Abstract
Soluble expression of recombinant therapeutic proteins in Escherichia coli (E. coli) has been a challenging task in biopharmaceutical development. In this study, a novel self-cleavable tag Zbasic–intein has been constructed for the soluble expression and purification of a recombinant cytokine, human interleukin-15 (IL-15). We screened several solubilizing tags fused with the self-cleavable Mycobacterium tuberculosis recA mini-intein ∆I-CM and demonstrated that Zbasic tag can significantly improve the solubility of the product with correspondent to the intein activity. The fusion protein “Zbasic–∆I-CM–IL-15” was expressed with high solubility and easily enriched by the cost-effective cation-exchange chromatography. The self-cleavage of the fusion tag Zbasic–∆I-CM was then induced by a pH shift, with an activation energy of 7.48 kcal/mol. The mature IL-15 with natural N-terminus was released and further purified by hydrophobic interaction and anion-exchange chromatography. High-resolution reverse-phase high-performance liquid chromatography and mass spectrometry analysis confirmed that the product was of high purity and correct mass. With a CTLL-2 cell proliferation-based assay, the EC50 was evaluated to be of about 0.126 ng/mL, similar to the product in clinical trials. By avoiding the time-consuming denaturing-refolding steps in previously reported processes, the current method is efficient and cost-effective. The novel tag Zbasic–∆I-CM can be potentially applied to large-scale manufacturing of recombinant human cytokines as well as other mammalian-sourced proteins in E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recombinant human cytokines, such as interleukin-15 (rhIL-15), are of great clinical potential for its lymphocyte homeostatic and NK cell development functions (Brincks and Woodland 2010; Liu et al. 2000; Ma et al. 2006). Currently, there are already multiple IL-15 clinical trials, in phase I or phase II, to develop rIL-15 for cancer immunotherapy or as an anti-HIV vaccine adjuvant (Mueller and Katsikis 2010; Ward et al. 2009). In order to fulfill the clinical demands, a simple, cost-effective method to produce rhIL-15 is urgently needed. However, low expression level and purification difficulties of rhIL-15 build up barriers for scalable clinical production (Béhar et al. 2011; Vyas et al. 2012). The expression of recombinant IL-15 has been studied in various expressing systems, including yeast, Escherichia coli (E. coli), and mammalian cells (Chertova et al. 2013; Han et al. 2011; Huang et al. 2006; Luo et al. 2015; Sun et al. 2016). As reported, the highest expression in mammalian cells was 890 ng/106 cells/24 h, which was achieved in HEK293 cells, but no purification data was provided (Huang et al. 2006). In yeast, c-Myc and FLAG-tagged IL-15 were expressed at about 75 mg/L/3 days, then deglycosylated and purified by Ni-NTA affinity chromatography (Sun et al. 2016). In E. coli, human and cynomolgus IL-15 could be expressed up to 100 mg/L when with a 6His tag (Ward et al. 2009). Due to its relatively short production cycle, high yield, and low investment on batch cultivation, E. coli is a feasible expression system for production of IL-15 (Nellis et al. 2012; Vyas et al. 2012).
Similar to most of the mammalian-sourced proteins, the IL-15 expressed in E. coli tends to form inclusion bodies, which requires a time-consuming denaturing and refolding process during purification. Solubilizing purification tags, such as glutathione S-transferase (GST) and maltose-binding protein (MBP), was employed to improve the solubility of target proteins (Pennati et al. 2014). In recent years, many types of novel purification tags are characterized. Some of them also improve target protein solubility, such as the hyperbasic tag “Zbasic” (Hedhammar and Hober 2007) and the human amyloid precursor protein (APP) hyperacidic region (Sangawa et al. 2013). With these novel tags, the fusion protein can be purified by conventional matrices like ion-exchange resin, which are relatively cost-effective than affinity matrices. However, tags must be removed precisely during purification according to the guideline of Food and Drug Administration (FDA), usually using proteases, such as enterokinases, thrombin, or factor Xa. But, these proteases can in turn cause significant cost increase and impurity cleanup afterward.
