Abstract
Partial nitrification (PN) has been considered as one of the promising processes for pretreatment of ammonium-rich wastewater. In this study, a kind of novel carriers with enhanced hydrophilicity and electrophilicity was implemented in a moving bed biofilm reactor (MBBR) to start up PN process. Results indicated that biofilm formation rate was higher on modified carriers. In comparison with the reactor filled with traditional carriers (start-up period of 21 days), it took only 14 days to start up PN successfully with ammonia removal efficiency and nitrite accumulation rate of 90 and 91%, respectively, in the reactor filled with modified carriers. Evident changes of spatial distributions and community structures had been detected during the start-up. Free-floating cells existed in planktonic sludge, while these microorganisms trended to form flocs in the biofilm. High-throughput pyrosequencing results indicated that Nitrosomonas was the predominant ammonia-oxidizing bacterium (AOB) in the PN system, while Comamonas might also play a vital role for nitrogen oxidation. Additionally, some other bacteria such as Ferruginibacter, Ottowia, Saprospiraceae, and Rhizobacter were selected to establish stable footholds. This study would be potentially significant for better understanding the microbial features and developing efficient strategies accordingly for MBBR-based PN operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional nitrification/denitrification nitrogen removal process has been extensively adopted in the past decades; however, it has inevitable disadvantages in treating high ammonium but low chemical oxygen demand (COD) wastewater considering that it needs external aeration and carbon source supply. In consequence, some novel technologies such as short-cut nitrification-anammox (Strous et al. 1997) or short-cut nitrification-denitrification process (Yoo et al. 1999) have been developed and implemented as more sustainable alternatives to treat low C/N liquors. In these processes, the pretreatment of partial nitrification (PN) is required to accumulate sufficient nitrite and avoid nitrate formation (Chu et al. 2015). Up to now, some feasible strategies have been introduced to start up PN process via the accurate control of temperature, free ammonium (FA), free nitrite acid (FNA), dissolved oxygen (DO) concentration, or sludge retention time (SRT) based on the different growth habits between ammonia-oxidizing bacterium (AOB) and nitrite-oxidizing bacterium (NOB) (Blackburne et al. 2008; Zeng et al. 2013). Most previous studies on PN start-up were carried out in activated sludge systems. Although PN can be established in these systems, it seems to take a long period to accumulate AOB (Gabarro et al. 2013). To overcome the deficiency of activated sludge systems, fixed biofilm system is generally a candidate because the long biomass retention time in biofilm guarantees the slow growth of AOB and the separate control of hydraulic retention time (HRT) and SRT is essential to achieve high-rate PN (Zhang et al. 2015). However, compared with activated sludge, fixed biofilm system presents lower volume loading and needs backwash at regular time intervals, leading to an increase of infrastructure and operating cost accordingly.
Moving bed biofilm reactor (MBBR), known as a promising process that combines the advantages of activated sludge and fixed biofilm (Zekker et al. 2012), is determined to be effective for AOB enrichment and further to achieve rapid start-up and high-rate ammonia oxidation of PN. In MBBR process, the property of carriers contributes to steady operation and nitrogen removal effect of the system. Currently, most commercial MBBR carriers are made of plastics with similar density to water like polyethylene, polypropylene, high-density polyethylene (HDPE), etc. However, the hydrophilicity and electrophilicity of these materials are relatively weak, which is unfavorable for pollutant transferring or electronegative biofilm formation (Chen et al. 2012). On the other hand, considering that nitrifying bacteria are not able to form strong biofilm due to their poor EPS production (Tsuneda et al. 2001), they can potentially trigger detachment events. Consequently, some novel carriers have been developed by physical/chemical surface modification to enhance biomass adhesion capacity and combat the drawbacks previously mentioned (Hibiya et al. 2000; Lackner et al. 2009). Nevertheless, the more improved hydrophilicity is still desired in order to improve hydrosoluble pollutant transferring within biofilms so as to accelerate the pollutant removal rates.
Up to now, few studies have focused on the dual improvement of both hydrophilicity and electrophilicity of carriers. In this study, we propose that AOB growth rate will be accelerated on an appropriate modified surface of a novel supporting material. Even though there are a variety of approaches for surface modification, we introduce a relatively facile approach to develop a novel type of carriers to start up the PN process in this study. These modified carriers were made of HDPE with a small proportion of polyquaternium-10 (PQAS-10) and Fe2O3 to enhance the hydrophilicity and electrophilicity, which arise from the superior water solubility and adsorption capacity of PQAS-10 and the positive electricity of Fe2O3. The nitrogen removal performance during the start-up of PN was analyzed in comparison with the other reactor filled with traditional carriers. Moreover, functional bacterial spatial distribution and microbial community structure shifts were also evaluated. This study is expected to inspire us on MBBR-based PN process operation and further promote the novel carrier implementation in industrial wastewater or domestic sewage treatment in the long term.
