Abstract
A stand-alone down-flow hanging sponge (DHS) system with a two-stage configuration was operated for 700 days to treat synthetic soft drink wastewater at 3000 mg/L chemical oxygen demand (COD). Throughout the operation, >90% COD and total organic carbon (TOC) removal efficiency was obtained by the first stage, and a final effluent of COD <60 mg/L (TOC <20 mg/L) was consistently maintained with the second stage. Lower organic removal efficiency was observed to closely correlate with lower pH, higher volatile fatty acid (VFA) concentration, and higher suspended solid (SS) in the effluent. Occasionally, biomass sloughing was observed as a cause to unstable reactor performance in the first stage. The microbial community of the retained biomass on the sponges differed significantly based on spatial locations of sponges, sampling time points, and loading shocks. In general, Proteobacteria were found to be more abundant in the reactor at an organic removal efficiency >80% than that at <50%. Specifically, operational taxonomic units closely related to Tolumonas auensis and Rivicola pingtungensis were identified as important populations that were responsible for degrading the major substrate in the soft drink wastewater toward to the end of the reactor operation. In addition, high abundance of Bacteroidetes in the reactor was speculated to be responsible for the VFA accumulation in the effluent. This study demonstrated that stand-alone DHS reactor could be used in treating high-strength wastewater efficiently.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On average, each American consumed approximately 41 gals of soft drinks in 2014 (Statista 2015). Accompanied with the soft drink consumption, wastewater with soluble and easily biodegradable carbohydrates as the main component is produced at a concentration ranging from 1200 to 8000 mg/L as chemical oxygen demand (COD) (Ait Hsine et al. 2005; Chen et al. 2005). Based on the Clean Water Act (Purdue University 2007), high-organic-strength soft drink wastewater needs to be treated to meet the regulation requirement before being discharged to the receiving water bodies. To do so, it is often discharged directly to a local sewage system and the food companies need to pay the treatment cost based on volume and organic loading to the local wastewater treatment facilities. Alternatively, the wastewater can be treated on-site prior to discharge to a local sewage system. For on-site treatments, aerobic processes, which are less favorable (Chen et al. 2005), can remove approximately 65% organic chemicals (Tebai and Hadjivassilis 1992). By contrast, anaerobic processes have been widely applied to treat high-organic-strength wastewater, primarily because of their characteristics including low operation cost, high organic loading tolerance, low excessive sludge yield, and reasonable treatment efficiency (around 80% COD removal) (Kalyuzhnyi et al. 1997). Among all the anaerobic treatment processes, the upper-flow anaerobic sludge blanket (UASB) process is the most favorable one to treat high-organic-strength wastewater (4–12 kg COD/day m3) compared to the other types like anaerobic contactor, anaerobic filter, and anaerobic fluidized bed reactor (Liu and Liptak 1999). However, the treated effluent from anaerobic processes still cannot be discharged directly into a natural water body. To further polish the effluent quality, aerobic activated sludge processes and membrane biological reactors have been used as post-treatment processes (Chen et al. 2005). Due to the high cost associated with these post-treatments, simple technology like trickling filter has been explored but with limited treatment efficiency (Chen et al. 2005).
In 1997, a post-treatment process or so-called down-flow hanging sponge (DHS) reactor was developed (Agrawal et al. 1997). This technology has the similar working principle as the trickling filter, but instead of stone, polymeric sponges are used as the supporting material (Agrawal et al. 1997; Machdar et al. 1997). The sponges are in direct contact with air (Tandukar et al. 2006; Tandukar et al. 2005) and can create an oxygen gradient along the sponge depth. This setting further allows the coexistence of aerobe and anaerobe growth inside the sponge as biofilms (Chuang et al. 2007). With its porous structure, the amount of biomass grown inside a sponge can be maximized. This further increases the solid retention time (SRT) and reduces sludge yield (Mahmoud et al. 2009). In comparison to traditional activated sludge process, the operation and maintenance cost for DHS is relatively low (Tandukar et al. 2005). In general, the combination of anaerobic processes and DHS reactors was reported to achieve at least 90% of organic removal in most studies (El-Kamah et al. 2011; Takahashi et al. 2011; Tawfik 2012; Wichitsathian and Racho 2010). So far, only limited studies have attempted to use DHS reactor as a standalone process to treat low-strength (<1000 mg/L COD) wastewater (Fleifle et al. 2013a; Fleifle et al. 2013b; Uemura et al. 2010).
In this study, DHS reactor was further explored to determine whether it can be effectively used to directly treat high-organic-strength wastewater such as soft drink wastewater. The performance was monitored based on parameters including pH, COD and total organic carbon (TOC) removal efficiencies, nitrogen species concentration, suspended solid (SS), and retained biomass concentration. Meanwhile, the biomass samples were taken at different heights of the DHS system at different time points, and the microbial community structures in those samples were analyzed using the next-generation sequencing technology targeting the 16S ribosomal RNA (rRNA) gene. The microbial community analyses revealed the dynamic through the operation and helped to identify the important microbes.