The C-terminal cleaving intein is an ideal alternative to apply together with the solubilizing tags. Intein is a self-catalytic segment, which can excise itself from a precursor protein posttranslationally (Perler 2002). Combined with affinity tags, such as chitin-binding domain (CBD), small ubiquitin-like modifier (SUMO), and elastin-like peptide (ELP), several kinds of self-cleavable purification tags have been reported (Setrerrahmane et al. 2014; Wood and Camarero 2014; Xie et al. 2013). Since only about 10 % of mammalian-sourced proteins could be solubly expressed in E. coli (Graslund et al. 2008), the intein-based soluble self-cleavable tagged protein expression is especially valuable to overcome the difficulties that resulted from inclusion bodies. Another important advantage of these intein-based tags is that they are generally enzymatic in nature and therefore exhibit highly specific activities under chosen physiological conditions (Wood and Camarero 2014). However, according to previous reports, intein activity is tightly related to its flanking exteins when fused in a precursor protein (Amitai et al. 2009; Kwong and Wong 2013; Wood et al. 2000), and the adequate tags should be optimized according to each target protein.
Here in this paper, we screened several novel tags fused with intein to construct the solubilizing self-cleavable chromatographic tag for the production of IL-15. We demonstrated that the novel tag “Zbasic–∆I-CM” successfully improved the solubility of IL-15 and accelerated the purification. With the new strategy, we successfully produced the N-terminal methionine-free rhIL-15 with high purity and bioactivity, without having to go through the inefficient and time-consuming denaturing-refolding steps.
Materials and methods
Reagents and materials
Competent cells of the E. coli strains DH5α and BL21 (DE3) were obtained from Microgene (Shanghai, China). CTLL-2 cell line was purchased from ATCC (no. TIB-214). Plasmids pTWIN1 and pMAL-C2 were obtained from New England Biolabs (NEB, USA). SP Sepharose Fast Flow (FF) resin, HiTrap Butyl HP, and Q Sepharose FF column were purchased from GE Healthcare (Piscataway, NJ, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). Taq DNA polymerase, T4 DNA ligase, and restriction enzymes were obtained from TaKaRa Biotechnology (Dalian, China). The PCR Purification Kit, Plasmid Mini Kit, and Gel Extraction Kit were purchased from Axygen (Hangzhou, China). The IL-15 monoclonal antibody was purchased from R&D Systems (MN, USA). The rhIL-15 (lot L1010008R/D) received from Biological Resource Branch at National Cancer Institute (BRB/NCI) as a gift was used as the reference standard and molecular weight (MW) standards. All other chemicals and reagents were obtained from Sinopharm Chemical Reagent (Beijing, China).
Plasmid construction
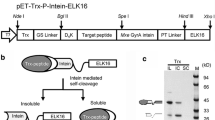
Multiple “tag–intein–IL15” vectors were constructed for expression in E. coli, as shown in Table 1.
We take Zbasic–intein–IL-15 (ZIIL15) and MBP–intein–IL-15 fusion proteins as examples to describe the vector construction (Fig. 1). The DNA fragment of ∆I-CM intein and Zbasic was synthesized following the published sequences (Hedhammar and Hober 2007; Wood et al. 1999). The DNA fragment of IL-15 was synthesized according to the NCBI reference NM_000585.4. MBP fragment was cloned from pMAL-C1. A His6 fragment was inserted into a pET30a vector at the upstream of MCS. Intein and IL-15 were connected by PCR (primer 1 5′-GAAGGGGTTGTCGTGCACAACAACTGGG TGAATGTAA-3′ for IL-15; primer 2 5′-TTACATTCACCCAGTTGTTGTGC ACGACAACCCCTTC-3′ for intein). The fragments that were inserted into the pET30a vector by type II restriction enzyme BsaI assisted “golden gate” cloning (Engler and Marillonnet 2014). Between Zbasic and intein, there is a flexible short-linker GGSG, encoded by GGTGGTTCTGGT. The expected rhIL-15 product is an 114aa cytokine containing two disulfide bonds, without the N-terminal methionine.
