Abstract
Driven by energy neutral/positive of wastewater treatment plants, significant efforts have been made on the research and development of mainstream partial nitritation and anaerobic ammonium oxidation (anammox) (PN/A) (deammonification) process since the early 2010s. To date, feasibility of mainstream PN/A process has been demonstrated and proven by experimental results at various scales although with the low loading rates and elevated nitrogen concentration in the effluent at low temperatures (15–10 °C). This review paper provides an overview of the current state of research and development of mainstream PN/A process and critically analyzes the bottlenecks for its full-scale application. The paper discusses the following: (i) the current status of research and development of mainstream PN/A process; (ii) the interactions among aerobic ammonium-oxidizing bacteria, aerobic nitrite-oxidizing bacteria, anammox bacteria, and heterotrophic bacteria; (iii) the suppression of aerobic nitrite-oxidizing bacteria; (iv) process and bioreactors; and (v) suggested further studies including efficient and robust carbon concentrating pretreatment, deepening of understanding competition between autotrophic nitrogen-converting organisms, intensification of biofilm anammox activity, reactor design, and final polishing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Motivations
Microbial anoxic ammonium oxidation (anammox) was first discovered in Delft in the 1980s (Mulder 1989) and regarded as “one of the most startling ones in environment biotechnology” (Rittmann and McCarty 2001). It is a remarkable microbial conversion process: ammonium is oxidized using nitrite, as electron acceptor, to produce nitrogen gas by anaerobic ammonium-oxidizing bacteria (anammox bacteria (AnAOB)), which belongs to the order of Planctomycetales (Strous et al. 1999). The partial nitritation and anammox (PN/A) (also termed deammonification) process consists of two consecutive reactions: ammonium is partially oxidized to nitrite aerobically (nitrite shunt or nitrogen shortcut) by ammonium-oxidizing bacteria (AOB), and subsequently, the remaining ammonium reacts with nitrite to form nitrogen gas anaerobically by AnAOB (van Loosdrecht 2008). Compared to conventional biological nitrogen removal (nitrification/denitrification), PN/A as an autotrophic nitrogen removal process has three unique advantages (Jetten et al. 1997; Wett 2007; Daigger 2014): (i) 60% of reduction in oxygen demand for partial oxidation of ammonium to nitrite, (ii) nearly 100% elimination of carbon demand for denitrification, and (iii) 80% reduction of excess sludge.
The PN/A process has been successfully implemented in removing ammonium in dewatering liquor from anaerobic digesters in the sidestream of municipal wastewater treatment and industrial wastewater treatment. In the latter case, PN/A is usually used to remove ammonium after anaerobic wastewater treatment. As of 2014, more than 200 full-scale facilities have been operating successfully in Europe, Asia (Lackner et al. 2014), and North America (WEF/WERF 2015). Introduction of mainstream PN/A process enables the decoupling of carbon and nitrogen removal and maximizes energy recovery through carbon concentrating pretreatment process that channels more carbon to anaerobic digester (or an up-flow anaerobic sludge blanket (UASB) reactor) for biogas generation. Calculations show that the wastewater treatment process with anammox in the main stream would yield 24 watt hours per person per day (Wh/p/day), compared to a 44 Wh/p/day consumption in conventional treatment (Siegrist et al. 2008). This provides a unique opportunity to achieve efficient nitrogen removal and energy neutral/positive wastewater treatment and substantial reduction in operating costs for the supplemental carbon dosing to efficiently remove nitrogen of low C/N ratio wastewater. Since late 1990s, proposals for mainstream PN/A applications have been suggested (Jetten et al. 1997; Siegrist et al. 2008; Kartal et al. 2010; Vlaeminck et al. 2012), and since the early 2010s (De Clippeleir et al. 2011; Ma et al. 2011; Winkler et al. 2012a), a great deal of efforts has been made in the research and development, and significant progress has been achieved (Stinson et al. 2013; Xu et al. 2015; Ma et al. 2015a).
Challenges in implementing PN/A in main stream
The first challenge is the high C/N ratio (7–12, g COD/g N) (Metcalf and Eddy 2003) of municipal wastewater after primary settling compared to that (<1 g COD/g N) (Lackner et al. 2014) of most sidestream PN/A process (Vlaeminck et al. 2012; Xu et al. 2015; Ma et al. 2015a). The high C/N ratio can result in a large fraction of heterotrophic bacteria (HB) in the sludge resulting in reductions of the populations and activities of AnAOB and AOB and low nitrogen removal rate (NRR). In contrast to sidestream process where the free ammonia (FA) and free nitrous acid (FNA) concentrations are higher than the threshold values (0.08–0.82 mg FA/L and 0.06–0.83 mg FNA/L) to inhibit nitrite oxidation bacteria (NOB) (Anthonisen et al. 1976) that results from the high ammonium concentration (500–1500 mg NH4-N/L) (Lackner et al. 2014), the lower ammonium concentration (30–100 mg NH4-N/L) (Metcalf and Eddy 2003) of municipal wastewater makes the NOB suppression difficult and becomes another major challenge in mainstream PN/A process (Vlaeminck et al. 2012; Stinson et al. 2013; Gilbert et al. 2014a; Lotti et al. 2015a; Xu et al. 2015; Ma et al. 2015a). Also, the low activity of AnAOB especially at low temperature (<15 °C) and seasonal temperature variation of municipal wastewater (10–25 °C) (Vlaeminck et al. 2012) compared to the high temperature (∼30 °C) of sidestream process are challenging (Kartal et al. 2010; Vlaeminck et al. 2012; Gilbert et al. 2014a; Xu et al. 2015; Ma et al. 2015a). The specific activity of AnAOB can drop approximately ten times when the temperature declined from approx. 30 to 10 °C (Lotti et al. 2015a) while a sharper activity decline was observed at 15 °C (Dosta et al. 2008; Lotti et al. 2015a). On the average, the maximum specific growth rate of AnAOB (0.05–0.09/day at 30 °C) (Strous et al. 1999; van der Star et al. 2008) is ten times lower than that of AOB (0.7–0.9/day, at 20–30 °C) (Wiesmann 1994; Park and Noguera 2007). Furthermore, the activity-temperature coefficient θ (defined by r 1 = r 2 × θ (T1 − T2), r: specific activity) of AOB, NOB, and AnAOB activities irregularly varied between 10.7 and 1.20 after acclimation to 10, 20, and 30 °C (Gilbert et al. 2015). These differences in temperature dependencies on growth rates can lead to imbalance of population and activities of key groups (Gilbert et al. 2015; Lotti et al. 2015a; Lackner et al. 2015), cause process vulnerability, and become hard to retaining enough AnAOB within the reactor that are extremely critical especially for “cold anammox” (20–10 °C) process. Last but not the least is the final effluent ammonium, nitrite, and nitrate concentrations of mainstream PN/A process in meeting the strict discharge standards and reuse requirements.
What has been achieved and the major barriers?
Table 1 shows the performance of laboratory-, pilot-scale, and different types of PN/A and anammox reactors fed with different influent C/N (g COD/g N) ratios. The laboratory systems fed with synthetic wastewater without additional carbon (C/N = 0) to avoid side effects of heterotrophic growth and operating between 30 and 20 °C (“warm anammox”) achieved high total nitrogen (TN) removal (70–90%) in anammox biofilm reactor (Guillén et al. 2015) and anammox and PN/A granular sludge reactor (Lotti et al. 2014a). Also, a similar performance was achieved in anammox granular sludge reactor (Ma et al. 2013), PN/A biofilm (Li et al. 2016), and suspended sludge process (Han et al. 2015; Han et al. 2016) fed with low BCOD/N influent ratio (≤2–3, g Chemical oxygen demand of biodegradable organics (BCOD) /g N) after carbon pretreatment process. Two full-scale mainstream PN/A processes: Strass Wastewater Treatment Plant (WWTP), Austria, which was augmented for AOB and AnAOB from the sidestream deammonification process to the mainstream process (Wett et al. 2013), and the 200,000 m3/day step-feed activated sludge process in Changi Water Reclamation Plant (WRP), Singapore, with wastewater temperature of 28–32 °C year-around under tropical climates (Daigger et al. 2008; Cao et al. 2013) achieved ≥85% of TN removal, demonstrating the high potential for full-scale application although the nitrogen removal was likely enhanced by the conventional denitrification significantly (75% of TN removal via nitrite path, Wett et al. 2015; 27.1% of TN removal through conventional denitrification for Changi WRP, Cao et al. 2014) due to the high influent C/N ratio (7–12 g COD/g N) (Regmi et al. 2014a; Cao et al. 2013). In general, the results of warm anammox are more promising compared with those of cold anammox. The performance of some laboratory PN/A systems such as granular sludge reactor operating at 18 °C (Winkler et al. 2012a) and hybrid moving bed biofilm reactor (MBBR) operating at 15 °C (Laureni et al. 2016) still achieved efficient TN removal (> 70%). Pilot-scale and prototype PN/A (reactor volume 50 m3) processes, fed with low C/N influent ratio after the carbon pretreatment process, have achieved effective TN removal (70–50%) and NRR (0.1–0.3 kg N/m3 day) at temperature between 23 and 17 °C (Lotti et al. 2014b; Geilvoet et al. 2015; Veuillet et al. 2015). The performance of the latter is similar to or even higher than that (0.05–0.10 kg N/m3 day) in conventional nitrification/denitrification process (ATV 1997). The reduction of TN removal efficiency of pilot-scale process compared to those of laboratory- scale process reflects the scale-up effects. Augmentation proved to be effective in NOB suppression and TN removal (Veuillet et al. 2015), but neither of the detail mechanisms involved, design made, and operational guidelines adopted was reported. The performance and NRR deteriorate mainly below 15 °C. Hendrickx et al. (2014), Lotti et al. (2014a), Gilbert et al. (2014a), Gao et al. (2015), and Lackner et al (2015) reported that both effluent nitrite and nitrate concentrations increased and NRR was insignificant at approximately 10 °C due to the drastic decrease of anammox activity approx. below 15 °C (Dosta et al. 2008; Gilbert et al. 2014a; Lotti et al. 2015a) and relatively active NOB at low temperatures (Isanta et al. 2015; Ma et al. 2015a). The studies on final polishing to remove residual ammonium, nitrate, the side product of anammox, and nitrite are also at its infancy and in absence of which large amount of disinfectant is expended in reuse application such as NEWater production in Singapore. In summary, the progress achieved so far demonstrates the feasibility of mainstream PN/A process applications even under low temperature, but more research and development are still needed.