Materials and methods
Preparation of traditional/modified carriers
The modified carriers were made of HDPE together with a small proportion of PQAS-10 (2 wt%) and Fe2O3 (2 wt%). The detailed protocol was as follows: PQAS-10 and Fe2O3 powders were dispersed in HDPE granules by mechanical mixing using a cylindrical blender (100 rpm for 5 min). Afterwards, the mixture was extruded by a single screw extruder (the ratio of length to diameter was 25:1) at a screw speed of 20 rpm. The barrel zone temperature of the four stages (feed stage, melt stage, harmonizing stage, and reaction stage) was 125, 135, 145, and 120 °C, respectively. The protocol for producing traditional carriers (made of only HDPE) was same as those modified carriers.
The carriers had a cylindrical shape (diameter of 10 mm, height of 10 mm) with a cross-shaped grid inside and “fins” on the exterior. The length, diameter, specific surface area, and density of the cylinders were 10 mm, 10 mm, 600 m2/m3, and 0.98 kg/L, respectively. To evaluate the hydrophilicity and electrophilicity of the newly developed carriers, two essential surface features of the carriers (surface zeta potential at pH value of 7.0 and contact angles of droplet placed on dried layers) were measured triply by using a SurPASS electrokinetic analyzer and a contact angle microscope, respectively.
Experimental setup and PN start-up
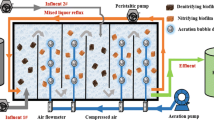
Two lab-scale moving bed biofilm reactors (MBBRs) made of polymethyl methacrylate were used in this study with outer diameter, inner diameter, height, and total volume of 20, 15, and 35 cm and 6.18 L, respectively. The reactors had double jacket for temperature control with the inflow and air pumped from the bottom and output from the upper outlet. Carriers were added in the reactors with the filling degree of 25%. In the experimental reactor (R1 in abbreviation), novel carriers were utilized; nevertheless, carriers made of only HDPE were used in the control reactor (R2 in abbreviation).
To start up the PN process, the sludge that is obtained from sludge refluxed liquid in a municipal sewage treatment plant was inoculated originally to the reactors (mixed liquid suspended solid (MLSS) was 4500 mg/L, and wet sludge inoculation dosage was 1.5 L). Tap water, (NH4)2SO4, and NaHCO3 were added to the domestic sewage to form low C/N wastewater with the detail contents of COD 30–53 mg/L, NH4 +-N 183–220 mg/L, NO2 −-N 0–1.1 mg/L, NO3 −-N 0–3.5 mg/L, and pH value 7.0–7.5. To promote the growth of AOB and eliminate the metabolism of NOB, constant temperature of 28 °C and relatively low DO concentration of 0.8–1.0 mg/L were maintained during the start-up (phase I). When nitrite accumulation rate (NAR) rose above 80%, AOB was supposed to be sufficient in the system and DO rose up to around 1.5 mg/L accordingly to realize stable operation (phase II). The two reactors were operated parallel with two cycles every day (aeration 11 h, sedimentation and drainage 1 h).
Chemical analysis of water quality
The concentrations of COD, NH4 +-N, NO2 −-N, and NO3 −-N in influent and effluent were measured daily according to the standard methods by ultraviolet and visible spectrophotometers (V550, JASCO, Japan) (APHA 1995). DO was measured by using an oxygen meter (Model 55/12, YSI, USA), while the pH value was detected by a pH meter (PB-10, Sartorius, Germany). Ammonia removal efficiency (ARE), nitrogen removal efficiency (NRE), and NAR were calculated according to the following three equations to evaluate the reactors’ performance.
Measurement of biomass on carriers during start-up
Total solid substances (TSS) on the two types of carriers during start-up were measured by removing the complete biomass from the carriers and taking the weight after drying at 105 °C. In order to have another indicative measurement of the biomass concentration, total polysaccharide and protein contents of biomass attached to the carriers were also determined. Polysaccharide determination was performed according to the sulfuric acid-anthrone method, while total protein concentration was determined by Lowry’s method (Bassin et al. 2012).