Materials and methods
Synthetic soft drink wastewater
The composition of the synthetic wastewater was prepared according to the composition measured in real wastewater discharged from the soft drink plant (Narihiro et al. 2015). Fructose, glucose, and polyethylene glycol (PEG) were the three main organic substrates (Table S1). The COD and TOC concentrations were approximately 3000 and 1000 mg/L, respectively. Ammonium bicarbonate and potassium phosphate dibasic were added to the synthetic wastewater to provide sufficient nutrient for microbial growth. Before the substrate was fed to the system, the media were autoclaved at 121 °C and 20 psi for 60 min. The pH of the influent substrate was adjusted to approximately 7 from day 0 and to 8.5–9 via the addition of sodium hydroxide from day 150. To prevent media deterioration during the feeding, it was submerged in an ice bath to keep the media bottle around 4 °C during the feeding.
Experimental setup and reactor operation
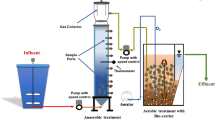
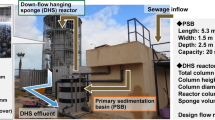
Figure 1 shows the configuration of DHS reactor used. The first stage treatment contained two identical DHS reactors, reactor A and reactor B, in parallel under the same condition. Each reactor was 2.2 L based on sponge volume. Thirty identical polyurethane rectangle sponge units (35 mm × 35 mm × 30 mm) were connected in series and placed inside a Plexiglass column (internal diameter 14 cm; height 145 cm) with two 4-cm gaps for air floatation. Both reactors A and B were fed with the same substrate from the top under the same flow rate during the entire experiment. In the second stage treatment, another DHS reactor (reactor 2) was used and the volume of the reactor was 4.4 L based on sponge volume. The reactor column contained two chains of 30 sponges connected in series and received effluent from reactors A and B. The organic loading rate (OLR) and hydraulic retention time (HRT) were calculated based on the sponge volume. Before the sponges were placed in the reactors A and B, they were briefly soaked with the activated sludge taken from a local wastewater treatment plant (Champaign, IL) as inoculum.
The schematic diagram of the DHS reactors with plots of TOC removal efficiency recorded in each reactor. a The configuration of the reactors. b The sponges and meshes used in the reactors. TOC removal efficiency and pH in c effluent from reactor A, d effluent from reactor B, and e effluent from reactor 2
The system was operated for 700 days under three different phases. During the start-up phase (day 0–day 138), the HRT was gradually decreased from 24 to 16.5 h. After the organic removal efficiency was stabilized, the system was evaluated with two different OLRs for 100 days by reducing HRT (phase I). At phase II starting at day 285, both reactors A and B were operated at an HRT of 15.3 h (4.9 kg COD/m3 sponge volume/day as OLR) for 350 days. Reactor 2 was set up using sponges without seed sludge on day 156, and since we mainly focused on the performance of the first stage reactors, the HRT was set at 24 h on reactor 2 throughout the entire operation. No external aeration was provided during the operation, and all reactors were covered by a black curtain to prevent algal growth from the light explosion. Detailed operation conditions are shown in Table 1.
Chemical and physical analytical methods
Effluent water samples from all three reactors were collected twice a week for analyses. Each water sample was filtered through a 0.22-μm filter before recording pH value and performing chemical measurements. COD was measured by using the Hach® COD digestion vial, and TOC was measured by using Shimadzu® TOC-V Analyzer. Volatile fatty acid (VFA) components were quantified by using the Shimadzu® HPLC LC-20AT equipped with Hi-Plex H column from Agilent Technologies®. Inorganic nitrogen species components were tested once a week. Ammonia, nitrate, and nitrite concentrations were measured by using Hach® DR 4000 Method 8155, 8171, and 8507, respectively.
The SS and volatile suspended solid (VSS) concentrations in the effluent of reactors A and B and in individual sponges collected at different height were measured using standard methods (APHA 1915). To collect the biomass in individual sponges, the biomass was first collected by scrambling the surface biomass and thoroughly squeezing and washing biomass in the sponge out using distilled water (Onodera et al. 2013). Then, biomass obtained in the distilled water was collected by centrifugation (8500 rpm, 10 min).
Biomass sampling, DNA extraction, PCR, and 16S rRNA sequencing analysis
Biomass in sponges was sampled at different heights along the reactor on days 476 and 666. In reactors A and B, the biomass was taken at three different heights (sponge nos. 5, 15, and 25 from the top). For reactor 2, no. 5 and no. 10 were sampled. On day 571, after a major sloughing happened in reactor A, the biomass was sampled again from sponge nos. 5, 15, and 25. Detailed sampling positions of sponges are shown in Fig. 1. After sampling, the biomass was mixed with 1× phosphate-buffered saline (PBS) before being centrifuged (10,000 rpm, 4 min); then, it was stored at −80 °C for further analysis.