This review paper attempts to update the readers with the overview of the current state of research and development of mainstream PN/A process, i.e., what has been achieved in knowledge and applications in microbiology and process technology and what are reaming as the bottlenecks for scale-up for full-scale application. Following the brief introduction, the microbiology knowledge generated on key microbial groups (AOB, NOB, AnAOB, and HB) involved in the PN/A process and their dynamics of interaction are described. Also, the effects of influent C/N ratio on the process performance are discussed. The suppression of NOB through kinetics and other factors is discussed and analyzed using practical cases after that in detail. Then, the major characteristics and performance of the different process (single-stage and two-stage processes) and the reactor configurations (suspended sludge, biofilm, and hybrid) implemented to date are analyzed. The final section highlights the key areas of further studies required to unblock the bottlenecks and to promote successful scale-up for full-scale application of mainstream PN/A process in wastewater treatment plants.
Key microbial groups and interactions
The AOB, NOB, AnAOB, and HB are the four major microbial groups involved in the mainstream PN/A process (Fig. 1), a typical multi-culture and multi-substrate process (van Loosdrecht et al. 2014). AOB, NOB, and HB (including heterotrophic denitrifiers and possibly phosphorus-accumulating organism (PAO) and glycogen-accumulating organism (GAO)) compete for oxygen; AOB and AnAOB compete for ammonium; NOB, AnAOB, and heterotrophic denitrifiers compete for nitrite, the limiting substrate of AnAOB in most instances; and heterotrophic denitrifiers and ordinary heterotrophic organism (OHO: HB except heterotrophic denitrifier) compete for organic carbon. The pattern and degree of variations in activity due to temperature change either at high (>20 °C) or low (15–10 °C) temperatures are microbial group- and species-dependent. Understanding the activity-temperature relationships of the key microbial groups is essential to develop potential optimization strategies of process design and operation.
Substrate competition among AOB, NOB, AnAOB, and HB (after Vangsgaard 2013)
Aerobic ammonium oxidizing bacteria and nitrite-oxidizing bacteria
Table 2 shows Monod growth kinetic parameters of AOB and NOB. Most of the parameter values are intrinsic (with little mass transfer effect when biomass floc size is approx. 40–50 μm (Beccari et al. 1992; Blackburne et al. 2007; Blackburne et al. 2008b) exhibiting the growth characteristics of the microorganisms. Inhibition and toxicity by FA and methane and high or low pH to AOB and free hydroxylamine, FA, and FNA to NOB observed in sidestream process (Lackner and Agrawal 2015) are not likely to occur for mainstream PN/A process (Vlaeminck et al. 2012). Nitrosomonas with μ max as high as 2.1/day has the potential to grow more than twice as fast as Nitrosospira with μ max varying between 0.7 and 0.9/day in the optimum temperature range (25––30 °C) (Siripong and Rittmann 2007). Nitrosomonas europaea/eutropha can outcompete other AOB species for NH4–N (Watson et al. 1989).
Recent studies indicate Nitrospira as the dominant NOB species in wastewater systems (Daims et al. 2001; Siripong and Rittmann 2007; Ward 2008). K NH4 of 0.64 mg NH4-N/L and K NO2 of 0.16 mg NO2-N/L of Nitrosospira and Nitrospira spp., respectively (Table 2), are lower than those of Nitrosomonas–AOB and most Nitrobacter spp. Hence, Nitrosospira and Nitrospira spp. could indeed be regarded as typical K strategists, while Nitrosomonas–AOB and Nitrobacter–NOB are regarded as the r strategists (Schramm et al. 1999; Dytczak et al. 2008). This may explain why Nitrobacter is a superior competitor when resources are abundant, while Nitrospira thrive under conditions of resource scarcity (Schramm et al. 1999; Nogueira and Melo 2006; Kim and Kim 2006; Blackburne et al. 2007; Huang et al. 2010; Nowka et al. 2015). It was reported that the specific nitrite oxidation activities of Nitrobacter and Nitrospira were 93.8 and 10.5 mg/g NOB h at 22–26 °C, respectively (Kim and Kim 2006).
The AOB and NOB microbial communities and corresponding intrinsic kinetics can shift and change along with changes of the environmental and imposed operational conditions. Temperature has significant effects on the diversity and kinetics (typically on μ max). The widely used relationships in sidestream PN/A process of the minimum sludge retention time (SRT) and temperature of AOB and NOB (Hellinga et al. 1998), which indicates the μ max.AOB >that of NOB at high temperatures (>∼20 °C), while the μ max.NOB >that of AOB at low temperatures (<∼20 °C), were based on Hunik (1993), where N. europaea and Nitrobacter agilis represented AOB and NOB, respectively. In mainstream PN/A research, it was found that at high temperature, the activities of AOB were higher than that of NOB (Regmi et al. 2014a; Yang et al. 2016) even under low dissolved oxygen (DO) condition (Gilbert et al. 2015). However, under the moderate and low temperatures, the activities (ex situ or in situ) of AOB could still be higher than NOB (De Clippeleir et al. 2013; Lotti et al. 2014a; Gilbert et al. 2014a; Gilbert et al. 2015). These discrepancies may be explained by two facts: (i) enrichment cultures of N. europaea and Nitrobacter agilis used by Hunik (1993) (Table 2) and a single temperature coefficient were adopted in formulating the temperature dependency relationship (Hellinga et al. 1998) and (ii) the dominance of Nitrospira–NOB rather than Nitrobacter–NOB was found in the mainstream process at moderate and cold temperature (Huang et al. 2010; De Clippeleir et al. 2013; Gilbert et al. 2014a; Gilbert et al. 2015), which could have different growth kinetics and temperature dependency from Nitrobacter–NOB. More studies are needed on the growth kinetics and temperature dependence of Nitrospira–NOB (and the mixed culture of Nitrospira and Nitrobacter) at low temperature (<15 °C).
Also, DO significantly affects the diversity and kinetics of AOB and NOB (Park and Noguera 2008; Bellucci et al. 2011; Liu and Wang 2013). After prolonged (300 days) operation at the high-DO (<8.5 mg/L) and the low-DO (0.12–0.26 mg/L) enrichment chemostat, AOB community belonging to N. europaea lineage exhibited different oxygen affinities and growth kinetics demonstrating that a direct correlation between AOB phylogeny and DO cannot be established at the lineage level (Park and Noguera 2004). Also, Nitrospira community shifted to group 2 from group 1 in the high-DO reactor after half year of operation, while there was no significant change to group 1 of Nitrospira community in the low-DO reactor (Park and Noguera 2008). It was reported that after more than a year of low DO operation (from the previous high-DO condition), N. europaea/eutropha remained as the dominant AOB, while the population increase of Nitrospira-like NOB was greater than Nitrobacter-like NOB as a result of the reduced nitrifier endogenous decay rate and low DO operation (Liu and Wang 2013).
Recently, Holger et al. (2015) and Van Kessel et al. (2015) reported the existence of “complete ammonia oxidizer (comammox),” a specific species of Nitrospira, which can perform both nitritation and nitratation. Key functional genes of these comammox Nitrospira have been detected in diverse terrestrial and aquatic environments as well as in new metagenomes from wastewater and drinking water treatment plants (Holger et al. 2015). Also, the coexistence of ‘comammox Nitrospira with AnAOB has been observed (Van Kessel et al. 2015). The exact role in PN/A processes of these organisms is yet to be evaluated.
Anammox bacteria
The optimum growth temperature for AnAOB was between 30 and 35 °C when enriched at 30 °C (Jetten et al. 2001). When the temperature reduced from approx. 30 to 10 °C, there was substantial decline in the ex situ maximum specific activity of granular AnAOB (0.08 at 30 °C to 0.01 g N2/g volatile suspended solid (VSS) day at 10 °C) in a laboratory air lift reactor; free cell AnAOB (1.6 g N2/g VSS day at 30 °C to 0.15 g N2/g VSS day at 10 °C) in a laboratory membrane biological reactor (MBR) (Lotti et al. 2015a) and in the in situ maximum anammox activity (465 mg N/L day at 29 °C versus 46 mg N/L day at 12.5 °C) in a laboratory hybrid reactor (Laureni et al. 2015). The activity-temperature dependency increased markedly at the lower temperatures between 15 and 10 °C, and the decline was greater than that of AOB (Lotti et al. 2015a). As shown in Table 1 and mentioned previously, AnAOB activity at low temperature of 12 and 10 °C was observed in anammox reactors (Hendrickx et al. 2014; Laureni et al. 2015) and in PN/A reactors (Hu et al. 2013; Gilbert et al. 2014a; Lotti et al. 2014a; Gao et al. 2015; Lackner et al. 2015), and Candidatus Brocadia fulgida were observed as the dominant AnAOB (Hu et al. 2013; Hendrickx et al. 2014; Lotti et al. 2014a). The optimum temperatures of AnAOB at low temperature observed by Hu et al. (2013) and Gilbert et al. (2014a) were 12 and 10 °C, respectively, likely due to acclimatization (Gilbert et al. 2014a). Low-yield and high maintenance energy requirement of AnAOB at low temperature were proposed from the observations of decrease of the ratio of nitrate formed to ammonium removed along with temperature reduction (Hu et al. 2013). The mechanistic reasons explaining activity decline from both kinetics and stoichiometry have not been elucidated yet. Nitrite was observed to accumulate in laboratory-scale anammox sequence batch reactor (SBR) at 15 °C (Dosta et al. 2008) and in PN/A MBBR and suspended sludge SBR with temperature decline from 13 to 10 °C (Gilbert et al. 2014a; Lackner et al. 2015). Also, it was reported that the system lost its stable structure of biofilm and granular sludge at 15 °C in an anammox SBR (Dosta et al. 2008). Corresponding to 0.03–0.05 g N2/g VSS day of specific activity of anammox floc sludge enriched at 10 °C (Hendrickx et al. 2014), an AnAOB biomass concentration of 3–5 g Mixed liquor suspended solids (MLSS)/L is needed to match a conventional nitrification/denitrification process performance at a wastewater temperature of 10 °C (Hendrickx et al. 2014). Obviously, conventional suspended system with conventional clarifier cannot maintain similar biomass concentration level in the reactor. Thus, it is essential to immobilize AnAOB (forming biofilm). Gilbert et al. (2015) reported that the biomass concentration in a MBBR at temperatures between 15 and 10 °C was maintained stably at approx. 9 g/L (more than twice that of the suspended sludge system). Furthermore, there was not much decrease in the population of AnAOB growing on the carrier material (Gilbert et al. 2015), whereas for a suspended sludge system, a 100-fold decrease in the AnAOB (16S ribosomal RNA (rRNA) copy numbers) was observed when the temperature decreased from 25 to 12 °C (Hu et al. 2013). Lackner et al. (2015) and Laureni et al. (2016) reported that the anammox activity in the biofilm of PN/A MBBR was recovered when temperature increased from 10 to 15 °C, while it did not in the suspended sludge SBR (Lackner et al. 2015). These experimental results illustrate the advantages and necessity of biomass immobilization. Furthermore, the low NRR and elevated nitrogen concentration in the effluent of mainstream PN/A that occurred at low temperature (Hendrickx et al. 2014; Lotti et al. 2014a; Gilbert et al. 2014a; Gao et al. 2015; Lackner et al. 2015) illustrate that the current biofilm process is still unable to maintain reasonable anammox activity and PN/A process performance at low temperature, and new approaches to enhance biofilm anammox activity are needed to develop.