Fluorescence in situ hybridization
The spatial distribution of AOB and NOB was examined by fluorescence in situ hybridization (FISH). Biomass samples of planktonic sludge and fixed biofilm were obtained randomly at stable phase (on day 60) from modified carrier-based R1 (Mod_planktonic and Mod_biofilm in abbreviation) and unmodified carrier-based R2 (Unm_planktonic and Unm-biofilm in abbreviation). The biomass in inoculated conventional active sludge (AS in abbreviation) was also detected. The biomass was dispersed to small clusters by ultrasonication for 10 min and fixed in a 4% (v/v) paraformaldehyde solution for 4 h. Afterwards, the samples were resuspended in a isometric mixture of phosphate-buffered saline (PBS) and 100% ethanol (1:1, v/v). Hybridization was carried out according to the previous report on a confocal scanning laser microscope (Leica TCS-SP2, Japan) (Amann et al. 1990). The oligonucleotide probe used in this experiment targeting AOB was NSO190 (5′-CGATCCCCTGCTTTTCTCC-3′) labeled with HEX dye, while the probe targeting NOB was NIT3 (5′-CCTGTGCTCCATGCTCCG-3′) labeled with Cy3 dye. The probes were prepared by a commercial service (TaKaRa, Japan).
DNA extraction, PCR, and high-throughput pyrosequencing data analysis
Biomass samples were obtained randomly at stable phase (on day 60), and DNA was extracted on the basis of the methods described previously (Liu et al. 2012). Primers for high-throughput pyrosequencing were Nobar-341F (5′-CCTACGGGNGGCWGCAG-3′) and Nobar-805R (5′-GACTACHVGGGTATCTAATCC-3′) targeting the V3–V4 region of bacterial 16S rRNA. The PCR protocol was as follows: 94 °C for 3 min; 5 cycles of 94 °C for 30 s, 45 °C for 20 s, and 65 °C for 30 s; 20 cycles of 94 °C for 30 s, 55 °C for 20 s, and 72 °C for 30 s; and 72 °C for 5 min. The PCR products were detected by 1.5% (w/v) agarose gel electrophoresis to confirm the product size and purified with the TIANgel Midi Purification Kit according to the manufacturer’s instructions. Finally, the high-throughput pyrosequencing was carried out on the Miseq Illumina by a commercial service (Sangon, China). Raw sequence data had been deposited to the NCBI Sequence Read Archive with accession no. SRR4302014.
For high-throughput pyrosequencing analysis, short- and low-complexity fragments were removed and paired-end reads were joined primarily by FLASH (http://sourceforge.net/projects/flashpage/). Afterwards, non-target sequences were removed and chimeras were detected by chimeras.uchime. The remained high-quality sequences were clustered and incorporated into operational taxonomic units (OTUs) by CD-HIT at the 97% sequence similarity threshold by using UCLUST (http://www.drive5.com/uclust/downloads1_1_579.html). Alpha-diversity (including richness, Shannon index, ACE index, Chao 1 index, Coverage and Simpson) was processed by using Mothur (http://www.mothur.org/) to evaluate the species variety in one sample. The software of Ribosomal Database Project (RDP) Classifier was performed for hierarchical clustering analysis with a confidence cutoff of 0.8 by Naïve Bayesian assignment arithmetic based on Bergey’s taxonomy that involved three levels of phylum, order, and genus, respectively.
Results
Novel carrier surface properties
The surface zeta potential of traditional HDPE carrier was −38.6 ± 0.8 mV (pH 7.0), indicating that this type of carrier was negatively charged. On the contrary, the modified carrier released a remarkable positive charge with the surface zeta potential of 11.7 ± 1.1 mV (pH 7.0), which was expected to greatly improve the electrophilicity and further to accelerate biofilm formation. Besides, the water contact angles of unmodified and modified carriers were 92.1 ± 2.7° and 57.5 ± 3.5°, respectively, implying the better hydrophilic feature of modified carrier.
Reactors’ performance during start-up
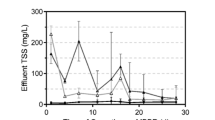
The performance of the two reactors filled with different carriers during the experiment is shown in Fig. 1. Distinct nitrification could be observed within the first few days after inoculation, which implied that both AOB and NOB existed in the seeding sludge. In the experimental reactor (R1), little nitrite (<10 mg/L) could be detected in the effluent while high nitrate generated during the first few days because of the immediate consumption of nitrite by NOB, whereas ARE kept over 60% and NAR stayed at low level. However, the effluent nitrite kept increasing while nitrate decreased gradually in the following days. To be specific, nitrite accumulated dramatically from day 7 to day 13 and NAR increased rapidly from 4 up to 62%. From day 14 to day 60 (phase II), R1 was operated in a steady way with the average effluent nitrite, effluent nitrate, ARE, and NAR of 131 and 14 mg/L and 90 and 91%, respectively, indicating the successful start-up of PN. The variation tendency of N-compounds in control reactor (R2) was similar to that in R1, while it took a longer period (21 days) to start up the PN process. From day 21 to day 60 (phase II), N-compounds in the effluent were stable with average ARE and NAR of 87 and 88%, respectively. The effluent nitrite in the two reactors could be further oxidized into nitrogen gas by either subsequent denitrification or anammox process.