The genomic DNA extraction and PCR amplification were performed as described in the previous protocol (Mei et al. 2016) with U515F forward and U909R reverse primer set (Wang and Qian 2009). The sequencing was performed using Illumina MiSeq Bulk 2 × 300 nt paired-end system. For sequencing analysis, the Mothur pipeline was first used to assemble the sequence to a length of approximately 394 bp and screen and trim the raw sequences by setting 20 as the quality score (Schloss et al. 2009). The sequences were further analyzed using QIIME 1.8.0 (Caporaso et al. 2010b) to pick operational taxonomic unit (OTU) by de novo strategy at 97% identity cutoff against the Greengenes database by using UCLUST algorithm (Edgar 2010). Most abundant sequences were used to represent each OTU and were aligned by using PyNAST (Caporaso et al. 2010a). The chimeric sequences were removed by ChimeraSlayer (Haas et al. 2011). After assigning taxonomy to each OTU by BLAST, the relative abundance of each OTU, alpha diversity (Chao1, observed OTUs, and good coverage indexes) and beta diversity (weighted Unifrac), was calculated by QIIME. The phylogenetic tree was constructed on ARB with neighbor-joining method (Ludwig et al. 2004). The sequence data obtained in this study were deposited at DDBJ under accession no. DRA005549.
Statistical methods
Shapiro-Wilk, Kruskal-Wallis, and Spearman correlation tests were performed in R (Kruskal and Wallis 1952; Shapiro and Wilk 1965; Spearman 1904). Permutational multivariate analysis of variance (PERMANOVA) was used to test the significances of the differences between community structures based on Bray-Curtis dissimilarity with 1000 permutation (Anderson 2005).
Results
Reactor operation and performance
Figure 1 shows the organic removal efficiency of all three reactors throughout the operation. During the start-up phase, the HRT for reactors A and B was gradually decreased from 24 to 16.5 h, and the TOC removal efficiency gradually increased to >90% at day 100. At phase I, the HRT was set at 16.5 h for 45 days and reduced to 13.5 h with the OLR increased from 4.5 to 5.3 kg COD/sponge-m3. This change led to a drop in the TOC removal efficiency from >90 to <50% and back to >90% after 50 days. In phase II, the system was re-started after a short holiday break, and the system performance was evaluated with an HRT and an OLR of 15.3 h and 4.9 kg COD/sponge-m3, respectively. The average TOC removal efficiencies for reactors A and B during this phase were 85 ± 11 and 83 ± 13%, respectively. Based on the Shapiro-Wilk tests, the distribution of TOC removal efficiency of both reactors during the entire operation did not follow a normal distribution and the Wilcoxon and Kruskal-Wallis tests further verified that these two efficiency data sets could be considered as nearly identical to each other.
Occasionally, poor TOC removal performance with reactors A and B was observed at different time points due to primarily biomass sloughing. On day 410, the TOC removal efficiency in reactor A dropped to <70% after a small amount of biomass sloughed from the sponges to the bottom of the reactor. Twenty days after the sloughing, the removal efficiency recovered to >80%. Biomass sloughing was also observed on day 525 in reactor A and day 400 in reactor B. In both cases, the efficiency recovered to >80% in 2 weeks after the sloughing. A major sloughing was observed in reactor A on day 571. Almost all the biomass on the surface of sponges no. 1 to no. 8 fell to the bottom of the reactor (pictures for the sponges covered with biomass at different conditions shows in Fig. S1), and the TOC removal efficiency decreased to <60%. It took 30 days for the removal efficiency recovered back to 80%. At around day 600, a sudden drop in TOC removal efficiency was observed with both reactors, and this was caused by forgetting to add inorganic components (ammonium and phosphorus) in the synthetic feed during preparation. The color of the biomass on the sponges became black from brown, and the efficiency dropped significantly to <50%. After correcting the mistake, it took 40 days for the efficiency recovered back to >80% for reactor A and even longer time for reactor B. Despite the efficiency fluctuation in reactors A and B, reactor 2 could consistently achieve an overall efficiency at 98% from day 300 and guaranteed the TOC of the effluent from reactor 2 consistently at <20 mg/L.
Correlations between TOC removal efficiency and related parameters
Spearman correlation test was used to correlate key operation parameters with the TOC removal efficiency. A positive correlation was observed between the effluent pH and the organic removal efficiency in both reactors A and B (Fig. 2a, b). Both TOC removal efficiencies and pH had negative correlations with VFA concentrations (p value <0.05). It was observed that VFA in the effluent from reactors A and B mainly consisted of acetate, propionate, and butyrate. When the effluent pH was >7.5, the VFA concentration was mostly <4 mM and the TOC removal efficiency was mostly >80%. The effluent SS concentrations were also measured at different TOC removal efficiencies from reactors A and B. The Spearman correlation test (p value <0.5 rho −0.65) indicated that a high effluent SS concentration correlated with a low TOC removal efficiency (Fig. 2e).