Soluble expression of ZIIL15
The fusion proteins were expressed in E. coli BL21. In detail, E. coli (BL21) cells containing the expressing plasmids were inoculated at 1:100 in LB media supplemented with 100 μg/mL kanamycin in shake flasks. The cells were then grown at 37 °C for 3–4 h, reaching an OD600 of ∼1.0. The expression was induced by 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 20 °C for 4 h. The cells were harvested by centrifugation, resuspended in phosphate-buffered saline (PBS) at 10 % weight/volume ratio, and disrupted by homogenization for six runs at 900 bar. After centrifugation at 12,000×g, 30 min at 4 °C, the soluble and insoluble fractions were analyzed by 12 % SDS-PAGE to evaluate the solubility and intein activity of each fusion protein. Based on the results, the fusion protein Zbasic–∆I-CM–IL-15 (ZIIL15) was selected for further studies.
Purification of fusion protein
Zbasic is a super alkaline tag with an isoelectric point (pI) of 11.49; so, we used the cation-exchange resin SP FF to purify the fusion protein. We took buffer A1 (100 mM Tris, 0.5 M arginine, 2 mM EDTA, 100 μM phenylmethylsulfonyl fluoride (PMSF), pH 9.0) as the homogenization buffer. Then, the soluble fusion protein was loaded onto the SP FF column (C V = 10 mL) at a flow rate of 1.0 mL/min. Then, the column was washed with buffer A1 until the absorbance at UV 280 nm reached baseline. The target protein was eluted by a linear gradient of buffer B1 (100 mM Tris, 0.5 M arginine, 1.2 M NaCl, 2 mM EDTA, 100 μM PMSF, pH 9.0) with a flow rate of 1.0 mL/min. The performance of SP FF chromatography was analyzed by 12 % SDS-PAGE and Western blot, with specific anti-human IL-15 primary antibodies and HRP-conjugated goat anti-mouse secondary antibodies (Ashley et al. 1988).
Cleavage activity of intein
The activity of the mini-intein ∆I-CM is pH and temperature dependent (Wood et al. 1999). To screen the optimal cleaving conditions, the fusion protein was incubated under various temperatures (4, 10, 16, 20, 25, 30, and 37 °C) at pH 6.0 or under various pH (6.0, 6.5, 7.0, 7.5, and 8.0) at 25 °C. Samples were collected hourly and electrophoresed on 12 % SDS-PAGE. The protein bands were quantified by scanning densitometry with the Bio-Rad Quantity One software. We used previous reported method to define the cleavage percentage (Wood et al. 2000). In brief, the cleavage percentage was defined as the intensity percentage of intein band in the sum of ZIIL15 and tag bands. To avoid the effect of molecular weight difference between fusion protein and cleaved fragment, we divided the optical density of bands by their respective molecular weights. The intein cleavage reaction was modeled as an irreversible first-order decay reaction of the formula C t = C∞•e−kt, where t is the incubation time, C t is the cleavage percentage at t, C∞ is the terminal cleavage percentage, and k is a function of pH and temperature, representing the first-order degradation constant (Trendline function, Microsoft Excel 2007).
Purification of released IL-15
IL-15 was released by the C-terminal cleavage of intein after the fusion protein was incubated at optimized conditions, 25 °C, pH 6.0 for 6 h. The released IL-15 was further purified by hydrophobic interaction chromatography (HIC). In detail, HiTrap Butyl HP column (C V = 1 mL) was equilibrated with buffer B1 and the sample was loaded at a flow rate of 1.0 mL/min. The target protein was eluted by a linear gradient to buffer B2 (20 mM Tris, 100 mM GuHCl, pH 7.4) in 10 C V .
Finally, the targeted protein rhIL-15 was concentrated using an AIEX Q FF (C V = 1 mL). The column was equilibrated with buffer A2 (50 mM Tris, 100 mM NaCl, 1 mM EDTA, pH 7.4). The conductivity sample from HIC was adjusted to a conductivity as that of buffer A2 and loaded onto the column at 1.0 mL/min. After the column was washed, rhIL-15 was eluted by a linear gradient to buffer B3 (50 mM Tris, 1 M NaCl, 1 mM EDTA, pH 7.4).
