength that leads to create a more active form of enzyme as discussed previously by Brander et al. (2014). In addition, there might be competition between OH− and Cl− for T2 and T3 sites of a laccase, as explained in the case of fluoride (Xu 1997).
CtLac activity was not affected by various metal ions such as Ca2+, Mg2+, Li2+, Ni 2+, and Al3+ making it suitable for applications in pulp and paper industry and in wastewater treatment containing heavy metals. The stimulation of laccase activity by Cu2+ observed in the study occurred probably due to the filling of type I and II copper binding sites with copper ions, highlighting the importance of Cu2+ ion in laccase function (Nagai et al. 2002; Kaushik and Thakur 2013; Sondhi et al. 2014). Inhibition of CtLac in the presence of Zn2+ was in accordance with the results from previously characterized fungal laccases (Murugesan et al. 2006; More et al. 2011). CtLac activity was inhibited in the presence of reducing agents suggesting reduction of the oxidized substrate along with the destabilization of disulfide bonds leading to changes in enzyme conformation (Johannes and Majcherczyk 2000). In addition, the inhibition of CtLac due to sodium azide was a result of its adverse effect on the internal electron transfer at the tri-nuclear copper center, thereby affecting the overall oxidation process (Ryan et al. 2003). Stimulation of CtLac activity in the presence of organic solvents was consistent as in the case of thermo-alkali stable recombinant laccases. Notably, recombinant laccases from B. pumilus and B. licheniformis showed an inhibitory effect at 30% (v/v) of DMSO retaining 55 and 8% of its 2,6-DMP and SGZ oxidation activities, respectively (Reiss et al. 2011; Lu et al. 2013). In contrast, CtLac was far more stable showing 80% of its 2,6-DMP oxidation activity.
The availability of phenolic compounds, such as pesticides (Hirai et al. 2004) and polycyclic aromatic hydrocarbons (Pozdnyakova et al. 2004), to oxidative degradation by the lignolytic enzymes was increased in the presence of surfactants. In addition, they also protect the enzymes from the reaction products during the oxidative degradation/polymerization of phenolic pollutants (Sakurai et al. 2003). A similar effect was observed in the case of horseradish peroxidase during conversion of pentachlorophenol where non-ionic surfactant Tween 80 was able to protect the enzyme from free radical attack and adsorption of products by precipitating them (Kim et al. 2007). The effect of ionic and non-ionic surfactants on CtLac activity is comparable with the laccases of Meripilus giganteus and B. tequilensis (Schmidt et al. 2012; Sondhi et al. 2014), suggesting that laccase could be very useful in the surfactant industry. Notably, CtLac is highly resistant to SDS while laccase from M. giganteus was inhibited severely in the presence of 1 mM of SDS. On the other hand, both Tween 20 and Tween 80 showed the stimulatory effect on CtLac while they are inhibitory to the SN4LAC of B. tequilensis (Sondhi et al. 2014).
Various fungal laccases and DyP-peroxidases have been investigated for their ability to oxidize phenolic and non-phenolic dimeric lignin model compounds (Rittstieg et al. 2002; Majumdar et al. 2014; Rahmanpour et al. 2016; Ramalingam et al. 2017). CID of oxidized products of GGGE indicated that CtLac catalyzes oligomerization of a dimeric lignin model compound. The major peak that appeared at 27.5 min represents the C5-C5 biphenyl tetramer or 5-O-4 biphenyl ether tetramer (Fig. 5). The C5-C5 biphenyl tetramer was more favored compared to the 5-O-4 biphenyl ether tetramer. The CID pattern of the GGGE authentic compound showed product ions with m/z of 271.1102, 256.0861, 195.0760, 165.0632, 149.0317, 121.0353, 109.0346, 93.0398, and 77.0443 from the losses of 18 Da (H2O, water), 30 Da (CH2O, formaldehyde), and 48 Da (H2O and CH2O) and cleavage of biphenyl linkage (Supplementary Fig. S2). However, CID spectra of CtLac-treated sample did not show any ions between 299.0661 ([M–H–2guaiacol–2CH2O–2CH3]−) and 123.0494 (guaiacol) and below 123.0494. It was reported that the C5-C5 biphenyl bond is not easily dissociated due to the partial double bond character, resulting in higher bond energy than in the 5-O-4 biphenyl ether (Jarrell et al. 2014; Huang et al. 2015). Moreover, a GGGE tetramer showed significant UV absorption shift to a higher wavelength (Fig. 4). The GGGE standard dimer peak at 22.5 min displayed little absorption at 310 nm. In contrast, the peak at 27.5 min which is considered as a tetramer shows absorption at 310 nm indicating that the number of the pi electrons participating in the conjugation system has been increased after radical coupling. These observations suggest that the C5-C5 biphenyl tetramer is a more favored product than the 5-O-4 biphenyl ether tetramer. The HPLC chromatogram showed several additional peaks of high molecular weight products following the major peak at 27.5 min. These findings suggest that CtLac causes oligomerization of GGGE rather than depolymerization of oxidized products followed by re-polymerization. As shown in Fig. 6, the oligomerization process was initiated by CtLac with the formation of a phenoxy-radical by one electron oxidation of GGGE and simultaneous reduction of oxygen to water, as a mechanism well known for the laccases (Riva 2006). Small laccases from Streptomyces can degrade a phenolic dimeric lignin model compound to a mixture of oxidative degradation products and vanillin; however, the detailed mechanism of these enzymes was not investigated (Majumdar et al. 2014). Recently, a similar mechanism of laccase that catalyzes oxidative oligomerization of a dimeric lignin model compound was investigated using commercially available fungal laccases from Trametes versicolor and Pleurotus ostreatus (Ramlingam et al. 2017).