Nitrogen species removal
To determine the fate of ammonia added in the influent, ammonia, nitrate, and nitrite in the influent and effluent from reactors A, B, and 2 were measured once a week from phase II (Fig. S2). At the beginning of phase II, the influent ammonia concentration fluctuated as well as the effluent ammonia concentration, which was later identified due to biological activity taking place in the feed bottle, and this issue was corrected by maintaining the feed bottle at 4 °C. Table 2 shows that more than 80% of ammonia was consumed in reactors A and B, with an average effluent ammonia at 6.4 and 7.5 mg-N/L, respectively. The nitrate concentrations in the effluent in both reactors were consistently below 1 mg-N/L, and the nitrite concentration was below detection limit. In reactor 2, all the residual ammonia from reactors A and B was almost consumed with an effluent ammonia at 0.3 mg-N/L. The effluent nitrate was also below the detection limit. The effluent nitrogen species removal efficiency in reactors in A and B did not correlate with the TOC removal efficiency.
Vertical profiles of reactor performance in the DHS reactors
Changes of TOC, nitrogen species, and TSS/VSS along the reactor height were only determined on day 666 when reactor A became stable and reactor B just suffered from sloughing (Fig. 3). To avoid any negative impact on the reactor performance, five out 30 sponges (nos. 5, 10, 15, 20, and 25) were taken from reactor A and reactor B. The vertical profiles of TOC, ammonia, and retained biomass concentrations shared similar trend along the reactors in both reactors. More than 50% of TOC in the influent was rapidly consumed by the first five sponges, and the TOC removal rate was largely reduced for the rest of the 25 sponges. More than 50% of ammonia in influent was also consumed at the first five sponges in reactors A and B, and the ammonia concentration was relatively stable in the lower parts of the reactors. The nitrite and nitrate concentrations were stable at <2 mg-N/L in all samples taken from different heights of both reactors.
The average VSS content attached on those five sponges was 1.72 g/sponge for reactor A and 1.07 g/sponge for reactor B, which corresponded to 47 and 20 mg-VSS/L-sponge, respectively. The retained biomass differences between these two reactors were also reflected on the TOC removal efficiency on day 666, at 93.8% with reactor A and only 48.1% for reactor B. In addition, the sponges at the lower part of reactors had the lowest amount of retained biomass for both reactors. It reduced from 2.1 g-VSS/sponge at no. 5 to 1.0 g-VSS/sponge at no. 25 in reactor A and from 1.1 to 0.9 g-VSS/sponge in reactor B.
Microbial population dynamics and composition
Microbial population dynamics of samples collected at different locations and different days in the DHS system was characterized using 16S rRNA-based sequencing method. On average, 468,736 16S rRNA gene sequence reads were generated in each biomass sample after removing chimeric sequences. Based on 97% sequence identity cutoff, these sequences were classified into 37,021 operational taxonomic units (OTUs). Chao1 index indicated that these samples contained 2.9- to 5.0-fold more OTUs than observed OTUs, and the high good coverage (average more than 91%) suggested that the obtained OTU data were sufficient to evaluate the microbial diversity in all samples (Table S2). In reactors A and B, the samples collected from the lower parts tended to have higher diversity comparing with that from upper parts on the same sampling day. The diversity in reactor A decreased due to the losing of biomass from the reactor after the major sloughing on day 571. Samples in reactor 2 had a similar level of diversity to that in the lower parts in reactors A and B. The microbial community in effluent SS samples was less diverse than that collected in the reactors.
The principal coordinate analysis (PCoA) plots (Fig. 4) based on weighted Unifrac were used to visualize the differences between samples. Table 3 summarizes the results of PERMANOVA on the heterogeneities of the community structures between different groups of samples. In general, the microbial communities of the samples taken on different days were significantly different from each other. The feeding accident significantly altered the communities in reactors A and B, while major sloughing happened on day 571 did not. Even though the sampling heights could significantly differentiate samples from different parts of the reactors, a few samples taken from the upper parts clustered with that from the middle parts or lower parts in the PCoA plots. The samples collected from reactor 2 were different from that in reactors A and B. Moreover, the microbial communities in reactors were distinct from that in effluent SS samples, and the effluent samples were separated among each by TOC removal efficiency.
Microbial community dynamics along reactors during the operation
Bacteroidetes, Proteobacteria, and Firmicutes were the three major phyla found in all samples from reactors A and B and represent 80% of all sequences (Fig. 5). The upper parts of both reactors tended to have more Bacteroidetes than that in other locations. The relative abundance of Proteobacteria in the upper parts of reactor A was the highest, while the middle parts of reactor B had more Proteobacteria. Generally, Firmicutes and Spirochaetes increased in both reactors during the operation, while Acidobacteria and Gemmatimonadetes decreased in the same period. For samples taken from reactor 2, Chlorobi and Gemmatimonadetes were two major phyla in addition to the Bacteroidetes and Proteobacteria on day 476, while the relative abundance of these two phyla on day 666 greatly decreased. In addition to Bacteroidetes and Proteobacteria, Firmicutes, Spirochaetes, and Actinobacteria, all increased during the operation.