Characterization of IL-15 by HPLC and LC-MS
We used a high-resolution RP-HPLC method (ExRP-HPLC) to determine the purity and deamidation of the product as described in previous publication (Nellis et al. 2012). Agilent 1260 HPLC system (Agilent, Santa Clara, CA) was fitted with tandem-plumbed C18 columns (X-Bridge BEH300, C18, 3.5 μm, 250 mm, Waters, Milford, MA) at 20 °C. Mobile phases were 0.08 % TFA and 0.02 % formic acid in HPLC-grade water or acetonitrile. Elution was performed at 0.14 mL/min and monitored at 210 nm (min-%B 0–10, 12–10, 16–47, 116–53, 126–100, 128–100, 130–10, 142–10). Peak areas were integrated using the Agilent Chem Station software. To confirm IL-15 preparation identities, a Waters Micromass QTOF Premier mass spectrometer interfaced with a Waters Acquity ultra high-performance liquid chromatography system was used for mass measurement. A gradient of 0.9 % B/min (A 0.1 % aqueous formic acid; B 0.1 % formic acid in acetonitrile) was used with an Acquity UPLC BEH C18 (1 × 100 mm) column. The column was heated to 40 °C. Mass spectrometer conditions are as follows: capillary was 2.5 kV, sampling cone was 45 V, source temperature was 100 °C, desolvation temperature was 200 °C, and desolvation gas flow was 350 L/min (Nellis et al. 2012).
Bioactivity analysis
The bioactivity of rhIL-15 can be determined as described previously (Soman et al. 2009). Briefly, CTLL-2 cells were cultured in the complete medium (RPMI-1640 supplemented with 10 % fetal bovine serum, 1000 U/mL IL-2, and 100 U/mL penicillin/streptomycin). Both the purified rhIL-15 and the reference standard were diluted to desired concentrations with assay medium (RPMI-1640 supplemented with 10 % fetal bovine serum and 100 U/mL penicillin/ streptomycin) and transferred to a 96-well plate at 50-μL volume. Next, 50 μL of prepared cell suspension (2 × 105 cells/mL) was also transferred to each well. This 100 μL assay medium (each well) containing 1 × 104 cells was then incubated at 37 °C, 5 % CO2 for 48 h. Finally, CCK-8 was added (10 μL/well) and incubated for another 1–4 h at 37 °C and 5 % CO2. The absorbance was then read at 450 nm, with a reference at 600 nm. With the GraphPad Prism software, we analyzed the data and calculated the EC50 value using the four-parameter non-linear logistic regression model as interpreted previously (Soman et al. 2009).
Results
Plasmid construction and soluble expression of ZIIL15
Using the self-cleavable intein ∆I-CM and Ssp DnaB, we constructed several self-cleavable “tag–intein” combinations to be fused at the N-terminus of rhIL-15 as shown in Table 1. The solubility and intein activity are summarized in Table 2.
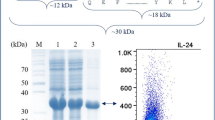
According to our studies on different tags as shown in Table 2, Zbasic–∆I-CM is the only tag that both improved solubility and showed high-intein activity. To demonstrate the capability of Zbasic on improving target protein solubility, MBP, which has been shown to increase protein solubility in previous studies, was used as a reference in this experiment. Both fusion proteins achieved high solubility when induced by 0.5 mM IPTG at 20 °C (Fig. 2a). The soluble fractions were incubated at 25 °C, pH 6.0 for 8 h for the intein cleavage. Along with the incubation, the band of ZIIL15 diminished and the band of Zbasic–∆I-CM accumulated, while no significant cleavage was observed for MBP–∆I-CM–IL-15 fusion (Fig. 2b). A separate Western blot using anti-IL-15 antibody was performed to confirm the identity of the fusion (data not shown). Since IL-15 monomer is instable in vitro and rapid cleavage is preferred for its production, Zbasic–∆I-CM–IL15 showed advantage over MBP–∆I-CM–IL-15 and was used for further study.