Suspended and free cell AnAOB have faster growth rate and higher activity than granular/biofilm anammox bacteria (van der Star et al. 2008; Cao et al. 2013; Gilbert et al. 2015; Lackner et al. 2015). One startling example is that the maximum specific activity (1.6 g N2/g VSS day) of free cell AnAOB enriched in a MBR operating at 30 °C was almost 20 times higher than that (0.08 g N2/g VSS day) of the granular sludge in the internal cycle (IC) anammox reactor operating at 30–35 °C in Rotterdam-Dokhaven WWTP (Lotti et al. 2015a). Recently, Lotti et al. (2015b) reported the highest maximal growth rate (μ max) of 0.334/day at 30 °C for Brocadia sp. 40. Also, the same species was found dominantly in the mainstream PN/A step-feed activated sludge process operating at an anoxic SRT of 3 days in the Changi WRP, Singapore (Cao et al. 2014). Thus, AnAOB can no longer be regarded as intrinsically slow-growing microorganisms (Lotti et al. 2015b). Despite the proof of higher activity of suspended/free cell AnAOB certainly at lower temperatures (Lackner et al. 2015), immobilization is still necessary due to oxygen inhibition in a single-stage mainstream PN/A. The potential influences of suspended AnAOB in a biofilm reactor performance of a mainstream PN/A process, which was largely overlooked in the past, have recently gained attentions (Hubaux et al. 2015; Corbalá-Robles et al. 2016). For inhibition and toxicity of oxygen, nitrite and organic substance (i.e., methanol, etc.) to AnAOB, Lackner and Agrawal (2015) may be referred.
Kartal et al. (2007) and Winkler et al. (2012b) observed AnAOB as the dominant species in reactors fed with influent containing HAc and NO3. High anammox nitrogen removal was also observed in the anoxic phase of laboratory granular anammox reactors fed with an influent acetate-COD/N ratio of up to 6 at both 22 and 30 °C (Guillén et al. 2014). This shows that AnAOB can effectively outcompete heterotrophic denitrifiers. Kartal et al. (2012) attributed to the substrate affinities of the AnAOB (K NO2.AnAOB 0.05 mg N/L) and denitrifier (K NO2.Denitrfier 0.3 mg N/L). However, the mechanisms, quantitative description, and analysis of these phenomena remain to be investigated (Kartal et al. 2012). Recent study shows that appropriate Fe(II) dosing (i.e., 0.09 mM) significantly enhanced the specific anammox growth rate up to 0.172/day compared to 0.118/day at regular Fe(II) level (0.03 mM) at 35 °C (Liu and Ni 2015). This new Fe(II)-based strategy would be likely to counteract the decrease of anammox activity resulting from low temperature, although this still warrants further experimental verification (Liu and Ni 2015).
Heterotrophic bacteria
In mainstream PN/A process, controlling heterotrophic growth is one of the decisive design aspects. The presence of HB in the reactor is inevitable (∼50% of population) even in the absence of exogenous carbon, due to release of the endogenous soluble microbial decay products (Kindaichi et al. 2004; Gilbert et al. 2014a). The influent C/N ratio is the most relevant parameter to control the growth of HB in mainstream PN/A process and to energy recovery. The carbon concentrating pretreatment process is the key unit to control influent C/N ratio. The tolerant range of C/N ratio in literature varies partially due to the inconsistent definitions of types and compositions of influent carbon matter. However, for an effective anammox-based process, an influent BCOD/N ratio of approx. ≤2–3 should be maintained for the suspended sludge system (Desloover et al. 2011; Vlaeminck et al. 2012; Guillén et al. 2014; Li et al. 2016; Han et al. 2016). Higher influent BCOD/N ratio compromises maximizing energy recovery although may increase TN removal (Han et al. 2016), benefits AnAOB due to oxygen depletion (Stinson et al. 2013), and accommodates excessive biological phosphorus removal (Cao et al. 2016a; Cao et al. 2016b). Under this situation, nitrite shunt (shortcut) may be a simple and proper alternative (Jimenez et al. 2014; Seuntjen et al. 2016). For biofilm and hybrid systems, a higher influent C/N ratio appears to be tolerated as HB in the liquid phase get washed out together with solids through short SRT control (Lotti et al. 2014b; Geilvoet and Hendrickx. 2015; Veuillet et al. 2015), but nitrite can react fast in the liquid phase should there be biodegradable COD overflow from carbon pretreatment unit, while recent study using a metagenomic assembly further supports the hypothesis of “nitrate loop,” i.e., some denitrifiers can reduce the excessive nitrate formed by NOB and AnAOB as electron acceptor for the degradation of certain organic matter (e.g., fermentation products) in the granule core (Winkler et al. 2012b) producing nitrite for AnAOB (Speth et al. 2016).
Suppression of nitrite oxidizing bacteria
Residual ammonium concentration
Maintaining the required level of residual ammonium concentration (RAC) is critical to the performance of mainstream PN/A process as it is the precondition to (i) suppress NOB through manipulating DO or oxygen supply (flux) (Pérez et al. 2014; Welker et al. 2016) and kinetic advantage of AeAOB (Poot et al. 2016) and (ii) maintain the activities of both AOB and AnAOB (Lotti et al. 2014a; Pérez et al. 2014). Recently, Wu et al. (2016) reported fast growing r strategists -AOB were enriched in a laboratory suspended activated sludge PN process operating under high RAC. The required RAC is maintained through control of aeration duration (Blackburne et al. 2008b; Ma et al. 2015b). A RAC of approx. 2 mg NH4-N/L is practiced (Stinson et al. 2013; Cao et al. 2013; Regmi et al. 2014a) for suspended sludge process. A value of RAC of >5 mg NH4-N/L was suggested for granular sludge system (Poot et al. 2016). Maintaining high ammonium levels above 2–5 mg NH4-N/L may request a final polishing when strict discharge standards were imposed although the residual ammonia may favor saving disinfection chemicals when chloramination is used for disinfection in the case of water reuse.
Dissolved oxygen and oxygen/ammonium flux ratio
In conventional nitrification, ammonium was limiting substrate and was completely oxidized to nitrate (Schramm et al. 1999). In a mainstream PN/A process, manipulation of the competition for oxygen between AOB and NOB under oxygen-limiting condition is one of the main strategies to suppress NOB (Pérez et al. 2014). For the suspended sludge system, AOB and NOB are present in liquid phase as flocs/small aggregates with limited mass transfer resistance. The competition for oxygen between AOB and NOB is, to a large extent, governed by intrinsic kinetics compared to biofilm system. The suppression of NOB primarily depends on faster oxygen uptake rate by AOB compared to that of NOB. In most cases, Nitrosomonas was reported to be the dominant genus of AOB (Bellucci et al. 2011) for nitrification in activated sludge process although the lineage and strains could be different (Park and Noguera 2004). The dominant genus of NOB varies. The specific growth rate of r strategists Nitrosomonas–AOB (Dytczak et al. 2008; Ahn et al. 2008) is higher than that of r strategists Nitrobacter–NOB corresponding to high operating dissolved oxygen (DO) (≥1.5 mg/L) (Dytczak et al. 2008; Ahn et al. 2008) leading to dominant AOB growth and suppression of NOB as reported by Dytczak et al. (2008), Ahn et al. (2008), Wett et al. (2013), and Cao et al. (2014). The operating moderate (<1.0 mg/L) and low (<0.5 mg/L) DO favors Nitrosomonas–AOB and suppresses NOB but selecting K strategists Nitrospira–NOB (grow at the rate close to the maximum) not r strategists Nitrobacter (grow at the rate far from the maximum) as reported by Huang et al. (2010), Dytczak et al. (2008), Sliekers et al. (2005), Kindaichi et al. (2007), and Gilbert et al. (2015). High nitrite concentration accumulated in the liquor phase often selects Nitrobacter as the dominant NOB due to its higher specific rate corresponding to the nitrite concentration than that of Nitrospira when nitrite affinity was involved in the kinetics (Blackburne et al. 2007; Blackburne et al. 2008a; Nowka et al. 2015) as reported by Nogueira and Melo (2006), Kim and Kim (2006), and Huang et al. (2010). Suppression of NOB is still achievable when DO varied approximately between 1.5 and 0.5 mg/L with the coexistence and change in relative abundance of Nitrobacter and Nitrospira as reported by Daims et al. (2001), Regmi et al. (2014a), and Ma et al. 2015b). However, suppression of NOB will become difficult when the specific growth rates of AOB and NOB at the operating DO set point are close to each other regardless of the controlling level of DO in the liquid phase if the process is oxygen limited only.
In biofilm system AOB, NOB and AnAOB compete for spatial space. Under aerated conditions, AOB grew on the outer surface layer, while NOB slightly deeper (several μm) and AnAOB inside the anoxic interior as verified by molecular microbial observation (Vlaeminck et al. 2010; Winkler et al. 2011; Poot et al. 2016) and mathematical simulation (Hao et al. 2002; Volcke et al. 2010). As such, substrates have to diffuse to these layers in order for conversions to occur. The substrate gradients, mass transfer, and kinetics determine the dominance, coexistence, or suppression/washout of bacteria. Importantly, layered structure of biofilm is needed in order to outcompete NOB (Picioreanu et al. 2016). Due to the mass transfer effect, more Nitrospira dominance (due to low DO) was reported (Kindaichi et al. 2007; De Clippeleir et al. 2011; De Clippeleir et al. 2013; Gilbert et al. 2015) compared to Nitrobacter-dominant cases (Isanta et al. 2015). Similarly, the dominance of Nitrosospira–AOB rather than Nitrosomonas–AOB was found in biofilm (Schramm et al. 1999) sometimes.
The oxygen/ammonium flux ratio-based approach of suppressing NOB by providing little oxygen to NOB (Sliekers et al. 2005; Pérez et al. 2014; Poot et al. 2016), mainly applicable for biofilm systems due to their layered structure, is more convincing than controlling only the DO in liquid phase. This approach has been verified in laboratory PN biofilms (Okabe et al. 2011; Isanta et al. 2015; Poot et al. 2016) and PN/A MBBR (Gilbert et al. 2015) and also studied via mathematical simulation (Vangsgaard et al. 2012). Model-based studies (Hao et al. 2002; Vangsgaard et al. 2012; Pérez et al. 2014; Isanta et al. 2015; Corbalá-Robles et al. 2015) illustrated that the interdependent relationship between the minimum ammonium flux (NH4min) and the DO flux in the bulk liquor constitutes the “operational window” of the process operation to suppress NOB for biofilm system. Growth kinetics of AnAOB, AOB, and NOB; temperature’ and thickness of biofilm influence the boundary of the “window” as conceptually proven by experimental results. This illustrates that changes in ammonium load (e.g., to cope with low anammox activity in winter) must cope with oxygen supply, etc. In fact, a higher ammonium flux or concentration may be used as a temporary intervention to suppress NOB in winter (Pérez et al. 2014).