The differences in microbial communities between samples could also be indicated by dynamics of major OTUs with relative abundance >3% (Fig. 6). Forty of the 53 major OTUs were affiliated to Bacteroidetes and Proteobacteria, and half of these 40 OTUs decreased during the operation in reactors A and B. On day 666, OTUs related to Dysgonomonas and Chryseobacterium decreased, while OTUs related to Paludibacter and Tolumonas greatly increased. The high abundance of Chlorobi observed on day 476 in reactor 2 was mainly contributed by OTU 78883, which almost disappeared by day 666. Same finding was observed with OTUs related to Gemmatimonadetes. In terms of the effluent biomass samples, Bacteroidetes, Proteobacteria, and Firmicutes took up >95% relative abundance in each sample. In the samples with >80% TOC removal efficiency, the relative abundance of Proteobacteria was >80% while in those with <50% efficiency, this number dropped to <50% (Fig. S3). Despite seeded with activated sludge, the microbial communities in DHS were different from typical microbial communities observed in activated sludge seed (Fig. S4). Specifically, genera Paludibacter, Dysgonomonas, and Chryseobacterium are more abundant in DHS reactors than in activated sludge seed.
The microbial communities in the upper parts of the reactors were likely responsible for most of the reactor performance since the top one-sixth part of the reactor could consume >60% organic content and nutrients in the feed. On day 666, with the significant efficiency differences between reactors A (93.7%) and B (48.1%), the total abundance of Proteobacteria-related OTUs present at the upper part of reactor A was 46.6% while it was only 21.6% in reactor B. Specifically, OTU146451 and OTU49038 had much higher abundance in reactor A than samples in reactor B. For the relative abundance of Bacteroidetes-related OTUs, the percentages in reactors A and B were 35.7 and 52.1%, respectively, and the relative abundance of OTU144240 and OTU89277 in the upper-part sample of reactor B was much higher than that of the other samples.
We further determined the abundance of microbial populations involving in nitrogen, including ammonia-oxidizing bacteria (AOB), nitrate-oxidizing bacteria (NOB), and complete ammonium oxidizer (comammox) in the DHS reactors. No AOB (Nitrosomonas sp. Nitrosococcus sp.), NOB (Nitrospira sp.), and comammox (Nitrospira sp.) (Gonzalez-Martinez et al. 2016) were detected in all samples from reactors A and B. In reactor 2, Nitrosomonas-related OTU was <0.01% on day 476, and Nitrospira-related OTU was <0.03% on day 666. The common denitrifiers such as Paracoccus spp. and Pseudomonas spp. were detected at low abundance (<0.01%) in all reactors.
Discussion
This study demonstrated the feasibility and efficiency of using a two-stage DHS reactor in treating high-strength wastewater. Compared with a previous anaerobic process used to treat the same synthetic wastewater, the DHS reactor in this study achieved a compatible organic removal efficiency (> 98%) at the same HRT (1.5 days) (Narihiro et al. 2015). As more than 50% of the sponges in the lower part of reactor 2 were not completely covered with biomass at the end of the experiment, this observation suggested that more biomass could be developed in sponges under a higher OLR or a lower HRT. In addition, the DHS used here did not require extra efforts that were needed in operating an anaerobic process, such as maintaining anaerobic conditions and mesophilic operation temperature, providing recirculation, and treating off-gas (Bal and Dhagat 2001; Bandara et al. 2011).
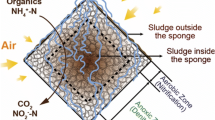
Being able to perform both anaerobic and aerobic reactions was the main factor to achieve high organic removal efficiency in the DHS reactor. Despite that the sponges in the reactor were in contact with air, the redox condition inside of the sponges was found to be anaerobic due to oxygen consumption by the microbes on the surface of the sponges (Kubota et al. 2014; Machdar et al. 2000). Therefore, part of the substrate was aerobically degraded on the surface of the sponges, and the remaining fraction was fermented and secreted as VFAs inside the sponges. Our finding suggested that less VFAs accumulated higher organic removal efficiency and pH value could be achieved (Fig. 2d). Subsequently, the fermented by-products could be degraded by both aerobes and anaerobes attached on the sponges downstream. To achieve good reactor performance, it would be crucial to maintain balanced cooperation between aerobic and anaerobic degradations and prevent the decrease in pH.