SDS-PAGE analysis of solubility and intein activity of Zbasic–intein–IL-15 and MBP–intein–IL-15. a Expression. M molecular weight, Sup soluble fraction after induction, IB inclusion bodies. b Cleavage activity. Lane 0 before cleavage, lane 2 after 2-h cleavage, lane 4 after 4-h cleavage, lane 6 after 6-h cleavage, lane 8 after 8-h cleavage. Predicted MW Zbasic ∼7 kDa, MBP ∼42 kDa, ∆I-CM ∼18 kDa, IL-15 ∼12 kDa. The black arrows point to the fusion proteins, and the white arrow points to the tag–intein fragment
Purification of fusion protein
Zbasic is a hyperbasic tag of 58aa and with a high pI of 11.49, which can be taken advantage of using a cation IEX (CIEX) for the purification. The expressed soluble fusion protein was loaded onto an SP FF column (Fig. 3c), and most of the negatively charged host cell proteins flowed through as shown in Fig. 3a. Due to highly positive-charged Zbasic fragment, the fusion protein was captured by SP FF resin and eluted with increasing conductivity as shown in Fig. 3b. We also observed that the fusion protein was easy to aggregate both in liquid or on column. To reduce the aggregation, we supplemented 0.5 M arginine to stabilize the protein in both buffers A1 and B1.
Impact of pH and temperature on cleavage efficiency of ZIIL15
According to previous reports, the activity of C-terminal cleaving intein is pH and temperature dependent. We tested the self-cleavage ability of intein in the fusion protein. Fusion protein of ZIIL15 was partially purified by SP FF. Preliminary kinetic experiments revealed that after 6-h incubation at pH 6.0 and 25 °C, about 80 % of the fusion protein were self-cleaved and IL-15 was released (Fig. 4a, b). Compared with the significant intensity change of the fusion protein band, the band of the released IL-15 is merely visible, which might have been caused by the instability of IL-15. We calculated the intensity percentage of intein band in the sum of ZIIL15 and intein bands to calculate the cleavage percentage. The cleavage of △I-CM intein was found indeed to be pH and temperature dependent. When the pH was at 6.0, the cleavage efficiency reached its peak. The self-cleavage of intein was observed at pH 6.0, 6.5, 7.0, 7.5, and 8.0, and its efficiency went down with the pH increase (data is shown in Fig. S1). Remarkably, ln(k) was linearly related to pH at pH > 7.0, thus exhibiting characteristics of a simple proton-catalyzed reaction (Wood et al. 2000).
Cleavage efficiency of Zbasic–∆I-CM. a SDS-PAGE and b Western blot analysis of ZIIL15 cleavage after different incubation durations. Lane M marker, lanes 0–6 incubation time (hours). After 6-h incubation at pH 6.0 and 25 °C, above 80 % of fusion protein was cleaved and IL-15 was released. c, d Efficiency of self-cleavage at different temperatures at pH 6.0. c The cleavage kinetic curves with the cleavage rate constant (k) as slope. d Arrhenius plot of ln(k) versus inverse temperature for determination of the cleavage activation energy, 0–6 cleaving time (hours). The black arrows point to the fusion proteins, and the gray arrows point to the released IL-15
We further studied the dependence of cleavage efficiency on temperature at the optimal cleavage pH 6.0. According to the calculated kinetics over a range of temperatures (4, 10, 16, 25, and 30 °C), cleavage efficiency increased when the temperature rose but the kinetics at 25 °C was similar to that at 30 °C (Fig. 4c). On the other hand, the fusion protein tended to aggregate at higher temperature (heavily aggregated at 37 °C; data not shown); therefore, 25 °C was determined to be the optimal temperature for cleavage.
According to previous studies (Wood et al. 2000), the cleavage reaction fits the classical Arrhenius equation, ln(k) = ln(A) − (E a /R) × (1/T), where k is the cleavage rate constant from the previous formula, A is the pre-exponential factor, R is the molar gas constant (1.985 [cal K−1 mol−1]), T is the thermodynamic temperature, and E a is the Arrhenius activation energy (cal/mol). In our studies about ZIIL15 (Fig. 4d), the activation energy of ∆I-CM was calculated to be 7.48 kcal/mol, which is close to the 3–5 kcal/mol typically reported for enzyme-catalyzed reactions (Bailey 1986).