Dynamics of biomass concentrations on carriers
The amount of biomass (TSS) on the two types of carriers is shown in Fig. 2a. For the modified carrier, a shorter period was required to establish a stable biomass on the support material and the amount of biomass was more than that on unmodified material. This advantage was mainly attributed to the improvement of the surface feature. Moreover, the concentrations of polysaccharide and protein followed a similar trend with TSS (Fig. 2b). These two parameters on the modified carrier were higher than those on traditional supporter, implying a higher biofilm formation rate on the modified carrier. When the contents reached a stable level, they slightly increased with the operation.
Spatial distribution of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria
It was known that the nitrogen removal performance had a very close relationship with the functional bacterial features; therefore, the N-removal bacterial distributions were detected by FISH (Fig. 3). Here, the samples of planktonic sludge and fixed biofilm were obtained from modified carrier-based R1 (Mod_planktonic and Mod_biofilm) and unmodified carrier-based R2 (Unm_planktonic and Unm-biofilm) on day 60 to evaluate whether differences of spatial distribution existed in planktonic sludge and fixed biofilm in the two MBBR systems. Since seeding sludge was derived from a conventional nitrification–denitrification wastewater treatment plant (WWTP), results indicated that almost the same amount of AOB and NOB existed in the seeding sludge (Fig. S1). However, the distribution of N-removal bacteria shifted remarkably and AOB was predominant when PN started up successfully (Fig. 3).
Microbial community structure and biodiversity at the reactors
The microbial community compositions in planktonic sludge and biofilm usually released differently in MBBR process according to a previous study (Biswas and Turner 2012). Consequently, the community structure and biodiversity of AS, Mod_biofilm, Mod_planktonic, Unm_biofilm, and Unm_planktonic had been evaluated by utilizing the Illumina high-throughput sequencing (HTS) technique. HTS results indicated that at least 31,907 effective sequences were obtained from each sample with an average length of 421 base pair after removing low-quality sequences and chimeras (Table 1). These effective sequences were normalized, and over 2000 (ranged from 2053 to 2673) OTUs were obtained (Table 1). To evaluate the relationship among the samples, the percentages of the shared OTUs and their corresponding sequences had been analyzed (Table S1), which indicated a relatively high proportion of shared OTUs (38.75%) among at least two of the five samples. Nevertheless, further analysis showed that only 255 of 6843 OTUs were shared by all samples (Table S1), whereas these OTUs accounted for 61.36% of the total sequences.
Alpha-diversity was used to evaluate the species variety. In this study, the Shannon indexes (H) were in the range of 5.06–5.52 (higher H value meant higher α-diversity), corresponding to the range of OTUs, Simpson values, and Chao 1 values (Table 1). The rarefaction curves of the sequencing results are shown in Fig. 4a. With the sequencing number increased, the curve of observed OTUs showed initial steep phase, then increased mildly, and finally became relatively flat.
Bacterial community analysis at high taxonomic levels
The major phyla in R1 and R2 were quite distinct from those in seed sludge (Fig. 5). It was displayed that the most abundant phylum was Proteobacteria in the samples except Mod_biofilm; nevertheless, bacterial diversity in the reactor shifted notably during the operation: Bacteroidetes increased sharply from 4.99 to over 32.17%, while Planctomycetes decreased from 20.4 to less than 2.54% in the two reactors. Besides, other phyla that were widespread in sewage treatment systems (Manefield et al. 2005) also held low proportions in these two reactors such as Gemmatimonadetes (0.66–1.32%), Acidobacteria (0.85–1.09%), Verrucomicrobia (0.71–2.30%), Chlamydiae (0.59–0.87%), Nitrospirae (0.11–1.62%), and Firmicutes (0.19–0.38%).
The identification and relative abundance of the bacterial communities at the order level are shown in Fig. 6 and Table 2. It was illustrated that the dominant orders in phyla Proteobacteria and Bacteroidetes presented an obvious shift from the seeding sludge to the biomass in the two reactors. Specifically, Sphingobacteriales and Burkholderiales belonging to phyla Bacteroidetes and Proteobacteria, respectively, enriched efficiently during the start-up, corresponding to the abundance of 31.12–48.75 and 13.22–37.29%, respectively (the abundance was 3.50 and 7.68%, respectively, in seeding sludge) (Fig. 6 and Table 2). Besides, within the phylum Planctomycetes, the abundance of Planctomycetales decreased remarkably from 20.39 to less than 2.52% during the start-up. Moreover, the population of the four orders (Sphingomonadales, Rhodospirillales, Clostridiales, and Verrucomicrobiales) declined considerably as well. Nitrospirales that belonged to the phylum of Nitrospirae reduced in planktonic sludge but increased in biofilm.