In conclusion, a novel laccase CtLac from C. thermarum stain TA2.A1 was cloned and successfully expressed in E. coli. Thermostability, resistance to the various surfactants, organic solvents, and halides are the unique properties of the CtLac. In addition, CtLac catalyzed oxidative dimerization of a dimeric lignin model compound GGGE. To the best of our knowledge, this is the first report on the mechanistic study of a thermoalkaliphilic bacterial laccase causing dimerization of a dimeric lignin model compound GGGE. Further works, such as the potential of CtLac in delignification and detoxification of lignocellulosic biomass and effects of various natural and synthetic laccase mediators on CtLac activity, need to be investigated to explore these finding in biorefinery and various industrial applications.
References
Baldrian P (2006) Fungal laccases occurrence and properties. FEMS Microbiol Rev 30:215–242
Brander S, Mikkelsen JD, Kepp KP (2014) Characterization of an alkali and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS One 2014, 9: e99402. doi:https://doi.org/10.1371/journal.pone.0099402
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bugg TD, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Couto R, Herrera T (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Diamantidis G, Effosse A, Potier P, Bally R (2000) Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol Biochem 32:919–927
Durao P, Chen Z, Fernandes AT, Hildebrandt P, Murgida DH, Todorovic S, Pereira MM, Melo EP, Martins LO (2008) Copper incorporation into recombinant CotA laccase from Bacillus subtilis: characterization of fully copper loaded enzymes. J Biol Inorg Chem 13:183–193
Fernandes AT, Soares CM, Pereira MM, Huber R, Grass G, Martins LO (2007) A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus. FEBS J 274:2683–2694
Freixo MR, Karmali A, Frazão C, Arteiro JM (2008) Production of laccase and xylanase from Coriolus versicolor grown on tomato pomace and their chromatographic behavior on immobilized metal chelates. Process Biochem 43:1265–1274
Gonzalo G, Colpab DI, Habibb MH, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119
Hirai H, Nakanishi S, Nishida T (2004) Oxidative dechlorination of methoxychlor by ligninolytic enzymes from white-rot fungi. Chemosphere 55:641–645
Huang J, Wu S, Cheng H, Lei M, Liang J, Tong H (2015) Theoretical study of bond dissociation energies for lignin model compounds. J Fuel Chem Technol 43:429–436
Ihssen J, Reiss R, Luchsinger R, Thöny-Meyer L, Richter M (2015) Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci Rep 5:10465–10478
Jarrell TM, Marcum CL, Sheng H, Owen BC, O’Lenick CJ, Maraun H, Bozell JJ, Kenttämaa HI (2014) Characterization of organosolv switchgrass lignin by using high performance liquid chromatography/high resolution tandem mass spectrometry using hydroxide-doped negative-ion mode electrospray ionization. Green Chem 16:2713–2727
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Jones SM, Solomon EI (2015) Electron transfer and reaction mechanism of laccases. Cell Mol Life Sci 72:869–883
Kalamorz F, Keis S, McMillan DG, Olsson K, Stanton JA, Stockwell P, Black MA, Klingeman DM, Land ML, Han CS, Martin SL, Becher SA, Peddie CJ, Morgan HW, Matthies D, Preiß L, Meier T, Brown SD, Cook GM (2011) Draft genome sequence of the thermoalkaliphilic Caldalkalibacillus thermarum strain TA2.A1. J Bacteriol 193:4290–4291
Kalyani DC, Munk L, Mikkelsen JD, Meyer AS (2016) Molecular and biochemical characterization of a new thermostable bacterial laccase from Meiothermus ruber DSM 1279. RSC Adv 6:3910–3918 Kaushik G, Thakur IS (2013) Purification, characterization and usage of thermotolerant laccase from Bacillus sp. for biodegradation of synthetic dyes. Appl Biochem Microbiol 49:352–359
Kim EY, Chae HJ, Chu KH (2007) Enzymatic oxidation of aqueous pentachlorophenol. J Environ Sci (China) 19:1032–1036
Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB (2008) Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol 79:217–224
Kudanga T, Le Roes-Hill M (2014) Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98:6525–6542
Lu L, Wang TN, Xu TF, Wang JY, Wang CL, Zhao M (2013) Cloning and expression of thermo alkali stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Bioresour Technol 134:81–86
Madhavi V, Lele SS (2009) Laccase: properties and applications. Bioresources 4:1694-1717 Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, Gerlt JA (2014) Roles of small laccases from Streptomyces in lignin degradation. Biochemistry 53:4047–4058