Microorganism growth kinetics provide a useful tool to study the competition, population shift, and dominance of AOB and NOB. However, cautions may be needed when using kinetics to analyze the competition between AOB and NOB. Using oxygen affinity such as K O2.AOB and K O2.NOB to analyze species dominance is feasible only when oxygen is limiting substrate, while ammonium for AOB and nitrite for NOB are in excess (Blackburne et al. 2007; Liu and Wang 2013; Pérez et al. 2014). Both ammonium and nitrite should be taken into account when the kinetics is under dual limitation as demonstrated by Al-Omari et al. (2015). In many cases, μ max/K S (S: limiting substrate) is an appropriate expression of specific growth rate and adopted in modeling (Sliekers et al. 2005; Pérez et al. 2014). Broadly speaking, taking both biomass populations and substrate conversions into account, the kinetic parameter evaluation should include μ max, K NH4.AOB., and K NO2.NOB in addition to K O2, b (decay constant), and Y (yield coefficient) (Bellucci et al. 2011). A wide range of kinetic parameter values is reported for AOB and NOB (Brockmann et al. 2008b; Pérez et al. 2014), but the prediction of nitrifying community is still not successful in many occasions (Okabe et al. 2011) due to lack of understanding the factors influencing the kinetics, namely sources and characteristics of the biomass (pure, enriched, mixed culture, lineages of the same genus, etc.) (Nowka et al. 2015; Park and Noguera 2004; Park and Noguera 2008); structure of floc and biofilm (Dytczak et al. 2008; Vazquez 2016); microcolony size, number, and spatial distribution of AOB and NOB in biofilm (Picioreanu et al. 2016) and wastewater (Okabe et al. 2011); overlap of influencing environment factors (Stinson et al. 2013; Ma et al. 2015a); and methods of experiment and parameter estimation (Guisasola et al. 2005). Apparently, deepening of understanding competition between autotrophic nitrogen-converting organisms is still critically essential for research and development of mainstream PN/A. A critical revisit and evaluation on the existing intrinsic kinetics is helpful. To be cost-effective, kinetic studies should be conducted under the relevant site conditions. The intrinsic growth kinetics and stoichiometry of Nitrospira-like NOB under low temperature (Gilbert et al. 2014a) can be an appropriate starting point of further studies.
Transient anoxia
Transient anoxia due to on/off aeration has been proven as an effective method to outcompete NOB. The lag phase in NOB activity at the beginning of the aeration phase is due to (Stinson et al. 2013) the following: (i) the absence of one or both of the substrates (nitrite and oxygen) (Gilbert et al. 2014b; Malovanyy et al. 2015) and (ii) inactivation of metabolic mechanisms in NOB recovery and NOB lag adaptation compared to AOB in aerobic conditions following transient anoxia (Kornaros et al. 2010). For suspended sludge systems, intermittent aeration based on on-line control of NO x (NO2 − + NO3 −)/NH4 + ratio (approx. 1) in the PN reactor of a two-stage PN/A process (Regmi et al. 2014a) is a typical example. Delay of Nitrospira activity after the anoxic phase (between 5 and 15 min) effectively suppressed NOB presence in a PN/A SBR process (Kornaros et al. 2010; Gilbert et al. 2014b). Intermittent aeration also seemed to be effective in integrated fixed-film and activated sludge (IFAS) (Trojanowicz et al. 2016). Simulation shows high TN removal (∼90%) with continuous when compared to intermittent aeration (68–80%) due to the oxygen inhibition during intermittent aeration to AnAOB (Corbalá-Robles et al. 2016). This oxygen inhibition will be less or absent when the nitrifying population is growing on the biofilm instead of in IFAS.
FNA inhibition
AOB were generally more tolerant to FNA than NOB under aerobic conditions (Vadivelu et al. 2006). Wang et al. (2014) found that under anoxic conditions, FNA was substantially more biocidal to NOB than to AOB. Wang et al. (2016) reported when one fourth of the sludge in the reactor was treated daily with FNA at 1.82 mg N/L in a sidestream unit for 24 h, approx. 80% of nitrite accumulation ratio and significant NOB population decrease were achieved in the mainstream nitritation reactor operating at low DO (0.3–0.8 mg/L) and a temperature of 22 °C. More experiments operating under site conditions and economic assessment are needed prior to its application.
Aerobic sludge retention time
Short aerobic SRT has been adopted to suppress and wash out NOB due to the higher growth rates of AOB compared to those of NOB in sidestream anammox process (Hellinga et al. 1998). An aerobic SRT of 2.5 days in a step-feed activated sludge process between 28 and 30 °C is one of the major contributing factors among others for the robust nitrogen shortcut at Changi WRP, Singapore (Cao et al. 2013). Full-scale nitrogen shortcut activated sludge process operating at an aerobic SRT of 3.5 days in the St. Petersburg Southwest WWTP, USA (Jimenez et al. 2014), is another reference of such operation. Currently, there is some tendency to use “aggressive” (short) aerobic SRT as an intervention to suppress NOB even under moderate and cold temperatures (Stinson et al. 2013), likely, taking the advantage of fast kinetics of AOB through a high ammonium concentration. However, to date, little experimental information on the duration required for the process to response and the ammonium concentration during the intervention period is reported.
Augmentation
Suppression of NOB and enhancement of AnAOB and AOB populations and activities in the mainstream PN/A process by transferring of AnAOB and AOB from the sidestream deammonification to the mainstream and retaining them in the mainstream by cyclone (Wett et al. 2013) or alternating feeding (cyclic exchange) of biomass (grow on carrier materials) and liquor between the mainstream and sidestream PN/A processes (Lemaire et al. 2013; Veuillet et al. 2015) have been implemented. The reduction of nitrite and nitrate concentrations in the effluent shows the feasibility. It may help to enhance the mainstream PN/A performance during the winter season. However, the differentiation on the contributions to mainstream PN/A process through increasing AOB and AnAOB population or FA and FNA inhibition from the sidestream is still unknown. Little information is available on the effects of temperature difference between the side and mainstream on the efficiency of augmentation. More studies on the mechanisms are needed to develop optimized operational strategies especially during the winter season.
Real-time aeration control
A variety of real-time aeration control strategies has been developed and applied to suppress NOB through DO setting or oxygen supply (flux). The response parameters linked to the DO or oxygen supply include the following: ammonium flux (Lackner et al. 2008), ratio of NO2 − to NO x , NO x to NH4 + (Regmi et al. 2014a), NO3 − produced to NH4 + removed (Veuillet et al. 2015), and pH change with time interval (dpH/dt) (Yang et al. 2007). In addition, the failures of mechanical equipment such as blowers (Veuillet et al. 2015) or pumps (Lotti et al. 2014b) resulted in serious operational disruption, illustrating the importance of key equipment reliability for a stable mainstream PN/A process.
In many cases, the suppression of NOB is the complexed integrated effect of multiple factors (Stinson et al. 2013; Cao et al. 2013; Wu et al. 2016). This increases the difficulties in the understanding and analysis of the mechanisms involved and is an area requiring further studies. Given that the PN/A process occurs at the aerobic/anoxic/anaerobic interphases, the potential involvement of ammonia-oxidizing archaea (AOA) (Park et al. 2006; Daigger and Littleton 2013), comammox (Holger et al. 2015,) and mixotrophic nitrification and denitrification (Stein 2011; Bellucci et al. 2011; Fitzgerald et al. 2015) should not be excluded from the scope of the studies.
Process and reactor
Carbon concentrating pretreatment process
The carbon concentrating pretreatment processes may include the following: (i) high-rate activated sludge (HRAS) such as the A-stage activated sludge process (SRT ∼0.5 days; hydraulic retention time (HRT) ∼0.5 h) in Strass WWTP with approx. 60% of COD removal (Wett et al. 2013); (ii) chemical-enhanced primary treatment (CEPT), which can remove about 80 to 90% for TSS and 50 to 70% for COD removal (Kroiss and Cao 2014); and (iii) UASB reactor aiming at maximizing energy recovery (Malovanyy et al. 2015; Guillén et al. 2015). In general, the influent BCOD/N ratio of approx. ≤2–3 can be met with application of these three types of properly designed pretreatment process. But different issues remain. HRAS is most widely applied as a carbon concentrating pretreatment process. Laureni et al. (2015) reported that the heterotrophic growth due to the residual carbon of the HRAS effluent is unable to impair AnAOB activity. However, carbon mineralization can account for up to 20 to 30% of the influent carbon (Roest et al. 2012; Jimenez et al. 2015), which minimizes the benefits of energy recovery, and a mechanistic understanding on influent characteristics and removal efficiency is still not available. The major issue for CEPT is the removal efficiency when wastewater contains high soluble COD concentration. The disadvantage of the UASB reactor is a large fraction of dissolved methane remaining in the liquid. Moreover, sulfide and methane removals in downstream process have yet to be resolved although some potential technologies and ideas have been proposed (Vela et al. 2015). The occasional high organic matter or suspended solid loading from the outflow pretreatment process into the downstream PN/A reactor can stimulate heterotrophic growth on the surface of the granules/biofilm (Liang et al. 2014) and causes the washout of active PN/A (granular) sludge (Geilvoet and Hendrickx 2015; Geilvoet et al. 2015) calling for a robust on-line control system.
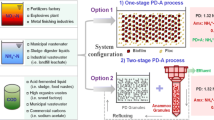
Single- and two-stage process
A single-stage PN/A process performs PN/A in one reactor, while a two-stage PN/A process separates the PN/A reactions in two reactors. Typical examples of two-stage PN/A process include the following: suspended sludge process for PN and biofilm for anammox (Ma et al. 2011; Regmi et al. 2014a), biofilm for both PN and anammox (Okabe et al. 2011), and biofilm for both PN and anammox with augmentation (Piculell et al. 2016). Combination of PN and anammox reactions in one reactor significantly reduces the costs of infrastructure and operation as compared to that of the two-stage process (Vlaeminck et al. 2012; De Clippeleir et al. 2013; Wett et al. 2013; Pérez et al. 2014). With respect to nitric and nitrous oxide gas emission (Sliekers et al. 2005; Winkler et al. 2011; De Clippeleir et al. 2013; Lotti et al. 2014a), the emission from the single-stage process is lower compared to that from the two-stage process (Kampschreur et al. 2009) as a single-stage reactor often operates under the condition of nitrite limitation and low DO concentration. The main advantage of splitting the PN/A in two different reactors is that the anammox reactor can be operated in an anoxic environment to avoid the competition for nitrite by NOB (Pérez et al. 2014), while it is unavoidable in a single-stage PN/A reactor (Winkler et al. 2012a; De Clippeleir et al. 2013). Another advantage is that the PN/A process can be optimized separately (Isanta et al. 2015). The following sections will concentrate on single-stage PN/A process as it is used more widely compared to two-stage PN/A process.