Analysis of the sequences at the genus level offered deep insights of the microbial community structure (Fig. 7). To be specific, two main genera were identified as predominance in the inoculums (Planctomyces and Candidatus), whereas almost all of them were eliminated during the start-up. On the contrary, a wide range of bacteria genera developed rapidly like Ferruginibacter, Ottowia, unclassified Saprospiraceae, Rhizobacter, Comamonas, Nitrosomonas, Dokdonella, unclassified Chitinophagaceae, Filimonas, etc.
Discussion
The successful start-up of PN verified the effective elimination of Nitrospira-related NOB and accumulation of Nitrosomonas-related AOB in the systems. It was reported that NOB could be inhibited in oxygen-limited condition attributed to the different oxygen saturation coefficients (Ks) of AOB and NOB (De Clippeleir et al. 2011). In this study, DO was kept at 0.8–1.0 mg/L, which was considered as one of the key factors to suppress NOB. Besides, AOB and NOB displayed distinct tolerances to free ammonium (FA): 0.6 mg/L of FA was sufficient to inhibit the bioactivity of NOB, while this concentration was over 5 mg/L for AOB (Alleman 1984). It was reasonable that the FA concentration of around 1.75 mg/L in these two reactors should be ideal to inhibit NOB’s growth but have little restrain on AOB. R1 had a much shorter start-up period with higher ARE and NAR than R2, indicating that the modified carriers with improved electrophilicity and hydrophilicity had distinct advantages of accelerating the PN start-up period and promoting PN performance. The dynamics of biomass concentrations on carriers (Fig. 2) also certified this advantage. It was evident in Fig. 1 that TN could be removed to some extent in both R1 and R2 throughout the operation period (NRE averaged 13.8 and 13.9%, respectively), implying the existence of denitrifying bacteria. It seemed that the aerobic systems in this study did not benefit for denitrifying bacterial metabolism; however, some anoxic/anaerobic microenvironment might be created inside the biofilm on carriers to protect these anaerobic microorganisms (Wang et al. 2006).
FISH images displayed in Fig. 3 that AOB was predominant, which was consistent with previous reports in similar systems to benefit the PN process (Calderon et al. 2012; Zhang et al. 2015). Nevertheless, NOB was minority but not thoroughly washed out. It was also worth mentioning that the distribution features released differently between planktonic sludge and fixed biofilm. Most N-removal bacteria exhibited free-floating cells in planktonic sludge (Fig. 3a, c), while these microorganisms trended to form small flocs in the biofilm (Fig. 3b, d). Besides, the N-removal bacterial distribution was almost the same in R1 and R2 except that the flocs in R1 were more compact than those in R2.
Percentages of the shared OTUs indicated a relatively low coherence of community, which proved the significant community differences among the five samples (Table S1). The biodiversity and richness of the samples in this study were similar to other municipal wastewater treatment systems (Ibarbalz et al. 2013; Zhu et al. 2013). Nevertheless, high biodiversity was identified in the seeding sludge, while Mod_planktonic showed the lowest α-diversity. Such selection was probably driven by the operation strategies and availability of substrate in the influent (Park et al. 2010). Noticeably, the richness and α-diversity were different between planktonic sludge and biofilm in R1, while they were approximate in R2 (Table 1), which might be attributed to different properties of carrier materials.
The rarefaction curves (Fig. 4a) indicated that the obtained sequencing data volume seemed reasonable to cover enough species. However, the rarefaction curves did not seem to reach the plateau, suggesting that there were still several minor proportions of OTUs unidentified. The Coverage values of the five samples also agreed with this phenomenon (in the range of 95.6–97.3%, Table 1). In addition, as shown in Fig. 4b, cluster analysis indicated a great difference of microbial community composition among seeding sludge, R1 and R2, and these taxonomic variances might result in diverse nitrogen removal performances.
The taxonomic identities of sequences at the phylum level showed that Proteobacteria was the most abundant phylum, which was in accordance with previous results in various municipal WWTPs (Ibarbalz et al. 2013; Wang et al. 2012; Zhang et al. 2012). Besides, Nitrospirae was a common nitrite-oxidizing phylum with a proportion of 0.49% in conventional sludge. With the reactor operation, Nitrospirae in planktonic sludge reduced to 0.11 and 0.20%, but it increased to 1.62 and 0.87% in biofilm, respectively, in R1 and R2. Interestingly, the carriers seemed to provide favorable conditions for Nitrospirae colonization; however, the PN process still runs stably without high nitrate generation, which might be attributed to inhibition of Nitrospirae activity according to the high FA concentration and oxygen-limited condition in the system.