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Masai E, Katayama Y, Fukuda M (2007) Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem 71:1–15
Mate DM, Alcalde M (2016) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10:1457–1467
Messerschmidt A, Huber R (1990) The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin modelling and structural relationships. Eur J Biochem 187:341–352
Miyazaki K (2005) A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 9:415–425
Morreel K, Dima O, Kim H, Lu F, Niculaes C, Vanholme R, Dauwe R, Goeminne G, Inzé D, Messens E, Ralph J, Boerjan W (2010) Mass spectrometry-based sequencing of lignin oligomers. Plant Physiol 153:1464–1478
More SS, Renuka PS, Pruthvi K, Swetha M, Malini S, Veena SM (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res 2011:7
Munk L, Sitarz AK, Kalyani DC, Mikkelsen JD, Meyer AS (2015) Can laccases catalyze bond cleavage in lignin? Biotechnol Adv 33:13–24
Murugesan K, Arulmani M, Nam IH, Kim YM, Chang YS, Kalaichelvan PT (2006) Purification and characterization of laccase produced by a white rot fungus Pleurotus sajorcaju under submerged culture condition and its potential in decolorization of azo dyes. Appl Microbiol Biotechnol 72:939–946
Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H (2002) Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol 60:327–335
Pozdnyakova NN, Rodakiewicz-Nowak J, Turkovskaya OV (2004) Catalytic properties of yellow laccase from Pleurotus ostreatus D1. J Mol Catal B Enzym 30:19–24
Rahmanpour R, Rea D, Jamshidi S, Fülöpb V, Bugga TD (2016) Structure of Thermobifida fusca DyP-type peroxidase and activity towards kraft lignin and lignin model compounds. Arch Biochem Biophy 564:54–60
Ramalingam B, Sana B, Seayad J, Ghadessy FJ, Sullivan MB (2017) Towards understanding of laccase-catalyzed oxidative oligomerization of dimeric lignin model compounds. RSC Adv 7:11951–11958
Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM (2009) Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci U S A 106:1760–1765
Rittstieg K, Suurnakki A, Suortti T, Kruus K, Guebitz G, Buchert J (2002) Investigations on the laccase-catalyzed polymerization of lignin model compounds using size-exclusion HPLC. Enzym Microb Technol 31:403–410
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Reiss R, Ihssen J, Thöny-Meyer L (2011) Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol 11:9–22
Ruijssenaars HJ, Hartmans S (2004) A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol 65:177–182
Ryan S, Schnitzhofer W, Tzanov T, Cavaco-Paulo A, Gübitz GM (2003) An acid stable laccase from Sclerotium rolfsii with potential for wool dye decolorization. Enzym Microb Technol 33:766–774
Sakurai A, Masuda M, Sakakibara M (2003) Effect of surfactants on phenol removal by the method of polymerization and precipitation catalyzed by Coprinus cinereus peroxidase. J Chem Technol Biotechnol 78:952–958
Santhanam N, Vivanco JM, Decker SR, Reardon KF (2011) Expression of industrially relevant laccases: prokaryotic style. Trends Biotechnol 29:480–489
Schmidt G, Krings U, Nimtz M, Berger RG (2012) A surfactant tolerant laccase of Meripilus giganteus. World J Microbiol Biotechnol 28:1623–1632
Sondhi S, Sharma P, Saini S, Puri N, Gupta N (2014) Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One 9:e96951. https://doi.org/10.1371/journal.pone.0096951
Telke AA, Ghodake GS, Kalyani DC, Dhanve RS, Govindwar SP (2011) Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR. Bioresour Technol 102:1752–1756
Torres-Salas P, Mate DM, Ghazi I, Plou FJ, Ballesteros AO (2013) Widening the pH activity profile of a fungal laccase by directed evolution. Chem Bio Chem 14:934–937
Tuor U, Winterhalter K, Fiechter A (1995) Enzymes of white-rot fungi involved in lignin degradation and ecological determinants for wood decay. J Biotechnol 41(1):1–17
Xu F (1997) Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem 272:924–928
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Acknowledgments
This work was supported by the GIST research institute (GRI) in 2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Sunil Ghatge and Youri Yang contribute equally to this work and should be considered as co-first authors.
Electronic supplementary material
ESM 1
(PDF 401 kb)
Rights and permissions
About this article
Cite this article
Ghatge, S., Yang, Y., Song, WY. et al. A novel laccase from thermoalkaliphilic bacterium Caldalkalibacillus thermarum strain TA2.A1 able to catalyze dimerization of a lignin model compound. Appl Microbiol Biotechnol 102, 4075–4086 (2018). https://doi.org/10.1007/s00253-018-8898-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8898-4