The ability of DHS in retaining biomass further enhanced the overall organic removal efficiency. In this study, the amount of retained biomass recorded was 46.8 g-VSS/L-sponge at an OLR of 4.9 kg COD/m3 per day, and these two numbers were higher than reported, 12.8 g-VSS/L-sponge and 2.9 COD/m3 per day, respectively, in a DHS reactor that treats agricultural drainage wastewater (Fleifle et al. 2013b). In addition, it was observed that the retained biomass in a DHS reactor treating gray wastewater increased when the OLR was increased (Tawfik et al. 2011). This study and other studies all suggested that the upper part of the DHS reactor contains higher retained biomass than the lower part due to higher OLR (Fleifle et al. 2013a; Kubota et al. 2014; Onodera et al. 2013). Once the retained biomass was developed, DHS reactor could effectively treat high-strength wastewater.
The feasibility of DHS in treating the high-strength wastewater here was also related to the organic composition of the wastewater (i.e., glucose, fructose, and PEG), which could be aerobically and anaerobically degraded by various microbes (Kawai 2002; Madigan et al. 1997). Different microbial communities were observed in the degradation of those organic substrates in this study due to differences in reactor operation time, OLR, and spatial locations. A high relative abundance of Proteobacteria was observed in the upper part of the reactor with >90% TOC removal efficiency. OTU49038 related to Tolumonas auensis (99% identity, NR_074805.1) and OTU146451 to Rivicola pingtungensis (99% identity, NR_133846.1) were often observed in samples taken at the upper part of the DHS system. T. auensis was reported to have the ability to catabolize the d-glucose and d-fructose under both anoxic and aerobic conditions (Fischer-Romero et al. 1996), and R. pingtungensis was reported to assimilate glucose and use various short-chain fatty acids as the carbon source (Sheu et al. 2014). The relative abundance of OTU49038 and 146451 was about 20 times higher than in the effluent SS samples with <50% efficiency. As higher SS was correlated with lower organic removal efficiency, losing these populations due to sloughing events from the upper part of the DHS system could lead to poor organic removal efficiency. For the phylum Bacteroidetes, it contained many anaerobes that could be responsible for the VFA production through fermentation (Uemura et al. 2010). Specifically, OTU144240, which was observed to dominate under treatment efficiency <50%, was closely related to Paludibacter spp. (Fig. S5) that could ferment sugars in anaerobic condition to VFA (Ueki et al. 2006). Other dominant OTUs found during the poor performance period such as OTU18891 were closely related to uncultured Bacterioidetes species found in anaerobic environments like gut and sludge samples. There, observations supported the occurrence of anaerobic conditions inside the sponge (Fig. S5).
Nevertheless, the DHS operation has faced biomass sloughing issue that could reduce reactor performance. While never reported in DHS reactors, the occurrence of biomass sloughing was commonly reported in biofilm-based membrane reactors (Le-Clech et al. 2006; Lesjean et al. 2005). In these membrane reactors, extracellular polymeric substance (EPS) could be produced when oxygen concentration gradient was developed across biofilm together with endogenous decay (Le-Clech et al. 2006), and excessive production of EPS could weaken the physical structure of biofilms, leading to the occurrence of sloughing (Garny et al. 2009). In this study, the relatively thick layer of retained biomass created by the high organic loading fed to the top of the DHS reactor facilitated the EPS accumulation and thus could increase the possibility of sloughing. By changing the reactor configuration from having sponges connected in series to random packing, the organic load of retained biomass per sponge could be reduced. As the result, excessive EPS production could be prevented as well as biomass sloughing. However, further investigations are required to understand the exact mechanism of sloughing in a DHS system treating high-strength wastewater. Last, due to high OLR and low N/C ratio, we did not observe that much nitrification and denitrification occurred in the DHS system. However, both nitrification and denitrification were reported as important pathways for nitrogen species in other DHS reactors (El-Kamah et al. 2011; Machdar et al. 2000; Tandukar et al. 2006). As the irregular sloughing could affect the estimate of biomass in the DHS system during mass balance calculation, further investigations with a more accurate estimation for the unaccounted nitrogen will be required.
Overall, this study demonstrated that the stand-alone DHS system could achieve >90% organic removal in treating high-strength soft drink wastewater at a concentration of 3000 mg/L COD and an OLR of 4.9 kg COD/m3 sponge volume/day. At least 98% removal efficiency could be guaranteed with two-staged configuration, and parameters like pH, VFA concentrations, and SS concentrations could be used to effectively evaluate the reactor performance. Microbial communities were significantly different at different sampling days and sampling positions in the reactors, and the reactor performance seems to correlate to the relatively high abundance of Proteobacteria-related populations including T. auensis and R. pingtungensis. Even though the sloughing hardly had effects on the microbial community structures, biomass loss could lead to unstable reactor performance. The mechanism behind the sloughing in DHS reactor should be further studied to develop solutions prior to full-scale applications.