The identification and relative abundance of bacterial communities at the order level (Fig. 5 and Table 2) illustrated that the dominant orders of Sphingobacteriales and Burkholderiales presented an obvious shift from the seeding sludge to the biomass in the two reactors. Previous studies indicated that Sphingobacteriales and Burkholderiales were common in various nitrogen removal systems, often known as heterotrophic denitrifiers in PN-SBR systems (Gabarro et al. 2012; Tian et al. 2015). In addition, some Burkholderiales was responsible for organic matter degradation in biofilm-colonized biofilters (Niemi et al. 2009). Moreover, recent research indicated that some heterotrophic bacteria like Comamonas with the order of Burkholderiales also had the function of oxidizing ammonia into nitrite (Chen and Ni 2011; Zhang et al. 2012). In this study, a certain amount of Comamonas had been found in R1 and R2 (Fig. 7). Therefore, the PN process might be attributed to the synergistic action of heterotrophic and autotrophic AOBs. Notably, Burkholderiales was a neighbor of Nitrosomonadales in the Ribosomal Database, and in many cases, some Nitrosomonadales sequences might be wrongly assigned by the RDP Classifier into the order of Burkholderiales and consequently lead to an overestimation of the abundance of Burkholderiales in the samples (Ye et al. 2011). The abundance of Planctomycetales decreased remarkably during the start-up. Planctomycetes were previously recognized as important members of activated sludge microbial communities but generally minority in PN systems (Juretschko et al. 2002), which was consistent with the results here. Nitrosomonadales, known as the predominant AOB in most sewage treatment plants (You et al. 2003), accounted for a small proportion in seeding sludge (0.93%). However, its abundance increased sharply when PN operated in stable phase (Table 2). It could be observed that the population of Nitrosomonadales was larger in biofilm than that in planktonic sludge (Table 2), implying a preference of AOB to inhabit in biofilm rather than sludge. Moreover, the proportion of Nitrosomonadales in Mod_biofilm was much higher than that in Unm_biofilm (**P < 0.01, two-tailed independent sample t test), which might lead to better PN performance in R1. Therefore, it could be concluded the modified carriers in R1 were indeed much beneficial for AOB’s growth as compared with those unmodified carriers in R2. Here, we had to say that the unexpected low richness of Nitrosomonadales in the reactors seemed to be contradictory with high PN efficiency in the reactors. Nevertheless, similar results had also been found in relevant systems (Liang et al. 2015; Ye et al. 2011).
Analysis of the sequences at the genus level illustrated that these genera compositions were quite similar with those obtained in a granular sludge-based PN reactor (Liang et al. 2015). Among these genera, Nitrosomonas was identified as functional genus for PN in the reactors. As mentioned before, Comamonas was regarded as a heterotrophic nitrifier as well as a promising denitrifier under various aeration conditions (Chen and Ni 2011; Zhang et al. 2012). The percentage of Comamonas was low (<1%) in the seeding sludge, but it increased obviously in the reactors (Fig. 7), which might play important roles together with Nitrosomonas in the PN process. Ferruginibacter, known as a type of heterotrophic freshwater bacterium, was abundant in the reactors (Fig. 7). Previous studies verified that the population of Ferruginibacter increased when the temperature rose (Kim et al. 2014) and some species were capable of hydrolyzing organic matter (Lim et al. 2009). Considering that the reactors operated under constant temperature of 28 °C and some organic substance coexisted in the effluent, they provide appropriate environment for Ferruginibacter inhabitation. Besides, Saprospiraceae and Ottowia also accounted for high proportions in PN systems. Previous studies revealed that some Saprospiraceae had the ability to degrade organic matters (Liang et al. 2015), while some species of Ottowia could reduce nitrate and nitrite in sewage treatment systems (Geng et al. 2014).