Suspended sludge process
The PN/A in 200,000 m3/day step-feed activated sludge process of the Changi WRP, Singapore (Cao et al. 2013; Cao et al. 2016a), and the deammonification process in Strass WWTP, Austria (Wett et al. 2013), are two existing typical suspended sludge PN/A process full-scale references. In these two cases, AnAOB were protected from oxygen in anoxic zone, and heterotrophic denitrification made significant contributions to nitrogen removal due to the high influent C/N ratio.
Enhancement of biofilm anammox activity
The high anammox activity in biofilm assists in maintaining a reasonable anammox activity at low temperature and in suppressing NOB at a wider range of DO/NH4 flux ratio due to nitrite unavailability (Vangsgaard et al. 2012; Pérez et al. 2014). Both are essential to unlock the bottlenecks of low anammox activity and low efficiency performance of mainstream PA/A at low temperatures. The intensification of anammox activity in the biofilm can be made through increase of AnAOBl population and activity. A thicker biofilm or large size of granular sludge is capable of harboring more AnAOBl population under aerated conditions (Vlaeminck et al. 2012; Winkler et al. 2012a; Pérez et al. 2014; Gilbert et al. 2015). A fixed biofilm, whose application is not popular, may help to grow thicker (and denser) anammox biofilm although the reactor volume may be larger than MBBR/IFAS. It was reported that a high NRR (2.28 kg N/m3 day) and high AnAOB population (16S rRNA 3.95 × 1011 copies/g VSS) of a granular sludge anammox UASB reactor treating low ammonium concentration wastewater at 16 °C were achieved due to the use of floc anammox sludge as seeds and the stepwise increase of up-flow velocity during granulation (Ma et al. 2013). This illustrates utilizing free/floc AnAOB, which has a high growth rate (0.2–0.3/day), as seeds of immobilization (Lotti et al. 2015b) may enhance biofilm anammox activity. Proper hydraulic control can enhance the density and integrity of biofilm and granular sludge. A wide range in the intrinsic maximum growth rates (μ max 0.06–0.10/day) at mesospheric temperatures of granular anammox sludge (Kartal et al. 2012) highlights the potentials to promote anammox cell activity in biofilm through optimizing immobilization process. The changes of granular sludge structure at low temperature (Dosta et al. 2008; Vazquez 2016), which could result in deeper penetration of oxygen impairing anammox, which can further deteriorate performance of mainstream PN/A, should be further studied in terms of phenomena and mechanisms. The potential solutions to mitigate or avoid it should be explored.
Granular sludge reactor
The pilot-scale mainstream PN/A granular sludge reactor (volume 4 m3, hydraulic capacity 1–3 m3/h) of Rotterdam-Dokhaven WWTP, which is continuously fed with the effluent of the chemically enhanced A-stage activated sludge process and without biomass augmentation, is a typical example of a granular sludge reactor. The flocculent biomass concentration in the liquid phase was about 30 mg/L only, which was dominated by heterotrophs. While the total biomass concentration in the reactor was about 4 g VSS/L, the diameter of granular sludge was in the range between 0.8 and 1.6 mm (Lotti et al. 2014b). Most of the AOB activity (80% based on maximum activity) and the NOB activity (84% based on maximum activity) were with the granules, but the actual (in situ) NOB activity in the reactor was maintained at a low level (10% based on maximum activity) due to the competition for oxygen between the AOB and NOB and for nitrite between AnAOB and NOB via optimization of the DO control (Geilvoet and Hendrickx 2015). High NRR and a satisfactory TN removal efficiency with temperature variations between 17 and 23 °C (Table 1) were achieved under the stable operational conditions (Geilvoet et al. 2015). Vazquez (2016) reported from PN/A ELAN®, another granular sludge process, that at low-temperature, granular sludge structure became loose with density reduction resulting in deeper oxygen penetration. The current research works focus on the suppression of NOB during the winter season (∼10 °C) (Geilvoet and Hendrickx 2015). It was proposed to build an overcapacity of anammox during the summer as to retain treatment capacity in the winter when anammox activity substantially drops (Lotti et al. 2014b). The recovery ability of AnAOB activity after 1 month of ammonium limitation (Sliekers et al. 2005) and the small decay constant b (0.003/day) (Hao et al. 2002) of the AnAOB sound to provide favorable conditions to build such a capacity. COD or solids overflowing from the chemically enhanced A-stage process into the granular sludge PN/A reactor during heavy rain caused heterotrophic growth on the biofilm surface (Geilvoet et al. 2015) indicating essentiality of robust on-line control.
Moving bed biofilm reactor
Bacteria grew mostly on the surface of the carriers such as Kaldnes rings (K1®, K3®, and K5®, etc.) in MBBR. As in the granular sludge reactor, low suspended sludge concentration was maintained in the liquid phase in the MBBR, e.g., 10–20 mg VSS/L, which accounted for about 3% of the total biomass and 6 to 33% of aerobic ammonium oxidation (Malovanyy et al. 2015). Microbial community was stable throughout the temperatures that varied between 20 and 10 °C (Gilbert et al. 2014a). The laboratory experiment showed that the operation was stable from 19 to 13 °C but became unstable at 10 °C (Gilbert et al. 2014a; Persson et al. 2014; Lackner et al. 2015). In order to overcome the mass transfer resistance, a higher DO (0.5–1.5 mg/L) was maintained in the liquid phase, which resulted in a high aeration energy consumption (Lemaire et al. 2013) and potential inhibition of anammox activity due to deeper oxygen penetration. Real-time aeration control strategy was implemented with an automatically adjusted DO set point based on online inlet and outlet concentrations of ammonium and nitrate so as to control the nitrate production below 11% of removed ammonium (i.e., stoichiometric nitrate production by anammox reaction, Strous et al. 1999) while maintaining a high ammonium oxidation conversion in the reactor (Veuillet et al. 2015). Exchanges of biofilm carriers and alternating feeding between the sidestream and mainstream PN/A (augmentation) process were used. Limited suppression of NOB was reported regardless of the applied intermittent aeration strategy (Gustavsson et al. 2015; Trojanowicz et al. 2016). With lower DO concentration, the thicker biofilm made it easier for AnAOB to compete with NOB for nitrite since inactivation of AnAOB by DO penetration is avoided (Gilbert et al. 2015). However, low DO can lead to low NRR (Hu et al. 2013; Lotti et al. 2014a). Hence, a balanced consideration is needed. More detachment of AOB from the biofilm and washout from the reactor could reduce the nitrogen removal from 50 to 40% at 25 °C (Malovanyy et al. 2015), indicating the importance of maintaining layered structure of biofilm and the appropriate hydraulics in reactor design (Lotti et al. 2014a; Winkler et al. 2012a).
Hybrid reactor
The major difference between the biofilm (granular sludge reactor and MBBR) and hybrid reactor is that a hybrid reactor has a much higher suspended sludge concentration in the liquid phase. The biomass in both liquid and solid (biofilm) phases plays an important role in microbial conversions. IFAS reactor is a typical hybrid reactor. In contrast to the biofilm reactor, AOB and NOB, in addition to the HB, are mainly spatially distributed in the liquid phase, while AnAOB are found mainly in the biofilm (Veuillet et al. 2015). It was reported that 60% of the aerobic capacity was realized in the liquid phase, while AnAOB activity was almost entirely (e.g., >96.5%) experienced in the biofilm (Malovanyy et al. 2015). The high suspended sludge concentration in the liquid phase allows significantly lower diffusion limitation compared to biofilm system, making it feasible to control the AOB/NOB competition through a low-level DO in the liquid phase. Moreover, the sludge age of suspended biomass can be controlled independently from the biofilm and provides an additional alternative to wash out HB and outselect NOB while tolerating a high influent COD/N ratio although solid removal may become a task of final polishing. As a result, sCOD/N ratio instead of COD/N ratio was used in the process design (Lemaire et al. 2013; Veuillet et al. 2015) although the ratio to be used in the design is yet to be formulated. However, few studies reported the nitrite reduction in the liquid prior nitrite diffusion to the surface of biofilm. IFAS ANITA Mox®, which has MLSS of up to 3 g/L (Veuillet et al. 2015) and the DO level controlled at less than 0.5 mg/L in the liquid phase (Lemaire et al. 2013), achieved a NRR thrice as much as that of the MBBR (Lemaire et al. 2013). A 3-day liquid phase SRT was efficient in suppressing the NOB activity (Veuillet et al. 2015). The pilot PN/A IFAS ANITA Mox® (2 m3) and another prototype IFAS ANITA Mox® (50 m3) achieved satisfactory nitrogen removal efficiency and NRR (Table 1). Real-time aeration control and argumentation were similar to the MBBR ANITA Mox® (Lemaire et al. 2013; Veuillet et al. 2015).
Further studies
It can be seen that the major bottlenecks for the scale-up of mainstream PN/A process are (i) unstable performance of the carbon concentrating pretreatment, (ii) suppression of NOB especially under low temperatures (15–10 °C), (iii) the low activity of AnAOB at low temperatures, and (iv) final polishing. Thus, the areas for further studies are proposed accordingly.
Carbon concentrating pretreatment process
More investigations should be carried out on the mechanisms of the carbon absorption/storage, particularly bio-flocculation/coagulation; minimization of carbon mineralization; and the relationships between the influent characteristics and HRAS/CEPT process efficiency under typical site operational conditions including SRT, HRT, DO, and temperatures, etc. The real-time control system and facilities to deal with variety of influent characteristics resulting from seasonal variations and heavy rain events should be developed and implemented.
Deepening of understanding competition between autotrophic nitrogen-converting organisms
Expanding the previous studies and models by accommodating the competition between AOB (mainly Nitrosomonas) and NOB (mainly Nitrospira and Nitrobacter), revisits and further studies on the wide range of kinetic parameters for AOB, NOB, and AnAOB in the literature are desired and crucial. The investigation of intrinsic kinetics and stoichiometry of Nitrospira at low temperatures can be an appropriate starting point. The potential involvement of ammonia-oxidizing archaea (AOA), comammox, and mixotrophic nitrifiers and denitrifiers should not be excluded from the scope of the studies.
Intensification of biofilm anammox activity
Intensification of anammox biofilm activity can be conducted through increase of population, cell activity of AnAOB, and integrity of biofilm, which helps to increase the anammox activity and NRR of mainstream PN/A at low temperature. To achieve these objectives, the following studies are proposed: (i) to utilize larger size of granular sludge or thicker biofilm; (ii) to improve the immobilization (granulation) process to culture biofilm with higher anammox population, density, and cell activity, including exploring the feasibility of using fast-growing free/flocculent AnAOB as seeds; and (iii) to develop effective operational strategies for augmentation implemented during the winter season. The possible structure change of granular sludge (and other types of biofilm) under low temperature should be studied. The impact of temperature on the activity of AnAOB and how it can be influenced by the cultivation history of the biomass and its aggregation status need to be investigated from both kinetics and stoichiometry for a proper mechanistic understanding to help the enhancement of anammox activity at low temperature.