Purification of released IL-15
IL-15 fragment was released by the C-terminal cleavage of intein tag after incubation at pH 6.0, 25 °C for 6 h. The released IL-15 was loaded onto a HiTrap Butyl HP column and eluted with a linear salt gradient. IL-15 was mainly in the second peak, where the conductivity was below 30 mS/cm (Fig. 5c). The HIC elution pool was then loaded onto a Q FF column, and the rhIL-15 was eluted with a linear salt gradient, as shown in Fig. 5d.
SP-cleavage-butyl-Q purification processes of rhIL-15. a, b SDS-PAGE and Western blot of purification of ZIIL15 and IL-15 at each step. Lane M marker, lane Ind total protein of bacteria after induction, lane IB inclusion bodies, lane Sup soluble fraction, lane SP SP FF elute, lane C post self-cleavage, lane HIC Butyl HP elute, lane Q Q FF elute. c Butyl HP chromatography profile. d Q FF chromatography profile
The entire process of expression and purification of IL-15 is shown in Fig. 5a, b. Fusion protein was expressed after IPTG induction (lane Ind), and the soluble fraction was selected for purification (lane Sup). After SP FF capture, off-column cleavage was conducted and the released IL-15 was further purified with butyl and Q columns. This process saved at least two steps compared with previous reports (Vyas et al. 2012).
Characterization of IL-15 by HPLC and LC-MS
The quality of rhIL-15 was analyzed by HPLC and LC-MS. Using high-resolution ExRP-HPLC, the purity of rhIL-15 final product was determined to be 92 %, among which about 6 % were deamidated, as shown in Fig. 6a. Benefited from intein functions, our product was with the natural N-terminus. That explains the slight difference in retention time in HPLC when comparing with reference, which contained an extra N-terminal methionine (Fig. 6b). ExRP-HPLC was also used for deamidation assessment of IL-15. Our product and the reference were both incubated at 25 °C for 3 days to induce deamidation before analysis. Each protein showed the following three consecutive peaks: an early isoaspartate form deamidated rhIL-15 peak (peak B), the intrinsic non-deamidated rhIL-15 peak (peak N), and a small peak of aspartate form deamidated rhIL-15 (peak D) (Fig. 6c, d), which was consistent with the previously reported results (Nellis et al. 2012).
ExRP-HPLC and LC-MS analyses of rhIL-15 final product. a rhIL-15 from intein system. b rhIL-15 reference standard. c Deamidated rhIL-15 from intein system. d Deamidated rhIL-15 reference standard. Peak B, isoaspartate form of deamidated rhIL-15; peak N, non-deamidated rhIL-15; peak D, aspartate form of deamidated rhIL-15. e Mass spectrometry analysis of rhIL-15
We used QTOF mass spectrometry to obtain the accurate molecular weight of the purified rhIL-15. As the result shown in Fig. 6e, the measured MW is 12,769.5 Da, 131.1 Da less than the reference MW 12,900.6 Da (Nellis et al. 2012), which is exactly the molecular weight of methionine residue. It demonstrated that Zbasic–∆I-CM was precisely cleaved, and no extra amino acids were left in the target protein.
Bioactivity analysis of prepared IL-15
CTLL-2 cell proliferation assay was used to evaluate the bioactivity of rhIL-15. The purified rhIL-15 was diluted with an assay medium by serial 1.5-fold dilutions. Of the prepared cell suspension containing 104 cells, 50 μL was transferred to each well of a 96-well plate containing 50 μL of rhIL-15 at different concentrations. The cells were incubated at 37 °C, 5 % CO2. Fourty-eight hours later, CCK-8 was added and incubated for another 1 h at 37 °C and 5 % CO2. The plate was then read at 450 and 600 nm (reference) as described in “Materials and methods” section. As shown in Fig. 7, the calculated EC50 of our product was 0.1261 ng/mL, close to that of the reference (Soman et al. 2009).
Discussion
In this study, we reported an intein-based novel self-cleavable solubilizing tag Zbasic–∆I-CM and its application in the soluble expression and purification of rhIL-15. E. coli is widely used in biopharmaceutical production. However, previous reports demonstrated that the preparation of rhIL-15 monomer in E. coli is very challenging due to the low expression and complicated purification (Vyas et al. 2012). Usually, tags like MBP and His6 are able to improve protein solubility or expression level, as well as simplify the purification. These tags also work well for IL-15 (Pennati et al. 2014; Sun et al. 2016). For clinical purpose, tags must be removed after purification to eliminate unintended effect or immunogenicity. Usually, those tags are cleaved with site-specific proteases, which can be expensive and time-consuming, and often introduce extra amino acids at the cleavage site to the final product.