References
Alleman JE (1984) Elevated nitrite occurrence in biological wastewater treatment systems. Water Sci Techno 17(2–3):409–419
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16s ribosomal-RNA-targeted oligonucleotide probes with flow-cytometry for analyzing mixed microbial-populations. Appl Environ Microb 56(6):1919–1925
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
Bassin JP, Kleerebezem R, Rosado AS, van Loosdrecht MCM, Dezotti M (2012) Effect of different operational conditions on biofilm development, nitrification, and nitrifying microbial population in moving-bed biofilm reactors. Environ Sci Technol 46(3):1546–1555. doi:10.1021/es203356z
Biswas K, Turner SJ (2012) Microbial community composition and dynamics of moving bed biofilm reactor systems treating municipal sewage. Appl Environ Microb 78(3):855–864. doi:10.1128/Aem.06570-11
Blackburne R, Yuan ZG, Keller J (2008) Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 19(2):303–312. doi:10.1007/s10532-007-9136-4
Calderon K, Martin-Pascual J, Poyatos JM, Rodelas B, Gonzalez-Martinez A, Gonzalez-Lopez J (2012) Comparative analysis of the bacterial diversity in a lab-scale moving bed biofilm reactor (MBBR) applied to treat urban wastewater under different operational conditions. Bioresource Technol 121:119–126. doi:10.1016/j.biortech.2012.06.078
Chen Q, Ni JR (2011) Heterotrophic nitrification-aerobic denitrification by novel isolated bacteria. J Ind Microbiol Biot 38(9):1305–1310. doi:10.1007/s10295-010-0911-6
Chen S, Cheng X, Zhang X, Sun DZ (2012) Influence of surface modification of polyethylene biocarriers on biofilm properties and wastewater treatment efficiency in moving-bed biofilm reactors. Water Sci Technol 65(6):1021–1026. doi:10.2166/wst.2012.915
Chu ZR, Wang K, Li XK, Zhu MT, Yang L, Zhang J (2015) Microbial characterization of aggregates within a one-stage nitritation-anammox system using high-throughput amplicon sequencing. Chem Eng J 262:41–48. doi:10.1016/j.cej.2014.09.067
De Clippeleir H, Yan XG, Verstraete W, Vlaeminck SE (2011) OLAND is feasible to treat sewage-like nitrogen concentrations at low hydraulic residence times. Appl Microbiol Biot 90(4):1537–1545. doi:10.1007/s00253-011-3222-6
Gabarro J, Ganigue R, Gich F, Ruscalleda M, Balaguer MD, Colprim J (2012) Effect of temperature on AOB activity of a partial nitritation SBR treating landfill leachate with extremely high nitrogen concentration. Bioresource Technol 126:283–289. doi:10.1016/j.biortech.2012.09.011
Gabarro J, Hernandez-del Amo E, Gich F, Ruscalleda M, Balaguer MD, Colprim J (2013) Nitrous oxide reduction genetic potential from the microbial community of an intermittently aerated partial nitritation SBR treating mature landfill leachate. Water Res 47(19):7066–7077. doi:10.1016/j.watres.2013.07.057
Geng S, Pan XC, Mei R, Wang YN, Sun JQ, Liu XY, Tang YQ, Wu XL (2014) Ottowia shaoguanensis sp nov., isolated from coking wastewater. Curr Microbiol 68(3):324–329. doi:10.1007/s00284-013-0481-8
Hibiya K, Tsuneda S, Hirata A (2000) Formation and characteristics of nitrifying biofilm on a membrane modified with positively-charged polymer chains. Colloid Surface B 18(2):105–112. doi:10.1016/S0927-7765(99)00141-1
Ibarbalz FM, Figuerola ELM, Erijman L (2013) Industrial activated sludge exhibit unique bacterial community composition at high taxonomic ranks. Water Res 47(11):3854–3864. doi:10.1016/j.watres.2013.04.010
Juretschko S, Loy A, Lehner A, Wagner M (2002) The microbial community composition of a nitrifying-denitrifying activated sludge from an industrial sewage treatment plant analyzed by the full-cycle rRNA approach. Syst Appl Microbiol 25(1):84–99. doi:10.1078/0723-2020-00093
Kim TG, Yun J, Hong SH, Cho KS (2014) Effects of water temperature and backwashing on bacterial population and community in a biological activated carbon process at a water treatment plant. Appl Microbiol Biot 98(3):1417–1427. doi:10.1007/s00253-013-5057-9
Lackner S, Holmberg M, Terada A, Kingshott P, Smets BF (2009) Enhancing the formation and shear resistance of nitrifying biofilms on membranes by surface modification. Water Res 43(14):3469–3478. doi:10.1016/j.watres.2009.05.011
Liang YH, Li D, Zeng HP, Zhang CD, Zhang J (2015) Rapid start-up and microbial characteristics of partial nitrification granular sludge treating domestic sewage at room temperature. Bioresource Technol 196:741–745. doi:10.1016/j.biortech.2015.08.003
Lim JH, Baek SH, Lee ST (2009) Ferruginibacter alkalilentus gen. nov., sp nov and Ferruginibacter lapsinanis sp nov., novel members of the family ‘Chitinophagaceae’ in the phylum Bacteroidetes, isolated from freshwater sediment. Int J Syst Evol Micr 59:2394–2399. doi:10.1099/ijs.0.009480-0