Reactor design
Plug flow or staged completely mixed stirred tanks, which help to suppress NOB and maintain high reaction rate, should be considered in biofilm reactor design. Multiple feed points, which enable the generation of “overcapacity zone” to allow process intensification/augmentation of anammox activity during winter season, should be explored. To maintain layered structure of biofilm, which is vital to suppress NOB and maintain anammox activity for biofilm process, design of reactor and configurations and selection of mechanical equipment should be made to minimize hydraulic patterns resulting in irregular detachment/sloughing and restructuring of biofilm. Also, the reactor must be equipped with robust real-time aeration control systems and reliable mechanical equipment.
Final polishing
Depending on the type of process used to implement the anammox conversions and the effluent requirements, a posttreatment might be needed for removal of suspended solids, nitrate, residual ammonia, and nitrite (its presence will lead to increased use of disinfection chemicals when reuse is imposed). Currently, it is difficult to state ,what type of polishing is needed, the state of technology is not developed well enough and the use of the treated water can be highly influential.
References
Ahn JH, Yu R, Chandran K (2008) Distinctive microbial ecology and biokinetics of autotrophic ammonia and nitrite oxidation in a partial nitrification bioreactor. Biotech Bioeng 100(6):1078–1087
Al-Omari A, Wett B, Nopens I, De Clippeleir H, Han M, Regmi P, Bott C, Murthy S (2015) Model-based evaluation of mechanisms and benefits of mainstream shortcut nitrogen removal processes. Wat. Sci Technol 71(6):840–847
Anthonisen A, Loehr R, Prakasam T, Srinath G (1976) Inhibition of nitrification by ammonia and nitrous acid. Water Pollut Control Fed 48(5):835–852
ATV (1997) Biologische und weitergehende Abwasserreinigung. Berlin
Beccari M, Dipinto AC, Ramadori R, Tomei MC (1992) Effects of dissolved oxygen and diffusion resistances on nitrification kinetics. Water Res 26:1099–1104
Bellucci M, Ofiteru ID, Graham DW, Head IM, Curtis TP (2011) Low-dissolved-oxygen nitrifying systems exploit ammonia-oxidizing bacteria with unusually high yields. Appl Environ Microbiol 77(21):7787–7796
Blackburne R, Vadivelua VM, Yuan Z, Keller J (2007) Kinetic characterisation of an enriched Nitrospira culture with comparison to Nitrobacter. Wat Res 41(3033):3042
Blackburne R, Yuan Z, Keller J (2008a) Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 19(2):303–312
Blackburne R, Yuan Z, Keller J (2008b) Demonstration of nitrogen removal via nitrite in a sequencing batch reactor treating domestic wastewater. Wat Res 42:2166–2176
Brockmann D, Rosenwinkel KH, Morgenroth E (2008) Practical identification of biokinetic parameters of a model describing two-step nitrification. Biotechnol Bioeng 103(3):497–514
Cao YS, Kwok BH, Yong W H, Chua SC, Wah YL, Yahya ABD GHANI (2013) The main stream autotrophic nitrogen removal in the largest full scale activated sludge process in Singapore: process analysis. In: Proceedings of WEF/IWA Nutrient Removal and Recovery 2013: Trends in Resource Recovery and Use, July 28–31, 2013, Vancouver
Cao YS, Kwok BH, Zhou Y, Lee Z, Liu Y, He JZ, van Loosdrecht MCM, Daigger GT, Lay W, Chua SC, Wah YL, Yahya AG (2014) Nitrogen removal activated sludge process from conventional to innovative process. In: Proceedings of IWA Specialist Conference on Global Challenges for Sustainable Wastewater Treatment and Resource Recovery. 26–30, Oct. 2014. Kathmandu
Cao YS, Kwok BH, van Loosdrecht MCM, Daigger GT, Chua SC, Wah YL, Yahya AG (2016a) The occurrence of EPBR in mainstream partial nitritation and anammox at a 200 000 m3/day activated sludge process, Singapore. Wat Sci Technol. doi:10.2166/wst.2016.565
Cao YS, Kwok BH, van Loosdrecht MCM, Daigger GT, Png HY, Chua SC, Wah YL, Yahya AG (2016b) Mainstream partial nitritation and anammox in a 200 000 m3/day activated sludge process in Singapore: scale-down by using laboratory fed-batch reactor. Wat Sci Technol. doi:10.2166/wst.2016.116
Corbalá-Robles L, Picioreanu C, van Loosdrecht MCM, Pérez J (2016) Analysing the effects of the aeration pattern and residual ammonium concentration in a partial nitritation-anammox process. Environ Technol 37(6):694–702
Daigger GT (2014) Oxygen and carbon requirements for biological nitrogen removal processes accomplishing nitrification, nitritation, and anammox. Wat Environ Res 86(2):204–209
Daigger GT, Littleton H (2013) Simultaneous biological nutrient removal: a state-of-the art review. In: Proceedings of WEFTEC. 2013.5–9 Oct 2013, Chicago
Daigger GT, Nicholson GA, Koh CLY, Moh WH, Young JC, Ghani YA, Yong WH (2008) Start-up and initial operation of Singapore’s 800,000 m3/day Changi Water Reclamation Plant. In: Proceedings of IWA--PUB Water Convention Conference, SIWW, 23–27, June 2008, Singapore
Daims H, Purkhold U, Bjerrum L, Arnold E, Wilderer PA (2001) Nitrification in sequencing biofilm batch reactors: lessons from molecular approaches. Wat Sci Technol 43:9–18
De Clippeleir H, Yan XG, Verstraete W, Vlaeminck SE (2011) OLAND is feasible to treat sewage-like nitrogen concentrations at low hydraulic residence times. Appl Microbiol Biotechnol 90:1537–1545
De Clippeleir H, Vlaeminck SE, de Wilde F, Daeninck K, Mosquera M, Boeckx P, Verstraete W, Boon N (2013) One-stage partial nitritation/anammox at 15 °C on pretreated sewage: feasibility demonstration at lab-scale. Appl Microbiol Biotechnol 97(23):10199–10210
Desloover J, De Clippeleir H, Boeckx P, Du LG, Colsen J, Verstraete W, Vlaeminck SE (2011) Floc-based sequential partial nitritation and anammox at full scale with contrasting N2O emissions. Wat Res. doi:10.1016/j.watres.2011.02.028
Dosta J, Fernández I, Vázquez-Padín JR, Mosquera-Corral A, Camposb JL, Mata-Álvarez J, Méndez R (2008) Short- and long-term effects of temperature on the anammox process. J Hazard Mater 154:688–693
Dytczak M, Londry KL, Oleszkiewicz JA (2008) Activated sludge operational regime has significant impact on the type of nitrifying community and its nitrification rates. Wat Res 42(8–9):2320–2328
Fitzgerald CM, Camejo J, Oshlag Z, Noguera DR (2015) Ammonia-oxidizing microbial communities in reactors with efficient nitrification at low-dissolved oxygen. Wat Res 70:38–51
Gao D-W, Huang X-L, Tao Y, Cong Y, Wang X-L (2015) Sewage treatment by an UAFB–EGSB biosystem with energy recovery and autotrophic nitrogen removal under different temperatures. Bioresour Technol 181:26–31
Geilvoet SP, Hendrickx T (2015) Latest development of mainstream anammox granular sludge process in Rotterdam-Dokhaven. Presented in the Workshop Mainstream Anammox. Amsterdam International Water Conference, 2–6 November 2015
Geilvoet SP, Van Erp Taalman Kip CS, Hendrickx TLG, Hoekstra M (2015) Mainstream deammonification at WWTP Rotterdam-Dokhaven. In: Proceedings of IWA Specialist Conference Nutrient Removal and Recovery: moving innovation into practice. 18–21 May, 2015 Gdańsk, Poland
Gilbert EM, Agrawal S, Karst SM, Horn H, Nielsen PH, Lackner S (2014a) Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ Sci Technol. doi:10.1021/es501649m
Gilbert EM, Agrawal S, Brunner F, Schwartz T, Horn H, Lackner S (2014b) Response of different Nitrospira species to anoxic periods depends on operational DO. Environ Sci Technol 48:2934–2941
Gilbert EM, Agrawal S, Schwartz T, Horn H, Lackner S (2015) Comparing different reactor configurations for partial Nitritation/anammox at low temperatures. Wat. Res. 81:92–100
Guillén JAS, Yimman Y, Vazquez CML, Brdjanovic D, van Lier JB (2014) Effects of organic carbon source, chemical oxygen demand/N ratio and temperature on autotrophic nitrogen removal. Wat Sci Technol 69(10):2079–2086
Guillén JAS, Guardado PRC, Vazquez CML, de Oliveira Cruz CM, Brdjanovic D, van Lier JB (2015) Anammox cultivation in a closed sponge-bed trickling filter. Bioresour Biotech 186:252–260
Guisasola A, Jubany I, Baeza JA, Carrera J, Lafuente J (2005) Respirometric estimation of the oxygen affinity constants for biological ammonium and nitrite oxidation. J Chem Technol Biotech 80:388–396
Gustavsson DJI, Persson F, Jansen JLC (2014) Manammox—mainstream anammox at Sjölunda WWTP. In: Proceedings of IWA World Water Congress and Exhibition. Lisbon, September 22, 2014
Gustavsson DJI, Okhravi A, Persson F, Alvarez NL, Jansen JLC (2015) Experiences of repression of nitrate production in nitritation-anammox on municipal wastewater. In: Proceedings of IWA Specialist Conference Nutrient Removal and Recovery: moving innovation into practice. 18–21 May, 2015 Gdańsk, Poland
Han M, De Clippeleir H, Al-Omari A, Stewart H, Keswani H, Wett B, Vlaeminck SE, Bott C, Murthy S (2015) Robustness evaluation for NOB out-selection in mainstream deammonification. In: Proceedings of IWA Specialist Conference Nutrient Removal and Recovery: moving innovation into practice. 18–21 May, 2015 Gdańsk, Poland. Conference Proceedings, 712–714
Han M, De Clippeleir H, Al-Omari A, Wett B, Vlaeminck SE, Bott C, Murthy S (2016) Impact of carbon to nitrogen ratio and aeration regime on mainstream deammonification. Wat Sci Tech. doi:10.2166/wst.2016.202
Hao XD, Heijnen JJ, van Loosdrecht MCM (2002) Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnol Bioeng 77(3):266–277
Hellinga C, Schellen AAJC, Mulder JW, van Loosdrecht MCM, Heijen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium rich waste water. Wat Sci Technol 37:135–142
Hendrickx TLG, Wang Y, Kampman C, Zeeman G, Temmink H, Buisman CJN (2012) Autotrophic nitrogen removal from low strength waste water at low temperature. Wat Res 46:2187–2193