References
Agrawal LK, Ohashi Y, Mochida E, Okui H, Ueki Y, Harada H, Ohashi A (1997) Treatment of raw sewage in a temperate climate using a UASB reactor and the hanging sponge cubes process. Wat Sci Tech 36:433–440
Ait Hsine E, Benhammou A, Pons M (2005) Water resources management in soft drink industry-water use and wastewater generation. Environ Technol 26:1309–1316
Anderson MJ (2005) Permutational multivariate analysis of variance. Department of Statistics, University of Auckland, Auckland
American Public Health Association (APHA), American Water Works Association, Water Pollution Control Federation, Water Environment Federation (1915) Standard methods for the examination of water and wastewater (Vol. 2). American Public Health Association
Bal A, Dhagat N (2001) Upflow anaerobic sludge blanket reactor: a review. Indian J Environ Health 43:1–82
Bandara WM, Satoh H, Sasakawa M, Nakahara Y, Takahashi M, Okabe S (2011) Removal of residual dissolved methane gas in an upflow anaerobic sludge blanket reactor treating low-strength wastewater at low temperature with degassing membrane. Water Res 45:3533–3540
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010a) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010b) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chen JP, Seng S-S, Hung Y-T (2005) Soft drink waste treatment. Wastewater Treatment 255–269
Chuang H-P, Ohashi A, Imachi H, Tandukar M, Harada H (2007) Effective partial nitrification to nitrite by down-flow hanging sponge reactor under limited oxygen condition. Water Res 41:295–302
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
El-Kamah H, Mahmoud M, Tawfik A (2011) Performance of down-flow hanging sponge (DHS) reactor coupled with up-flow anaerobic sludge blanket (UASB) reactor for treatment of onion dehydration wastewater. Bioresour Technol 102:7029–7035
Fischer-Romero C, Tindall BJ, Jüttner F (1996) Tolumonas auensis gen. nov., sp. nov., a toluene-producing bacterium from anoxic sediments of a freshwater lake. Int J Syst Evol Microbiol 46(1):183–188
Fleifle A, Tawfik A, Saavedra O, Yoshimura C, Elzeir M (2013a) Modeling and profile analysis of a down-flow hanging sponge system treating agricultural drainage water. Sep Purif Technol 116:87–94
Fleifle A, Tawfik A, Saavedra OC, Elzeir M (2013b) Treatment of agricultural drainage water via downflow hanging sponge system for reuse in agriculture. Water Sci Technol Water Supply 13:403–412
Garny K, Neu TR, Horn H (2009) Sloughing and limited substrate conditions trigger filamentous growth in heterotrophic biofilms—measurements in flow-through tube reactor. Chem Eng Sci 64:2723–2732
Gonzalez-Martinez A, Rodriguez-Sanchez A, van Loosdrecht MM, Gonzalez-Lopez J, Vahala R (2016) Detection of comammox bacteria in full-scale wastewater treatment bioreactors using tag-454-pyrosequencing. Environ Sci Pollut Res:1–11
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504
Kalyuzhnyi S, Saucedo JV, Martinez JR (1997) The anaerobic treatment of soft drink wastewater in UASB and hybrid reactors. Appl Biochem Biotechnol 66:291–301
Kawai F (2002) Microbial degradation of polyethers. Appl Microbiol Biotechnol 58:30–38. doi:10.1007/s00253-001-0850-2
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. JASA 47:583–621
Kubota K, Hayashi M, Matsunaga K, Iguchi A, Ohashi A, Li Y-Y, Yamaguchi T, Harada H (2014) Microbial community composition of a down-flow hanging sponge (DHS) reactor combined with an up-flow anaerobic sludge blanket (UASB) reactor for the treatment of municipal sewage. Bioresour Technol 151:144–150
Le-Clech P, Chen V, Fane TAG (2006) Fouling in membrane bioreactors used in wastewater treatment. J Membr Sci 284:17–53. doi:10.1016/j.memsci.2006.08.019
Lesjean B, Rosenberger S, Laabs C, Jekel M, Gnirss R, Amy G (2005) Correlation between membrane fouling and soluble/colloidal organic substances in membrane bioreactors for municipal wastewater treatment. Wat Sci Tech 51:1–8
Liu DH, Liptak BG (1999) Environmental engineers’ handbook on CD-ROM. CRC press
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Buchner A, Lai T, Steppi S, Jobb G, Förster W (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Machdar I, Harada H, Ohashi A, Sekiguchi Y, Okui H, Ueki K (1997) A novel and cost-effective sewage treatment system consisting of UASB pre-treatment and aerobic post-treatment units for developing countries. Wat Sci Tech 36:189–197. doi:10.1016/S0273-1223(97)00739-7
Machdar I, Sekiguchi Y, Sumino H, Ohashi A, Harada H (2000) Combination of a UASB reactor and a curtain type DHS (downflow hanging sponge) reactor as a cost-effective sewage treatment system for developing countries. Wat Sci Tech 42(3–4):83–88
Madigan MT, Martinko JM, Parker J (1997) Brock biology of microorganisms, vol 11. Prentice hall, Upper Saddle River, NJ