Liu T, Li D, Zeng HP, Li XK, Zeng TT, Chang XY, Cai YA, Zhang J (2012) Biodiversity and quantification of functional bacteria in completely autotrophic nitrogen-removal over nitrite (CANON) process. Bioresource Technol 118:399–406. doi:10.1016/j.biortech.2012.05.036
Manefield M, Griffiths RI, Leigh MB, Fisher R, Whiteley AS (2005) Functional and compositional comparison of two activated sludge communities remediating coking effluent. Environ Microbiol 7(5):715–722. doi:10.1111/j.1462-2920.2004.00746.x
Niemi RM, Heiskanen I, Heine R, Rapala J (2009) Previously uncultured beta-Proteobacteria dominate in biologically active granular activated carbon (BAC) filters. Water Res 43(20):5075–5086. doi:10.1016/j.watres.2009.08.037
Park H, Rosenthal A, Jezek R, Ramalingam K, Fillos J, Chandran K (2010) Impact of inocula and growth mode on the molecular microbial ecology of anaerobic ammonia oxidation (anammox) bioreactor communities. Water Res 44(17):5005–5013. doi:10.1016/j.watres.2010.07.022
Strous M, Van Gerven E, Zheng P, Kuenen JG, Jetten MSM (1997) Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (anammox) process in different reactor configurations. Water Res 31(8):1955–1962. doi:10.1016/S0043-1354(97)00055-9
Tian M, Zhao FQ, Shen X, Chu KH, Wang JF, Chen S, Guo Y, Liu HH (2015) The first metagenome of activated sludge from full-scale anaerobic/anoxic/oxic (A2O) nitrogen and phosphorus removal reactor using Illumina sequencing. J Environ Sci-China 35:181–190. doi:10.1016/j.jes.2014.12.027
Tsuneda S, Park S, Hayashi H, Jung J, Hirata A (2001) Enhancement of nitrifying biofilm formation using selected EPS produced by heterotrophic bacteria. Water Sci Technol 43(6):197–204
Wang XJ, Xia SQ, Chen L, Zhao JF, Renault NJ, Chovelon JM (2006) Nutrients removal from municipal wastewater by chemical precipitation in a moving bed biofilm reactor. Process Biochem 41(4):824–828. doi:10.1016/j.procbio.2005.10.015
Wang XH, Hu M, Xia Y, Wen XH, Ding K (2012) Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl Environ Microb 78(19):7042–7047. doi:10.1128/Aem.01617-12
Ye L, Shao MF, Zhang T, Tong AHY, Lok S (2011) Analysis of the bacterial community in a laboratory-scale nitrification reactor and a wastewater treatment plant by 454-pyrosequencing. Water Res 45(15):4390–4398. doi:10.1016/j.watres.2011.05.028
Yoo K, Ahn KH, Lee HJ, Lee KH, Kwak YJ, Song KG (1999) Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res 33(1):145–154
You SJ, Chuang SH, Ouyang CF (2003) Nitrification efficiency and nitrifying bacteria abundance in combined AS-RBC and A2O systems. Water Res 37(10):2281–2290. doi:10.1016/S0043-1354(02)00636-X
Zekker I, Rikmann E, Tenno T, Lemmiksoo V, Menert A, Loorits L, Vabamae P, Tomingas M, Tenno T (2012) Anammox enrichment from reject water on blank biofilm carriers and carriers containing nitrifying biomass: operation of two moving bed biofilm reactors (MBBR). Biodegradation 23(4):547–560. doi:10.1007/s10532-011-9532-7
Zeng TT, Li D, Zeng HP, Zhang Z, Liu LQ, Zhang XJ, Zhang J (2013) Analysis of microbial population dynamics in a partial nitrifying SBR at ambient temperature. Curr Microbiol 66(6):614–620. doi:10.1007/s00284-013-0317-6
Zhang T, Shao MF, Ye L (2012) 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. Isme J 6(6):1137–1147. doi:10.1038/ismej.2011.188
Zhang XJ, Li D, Liang YH, Zeng HP, He YP, Fan D, Zhang J (2015) Start-up, influence factors, and the microbial characteristics of partial nitrification in membrane bioreactor. Desalin Water Treat 54(3):581–589. doi:10.1080/19443994.2014.891081
Zhu XB, Tian JP, Liu C, Chen LJ (2013) Composition and dynamics of microbial community in a zeolite biofilter-membrane bioreactor treating coking wastewater. Appl Microbiol Biot 97(19):8767–8775. doi:10.1007/s00253-012-4558-2
Acknowledgements
We thank Hong-lei Zhan from Stevens Institution of Technology for providing language help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (grant number 51408095), the China Postdoctoral Science Foundation (grant numbers 2014M561236, 2015T80257), the Foundation of Liaoning Educational Committee (grant number L2014021), and the Fundamental Research Funds for the Central Universities (grant number DUT16RC(4)13).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 35 kb)
Rights and permissions
About this article
Cite this article
Liu, T., Mao, Yj., Shi, Yp. et al. Start-up and bacterial community compositions of partial nitrification in moving bed biofilm reactor. Appl Microbiol Biotechnol 101, 2563–2574 (2017). https://doi.org/10.1007/s00253-016-8003-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-8003-9