Hendrickx TLG, Kampmana C, Zeeman G, Temmink H, Hu Z, Kartal B, Buisman CJN (2014) High specific activity for anammox bacteria enriched from activated sludge at 10 °C. Bioresour Technol 163:214–221
Holger D, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature. doi:10.1038/nature16461
Hu Z, Lotti T, Kreuk MD, Kleerebezem R, van Loosdrecht MSM, Kruit J, Jetten MSM, Kartala B (2013) Nitrogen removal by a nitritation-anammox bioreactor at low temperature. Appl Environ Microb 79(8):2807–2812
Huang ZH, Gedalang PB, Asvapathanagul P, Olson BH (2010) Influence of physicochemical and operational parameters on Nitrobacter and Nitrospira communities in an aerobic activated sludge bioreactor. Wat Res. 44:4351–4358
Hubaux N, Wells G, Morgenroth E (2015) Impact of coexistence of flocs and biofilm on performance of combined nitritation-anammox granular sludge reactors. Wat. Res. 68:127–139
Hunik JH (1993). Engineering aspects of nitrification with immobilized cells. Ph.D. thesis, Wageningen UR, Wageningen
Isanta E, Reino C, Carrera J, Pérez J (2015) Stable partial nitritation for low-strength wastewater at low temperature in an aerobic granular reactor. Wat. Res. 80:149–158. doi:10.1016/j.watres.2015.04.028
Jetten MSM, Horn SJ, van Loosdrecht MCM (1997) Towards a more sustainable municipal wastewater treatment system. Wat Sci Tech 35(9):171–180
Jetten MSM, Wagner M, Fuerst J, van Loosdrecht MCM, Kuenen G, Strous M (2001) Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr Opin Biotechnol 12:283–288
Jimenez J, Wise G, Burger G, Du WW, Dold P (2014) Mainstream nitrite-shunt with biological phosphorus removal at the city of ST Petersburg Southwest. In: Proceedings of WEFTEC 2014 September 28–October 1, 2014. New Orleans, USA
Jimenez J, Miller M, Bott C, Murthy S, De Clippeleir H, Wett B (2015) High-rate activated sludge system for carbon management evaluation of crucial process mechanisms and design parameters. Wat Res. doi:10.1016/j.watres.2015.07.032
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM (2009) Review nitrous oxide emission during wastewater treatment. Wat Res 43:4093–4103
Kartal B, Kuypers MMM, Lavik G, Schalk J, Op den Camp HJM, Jetten MSM, Strous M (2007) Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol 9:635–642
Kartal B, Kuenen JG, van Loosdrecht MCM (2010) Sewage treatment with anammox. Science 328(5979):702–703. doi:10.1126/science.1185941
Kartal, B, van Niftrik L, Keltjens JT, Op den Camp HJM, Jetten MSM (2012) Anammox—growth physiology, cell biology, and metabolism, In: Advances in microbial physiology (Edited by: Robert Poole), 60:212–262. ISSN: 0065–2911.DOI: 10.1016/B978-0-12-398264-3.00003-6
Kessel V, Maartje AHJ, Speth DR, Albertsen M, Nielsen PH, den Camp HJM O, Kartal B, Jetten MSM, Lücker S (2015) Complete nitrification by a single microorganism. Nature 528:555–559. doi:10.1038/nature16459
Kim D-J, Kim S-H (2006) Effect of nitrite concentration on the distribution and competition of nitrite-oxidizing bacteria in nitratation reactor systems and their kinetic characteristics. Wat Res 40:887–894
Kindaichi T, Ito T, Okabe S (2004) Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by MAR-FISH. Appl Environ Microbial 70(3):1641–1650
Kindaichi T, Tsushima I, Ogasawara Y, Shimokawa M, Ozaki N, Satoh H, Okabe S (2007) In situ activity and spatial organization of anaerobic ammonium-oxidizing (anammox) bacteria in biofilms. Appl Environ Microbial 70(3):4931–4939
Kornaros M, Dokianakis SN, Lyberatos G (2010) Partial nitrification/denitrification can be attributed to the slow response of nitrite oxidizing bacteria to periodic anoxic disturbances. Environ Sci Technol 44(19):7245–7253
Kroiss H, Cao YS (2014) Energy considerations. In: Wanner J, Jenkins D (eds) Activated sludge—100 years and counting. IWA Publishing, London ISBN: 9781780404936. 424 pages
Lackner S, Agrawal S (2015) Process fundamentals-microbiology, stoichiometry, kinetics, and inhibition. In: WEF/WERF (2015) Shortcut nitrogen removal—nitrite shunt and deammonification. Publisher: Water Environment Federation. ISBN: 978-1-57278-313-3
Lackner S, Terada A, Smets BF (2008) Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: results of a modelling study. Wat Res 42:1102–1112
Lackner S, Gilbert EM, Vlaeminck SE, Joss A, Horn H, van Loosdrecht MCM (2014) Full-scale partial nitritation/anammox experiences—an application survey. Wat. Res. 55:292–303
Lackner S, Welker S, Gilbert EM, Horn H (2015) Influence of seasonal temperature fluctuations on two different partial nitritation-anammox reactors treating mainstream municipal wastewater. Wat Sci Technol 72(8):1358–1365
Laureni M, Weissbrodt DG, Szivak I, Robin O, Nielsen JL, Morgenroth E, Joss A (2015) Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater. Wat Res 80:325–336
Laureni M, Falås P, Robin O, Wick A, Weissbrodt DG, Nielsen JL, Ternes TA, Morgenroth E, Joss A (2016) Mainstream partial nitritation and anammox: long-term process stability and effluent quality at low temperatures. Wat Res. doi:10.1016/j.watres.2016.05.005
Lemaire R, Thomson C, Christensson M, Zhao H, Thesing G (2013) Mainstream deammonification using ANITA™Mox process W15: mainstream deammonification and shortcut TN removal—innovation and implementation. In: Proceedings of WEFTEC 2013, October 6th, Chicago
Li XJ, Sun S, Badgley BD, Sung SH, Zhang HS, He Z (2016) Nitrogen removal by granular nitritation/anammox in an upflow membrane-aerated biofilm reactor. Wat Res 94:23–31
Liang YH, Li D, Zhang XJ, Zeng HP, Yang Z, Zhang J (2014) Microbial characteristics and nitrogen removal of simultaneous partial nitrification, anammox and denitrification (SNAD) process treating low C/N ratio sewage. Bioresour Biotech 194:103–109
Liu YW, Ni BJ (2015) Appropriate Fe (II) addition significantly enhances anaerobic ammonium oxidation (anammox) activity through improving the bacterial growth rate. Sci Rep 5:8204. doi:10.1038/srep08204
Liu GQ, Wang JM (2013) Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ Sci Technol 47:5109–5117
Lotti T, Kleerebezem R, Hu Z, Kartal B, Jetten MSM, van Loosdrecht MCM (2014a) Simultaneous partial nitritation and anammox at low temperature with granular sludge. Wat Res 66:111–121
Lotti T, Kleerebezem R, Hub Z, Kartal B, de Kreuk MK, van Erp Taalman Kip C, Kruit J, Hendrickx TLG, van Loosdrecht MCM (2014b) Pilot-scale evaluation of anammox based main-stream nitrogen removal from municipal wastewater. Environ Technol 36(9):1167–1177
Lotti T, Kleerebezem R, Abelleira-Pereira J, Abbas B, van Loosdrecht MCM (2015a) Faster through training: the anammox case. Wat. Res. 81:261–268
Lotti T, Kleerebezem R, van Loosdrecht MCM (2015b) Effect of temperature change on anammox activity. Biotechnol Bioeng 112(1):98–103
Ma B, Peng YZ, Zhang SJ, Wang JM, Gan YP, Chang J, Wang S, Wang SY, Zhu GB (2013) Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour Technol 129:606–611
Ma B, Wang SY, Cao SB, Miao YY, Jia FX, Du R, Peng YZ (2015a) Review biological nitrogen removal from sewage via anammox: recent advances. Bioresour Technol. doi:10.1016/j.biortech.2015.10.074
Ma B, Bao P, Wei Y, Zhu GB, Yuan ZH, Peng YZ (2015b) Suppressing nitrite-oxidizing bacteria growth to achieve nitrogen removal from domestic wastewater via anammox using intermittent aeration with low dissolved oxygen. Sci Rep 5:13048. doi:10.1038/srep13048
Ma B, Zhang S, Zhang L, Yi P, Wang J, Wang S, Peng Y (2011) The feasibility of using a two-stage autotrophic nitrogen removal process to treat sewage. Bioresour Technol 102(17):8331–8334
Malovanyy A, Yang JJ, Trela J, Plaza E (2015) Combination of up-flow anaerobic sludge blanket (UASB) reactor and partial nitritation/anammox moving bed biofilm reactor (MBBR) for municipal wastewater treatment. Bioresour Technol 180:144–153
Metcalf and Eddy (2003) Wastewater engineering treatment and reuse. 4th Edition, McGraw Hill
Mulder A (1989) Anoxic Ammonium Oxidation, Patent EP 0327184 A1
Nogueira R, Melo LF (2006) Competition between Nitrospira spp. and Nitrobacter spp. in nitrite-oxidizing bioreactors. Biotech Bioeng. doi:10.1002/bit. 21004
Nowka B, Daims H, Spieck E (2015) Comparison of oxidation kinetics of nitrite-oxidizing bacteria. Appl Environ Microbiol 81:745–753
Okabe S, Oshiki M, Takahashi Y, Satoh H (2011) Development of long-term stable partial nitrification and subsequent anammox process. Bioresour Technol 102:6801–6807
Park H-D, Noguera DR (2004) Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Wat Res. 38:3275–3286
Park H-D, Noguera DR (2007) Characterization of two ammonia-oxidizing bacteria isolated from reactors operated with low dissolved oxygen concentrations. J Appl Microb 102(5):1401–1417
Park H-D, Noguera DR (2008) Nitrospira community composition in nitrifying reactors operated with two different dissolved oxygen levels. J Microbiol Biotechnol 18(8):1470–1474
Park H-D, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl Environ Microbiol 72(8):5643–5647
Pérez J, Lotti T, Kleerebezem R, Picioreanu C, van Loosdrecht MCM (2014) Outcompeting nitrite-oxidizing bacteria in single stage nitrogen removal in sewage treatment plants: a model-based study. Wat Res 66:208–218
Persson F, Sultana R, Suarez M, Hermansson M, Plaza E, Wilén B-M (2014) Structure and composition of biofilm communities in a moving bed biofilm reactor for nitritation–anammox at low temperatures. Bioresour Technol 154:267–273
Picioreanu C, Pérez J, van Loosdrecht MCM (2016) Impact of cell cluster size on apparent half-saturation coefficients for oxygen in nitrifying sludge and biofilms. Wat. Res. 106:371–382