Mahmoud M, Tawfik A, Samhan F, El-Gohary F (2009) Sewage treatment using an integrated system consisting of anaerobic hybrid reactor (AHR) and downflow hanging sponge (DHS). Desalin Water Treat 4:168–176
Mei R, Narihiro T, Nobu MK, Liu WT (2016) Effects of heat shocks on microbial community structure and microbial activity of a methanogenic enrichment degrading benzoate. Lett Appl Microbiol 63(5):356–362
Narihiro T, Kim N-K, Mei R, Nobu MK, Liu W-T (2015) Microbial community analysis of anaerobic reactors treating soft drink wastewater. PLoS One 10:e0119131
Onodera T, Matsunaga K, Kubota K, Taniguchi R, Harada H, Syutsubo K, Okubo T, Uemura S, Araki N, Yamada M (2013) Characterization of the retained sludge in a down-flow hanging sponge (DHS) reactor with emphasis on its low excess sludge production. Bioresour Technol 136:169–175
Purdue University (2007) Clean Water Act (CWA). Food Processing Environmental Assistance Center. http://www.fpeac.org/cwa.html. Accessed 1 Oct 2016
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Shapiro S, Wilk M (1965) An analysis of variance test for normality. Biometrika 52:591–611
Sheu S-Y, Chen J-C, Young C-C, Chen W-M (2014) Rivicola pingtungensis gen. nov., sp. nov., a new member of the family Neisseriaceae isolated from a freshwater river. Int J Syst Evol Microbiol 64:2009–2016
Spearman C (1904) The proof and measurement of association between two things. Am J Psychol 15:72–101
Statista (2015) Per capita consumption of soft drinks in the United States from 2010 to 2014 (in gallons). https://www.statista.com/statistics/306836/us-per-capita-consumption-of-soft-drinks/. Accessed Sep. 2016
Takahashi M, Yamaguchi T, Kuramoto Y, Nagano A, Shimozaki S, Sumino H, Araki N, Yamazaki S, Kawakami S, Harada H (2011) Performance of a pilot-scale sewage treatment: an up-flow anaerobic sludge blanket (UASB) and a down-flow hanging sponge (DHS) reactors combined system by sulfur-redox reaction process under low-temperature conditions. Bioresour Technol 102:753–757
Tandukar M, Uemura S, Machdar I, Ohashi A, Harada H (2005) A low-cost municipal sewage treatment system with a combination of UASB and the “fourth-generation” downflow hanging sponge reactors. Water Sci Technol 52:323–329
Tandukar M, Machdar I, Uemura S, Ohashi A, Harada H (2006) Potential of a combination of UASB and DHS reactor as a novel sewage treatment system for developing countries: long-term evaluation. J Environ Eng 132:166–172. doi:10.1061/(ASCE)0733-9372(2006)132:2(166)
Tawfik A (2012) Polyurethane trickling filter in combination with anaerobic hybrid reactor for treatment of tomato industry wastewater. In: Zafar F, Sharmin E (eds) Polyurethane. INTECH, Chapter 16. doi:10.5772/47982
Tawfik A, Wahab RA, Al-Asmer A, Matary F (2011) Effect of hydraulic retention time on the performance of down-flow hanging sponge system treating grey wastewater. Bioprocess Biosyst Eng 34:767–776
Tebai L, Hadjivassilis I (1992) Soft drinks industry wastewater treatment. Wat Sci Tech 25(1):45–51
Ueki A, Akasaka H, Suzuki D, Ueki K (2006) Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, Gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int J Syst Evol Microbiol 56:39–44
Uemura S, Suzuki S, Abe K, Kubota K, Yamaguchi T, Ohashi A, Takemura Y, Harada H (2010) Removal of organic substances and oxidation of ammonium nitrogen by a down-flow hanging sponge (DHS) reactor under high salinity conditions. Bioresour Technol 101:5180–5185
Wang Y, Qian P-Y (2009) Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One 4:e7401
Wichitsathian B, Racho P (2010) Quantification of organic and nitrogen removal in downflow hanging sponge (DHS) systems as a post-treatment of UASB effluent. Water Sci Technol 62(9):2121–2127
Acknowledgements
We thank Kyohei Kuroda, Na-Kyung Kim, and Ran Mei at the University of Illinois at Champaign-Urbana for the valuable advice. The opinion expressed here is the authors’ perspective and does not represent PepsiCo Inc.
Author information
Authors and Affiliations
Contributions
JY, AS, and WTL designed the experiment, JL and CF conducted the experiment, and all authors contributed to the manuscript writing.
Corresponding author
Ethics declarations
Funding
This study was supported by PepsiCo Inc. to Dr. Wen-tso Liu.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 5504 kb)
Rights and permissions
About this article
Cite this article
Liao, J., Fang, C., Yu, J. et al. Direct treatment of high-strength soft drink wastewater using a down-flow hanging sponge reactor: performance and microbial community dynamics. Appl Microbiol Biotechnol 101, 5925–5936 (2017). https://doi.org/10.1007/s00253-017-8326-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8326-1