Piculell M, Christensson M, Jönsson K, Welander T (2016) Partial nitrification in MBBRs for mainstream deammonification with thin biofilms and alternating feed supply. Wat Sci Technol 73(6):1253–1260
Poot V, Hoekstra M, Geleijnse MAA, van Loosdrecht MCM, Pérez J (2016) Effects of the residual ammonium concentration on NOB repression during partial nitritation with granular sludge. Wat Res 106:518–530
Prosser JI (1989) Autotrophic nitrification in bacteria. Adv Microb Physiol 30:125–181
Regmi P, Miller MW, Holgate B, Bunce R, Park H, Chandran K, Wett B, Murthy S, Bott C (2014a) Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Wat Res 57:162–171
Regmi P, Holgate B, Fredericks D, Miller MW, Wett B, Murthy S, Bott C (2014b) A pilot-scale mainstream nitritation-denitritation process followed by an anammox MBBR operated within wide range of operating conditions., In: Proceedings of IWA World Water Congress, 21–26 Sept. 2014, Lisbon
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill, New York
Roest K, Daamen B, de Graaff MS, Hartog L, Zandvoort MH, Uijterlinde CA, Dilven S, van Lierg JB, van Loosdrecht MCM (2012) Energy production from wastewater—dynamic filtration of activated sludge. In: Proceedings of IWA Water Congress, 15–20, September, 2012, Busan, Korea
Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R (1999) Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol 65(8):3690–3696
Seuntjen D, Vlaeminck S E, Bundervoet B, Mollen H, Wypkema E, Colsen J. (2016) Measures to influence partial nitritation/anammox. WEF-IWA Nutrient Removal and Recovery (NRR) Conference. 16–20 July 2016. Denver, USA
Siegrist H, Salzgeber D, Eugster J, Joss A (2008) Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci Technol 57:383–388
Siripong S, Rittmann B (2007) Diversity study of nitrifying bacteria in full-scale municipal wastewater treatment plants. Wat Res 41(5):1110–1120
Sliekers A, Haaijer S, Stafsnes M, Kuenen J, Jetten MSM (2005) Competition and coexistence of aerobic ammonium- and nitrite oxidizing bacteria at low oxygen concentrations. Appl Microbiol Biotechnol 68(6):808–817
Speth DR, Guerrero-Cruz S, Dutilh BE, Jetten MS (2016) Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system. Nature Communications Nature Communications. doi:10.1038/ncomms11172
Stein LY (2011) Heterotrophic nitrification and nitrifier denitrification. In: Nitrification (ed by Ward B. B. et al.) IWA Publishing ISBN-13: 978-1-55581-481-6
Stinson B, Murthy S, Bott C, Wett B, Al-Omari A, Bowden G, Mokhyerie Y, De Clippeleir H (2013) Roadmap toward energy neutrality & chemical optimization at enhanced nutrient removal facilities. In: Proceedings of WEF/IWA Nutrient Removal and Recovery 2013: Trends in Resource Recovery and Use, July 28–31, 2013, Vancouver
Strous M, Kuenen JG, Jetten MSM (1999) Key physiology of anaerobic ammonium. Appl Environ Microbiol 65(7):3248–3250
Trojanowicz K, Plaza E, Trela J (2016) Pilot scale studies on nitritation-anammox process for mainstream wastewater at low temperature. Wat Sci Technol 73(4):761–768
Vadivelu VM, Yuan ZG, Fux C, Keller J (2006) The inhibitory effects of free nitrous acid on the energy generation and growth processes of an enriched Nitrobacter culture. Environ Sci Technol 40(14):4442–4448
Van der Star WRL, Abma WR, Blommers D, Mulder JW, Tokutomid T, Strouse M, Picioreanua C, van Loosdrecht MCM (2008) Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Wat Res 41:4149–4163
Van Loosdrecht MCM (2008) Innovative biological nitrogen removal. In Biological wastewater treatment principle, modelling and design, (edited by: Henze and van Loosdrecht et al.). IWA Publishing. ISBN: 1843391880
Van Loosdrecht MCM, Seah H, Wah YL, Cao YS (2014) The next 100 years. In: Activated sludge—100 years and counting (Editors: Jiri Wanner and David Jenkins) IWA Publishing, London, ISBN: 9781780404936. 424 pages
Vangsgaard AK (2013) Modeling, experimentation, and control of autotrophic nitrogen removal in granular sludge systems. Technical University of Denmark. PhD Thesis
Vangsgaard AK, Mauricio-Iglesias M, Gernaey KV, Smets BF, Sin G (2012) Sensitivity analysis of autotrophic N removal by a granule based bioreactor: influence of mass transfer versus microbial kinetics. Bioresour Technol 123:230–241
Vazquez J (2016) Mainstream ELAN® process WWTP. The 13th IWA Leading Edge Conference on Water and Wastewater Technology. Workshop: the future is here: experiences in the full-scale implementation of mainstream deammonification for leading edge nitrogen control. 13–16 June 2016. Jerez de la Frontera, Spain
Vela JD, Stadler LB, Martin KJ, Raskin L, Bott CB, Love NG (2015) Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment. Environ Sci Technol Lett 2(9):234–244
Veuillet F, Zozor P, Stefansdottir D, Christensson M, Skonieczny T, Ochoa J, Lemaire R (2015) Mainstream deammonification using ANITA™Mox process. In: Proceedings of IWA Specialist Conference Nutrient Removal and Recovery: moving innovation into practice. 18–21 May, 2015 Gdańsk, Poland
Vlaeminck SE, Terada A, Smets BF, De Clippeleir H, Schaubroeck T, Bolca S, Demeestere L, Mast J, Boon N, Carballa M, Verstraete W (2010) Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox. Appl Environ Microbiol 76(3):900. doi:10.1128/AEM.02337-09
Vlaeminck SE, Clippeleir HD, Verstraete W (2012) Microbial resource management of one-stage partial nitritation/anammox. Microb Biotechnol 5(3):433–448
Volcke EIP, Picioreanu C, De Baets B, van Loosdrecht MCM (2010) Effect of granule size on autotrophic nitrogen removal in a granular sludge reactor. Environ Technol 31(11):1271–1280
Wang Q, Jiang G, Ye L, Hu S, Yuan ZG (2014) Side-stream sludge treatment using free nitrous acid selectively eliminates nitrite oxidizing bacteria and achieves the nitrite pathway. Water Res 55:245–255
Wang DG, Wang QL, Laloo A, YF X, Bond PL, Yuan ZG (2016) Achieving stable nitritation for mainstream deammonification by combining free nitrous acid-based sludge treatment and oxygen limitation. Sci Rep 6:25547. doi:10.1038/srep25547
Ward B (2008) Nitrification. In: Sven Erik Jørgensen, Brian D. Fath (Editor-in-Chief). Ecological processes. Vol. [3] of Encyclopedia of ecology, 5 vols. 2511–2518. Oxford: Elsevier
Watson, S.W., Bock, E., Harms, H., Koops, H.-P., Hoper, A.B (1989) Nitrifying bacteria. In: Staley, J.T., Bryant, M.P., Pfenning, N. (Eds.), Bergey’s manual of systematic bacteriology. Williams and Wilkins Publication, Baltimore, pp. 1808–1834
WEF/WERF (2015) Shortcut nitrogen removal—nitrite shunt and deammonification. Publisher: Water Environment Federation. ISBN: 978-1-57278-313-3
Welker S, Horn H, Lackner S (2016) Substrate contentment: influence of residual ammonium and dissolved oxygen concentrations on autotrophic nitrogen removal. WEF/IWA NRR, 10–13 July 2016. Denver USA
Wett B (2007) Development and implementation of a robust deammonification process. Wat. Sci Technol 56(7):81–88
Wett B, Omari A, Podmirseg SM, Han M, Akintayo O, Brandón MG, Murthy S, Bott C, Hell M, Takács I, Nyhuis G, O’Shaughnessy M (2013) Going for mainstream deammonification from bench- to full-scale for maximized resource efficiency. Wat Sci Technol 58(6):1155–1171
Wett B, Podmirseg SM, Gómez-Brandón M, Hell M, Nyhuis G, Bott C, Murthy S (2015) Expanding DEMON sidestream deammonification technology towards mainstream application. Water Environ Res 87:2084–2094
Wiesmann (1994) Biological nitrogen removal from wastewater. In: Advances in biochemical engineering biotechnology, Vol. 51 Managing Editor: A. Fiechter. Springer-Verlag Berlin Heidelberg. 114–153
Winkler MKH, Kleerebezem R, Kuenen JG, Yang JJ, van Loosdrecht MCM (2011) Segregation of biomass in cyclic anaerobic/aerobic granular sludge allows the enrichment of anaerobic ammonium oxidizing bacteria at low temperatures. Environ Sci Technol 45:7330–7337
Winkler MKH, Kleerebezem R, van Loosdrecht MCM (2012a) Integration of anammox into the aerobic granular sludge process for main stream wastewater treatment at ambient temperatures. Wat Res 46:136–144
Winkler MKH, Bassin JP, Kleerebezem R, Sorokin DY, van Loosdrecht MCM (2012b) Unravelling the reasons for disproportion in the ratio of AOB and NOB in aerobic granular sludge. Appl Microbiol Biotechnol 94:1657–1666
Wu J, He CD, van Loosdrecht MCM, Pérez J (2016) Selection of ammonium oxidizing bacteria (AOB) over nitrite oxidizing bacteria (NOB) based on conversion rates. Chem Eng J. doi:10.1016/j. cej.2016. 07. 019
Xu G, Zhou Y, Yang Q, Lee Z-P, Gu J, Lay W, Cao YS, Liu Y (2015) The challenges of mainstream deammonification process for municipal used water treatment. Appl Microbiol Biotechnol 99(6):2485–2490
Yang Q, Peng YZ, Liu X, Zeng W, Mino T, Satoh H (2007) Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities. Environ Sci Technol 41:8159–8164
Yang Q, Shen N, Lee ZM-P, Xu GJ, Cao YS, Kwok BH, Lay W, Liu Y, Zhou Y (2016) Simultaneous nitrification, denitrification and phosphorus removal (SNDPR) in a full-scale water reclamation plant located in warm climate. Wat Sci Technol. doi:10.2166/wst.2016.214
Zhang SJ, Zhang L, Shao H, Gan YP, Peng YZ (2014) Nitrogen removal from mainstream by one-stage hybrid anammox system. In: Proceedings of Water Convention, SIWW 2014. June 30, 2014. Singapore
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cao, Y., van Loosdrecht, M.C.M. & Daigger, G.T. Mainstream partial nitritation–anammox in municipal wastewater treatment: status, bottlenecks, and further studies. Appl Microbiol Biotechnol 101, 1365–1383 (2017). https://doi.org/10.1007/s00253-016-8058-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-8058-7