For its self-splicing or self-cleaving ability, intein has been widely used in protein purification, ligation, and circularization (Böcker et al. 2015; Perler and Allewell 2014; Shi et al. 2014). The engineered C-terminal self-cleaving intein is especially valuable for protein expression and purification (Wood and Camarero 2014). This type of intein is pH and temperature sensitive. It is relatively stable at a higher pH (e.g., 8.5), allowing its purification at a stable pH range and cleavage by a shift to lower pH (e.g., 6.5). The cleavage efficiency also increases with temperature and peaks at 37 °C (Wood et al. 2000). A number of affinity tags or non-chromatographic purification tags were reported to be fused with these C-terminal cleaving inteins to construct new tags (Setrerrahmane et al. 2014; Wood and Camarero 2014; Xie et al. 2013). Once the fusion protein is purified, the intein can be induced to cleave precisely and release the target protein with a correct N-terminus. The C-terminal cleaving intein-based system makes it possible to produce N-terminal methionine-free mature biopharmaceuticals in E. coli.
Since biopharmaceuticals are usually aggregate prone when expressed in E. coli, the expression solubility is always an important aspect to be considered. In previous reports, many fusion proteins were expressed in the form of inclusion bodies, such as GLP-1 and hEGF (Esipov et al. 2008; Jiang et al. 2015). Solubilizing tags, such as MBP and GST, are effective to improve fusion solubility. MBP–∆I-CM fusion tag successfully simplified the purification of acidic fibroblast growth factor (aFGF), as described in previous literatures (Wood et al. 2000). Another criterion to evaluate intein-based system is the activity of self-cleavage. In some cases, the half-life of the precursor protein in vitro was less than 1 h at 37 °C, pH 6.0 (Wood et al. 2000). However, the cleavage efficiency of intein is highly related to the structure of the N- and C-exteins (Liu et al. 2014; Mujika et al. 2012). When MBP–∆I-CM was used in our studies for rhIL-15 purification, as shown in Fig. 2, its cleavage efficiency was very limited. In addition, most affinity tags can simplify the purification, but the corresponding resins are more expensive than the conventional chromatography resins, such as HIC and IEX. The potential high cost limits the application of these tags in large-scale production of biopharmaceuticals. In recent years, some hyperbasic or acidic solubilizing tags were discovered, which are very effective to facilitate the purification by IEX (Hedhammar and Hober 2007; Sangawa et al. 2013). It is promising to configure new tags with these highly charged fragments in combination with C-terminal cleaving inteins.
For the purification of N-terminal methionine-free rhIL-15, we used the C-terminal cleaving mini-intein ∆I-CM and Ssp DnaB to construct tandem tags with MBP, CBD, His6, or Zbasic, respectively. We studied all these tags on their abilities to improve the solubility and self-cleavage activity (Table 2). The results revealed that with the same C-extein, IL-15, cleavage activity of intein was apparently influenced by its N-extein. Zbasic–∆I-CM tag showed the best solubility and cleavage activity. With this tag, we developed a three-step chromatography process (SP FF, Butyl HP, Q FF) to purify the rhIL-15, which saved two steps of chromatography compared to the previously published process (S-200, Butyl 650-S, Source 15Q, QXL, Superdex 75) (Vyas et al. 2012). Most importantly, we eliminated the time-consuming denaturing and refolding process and the final product was with natural N-terminus, without an extra methionine.
To evaluate the quality of rhIL-15 produced by the intein-based system, we analyzed its purity with ExRP-HPLC, characterized the molecule with UPLC-MS, and evaluated its bioactivity with a CTLL-2 cell proliferation assay. Recombinant human IL-15 drug product from NCI at Frederick was used as a reference standard in our assays. The purity of our product is 92 % with 6 % deamidation. The MW is 12,769.5 Da, just 131.1 Da less than the reference (MW 12,900.6 Da), indicating the absence of one methionine as compared to the reference. The EC50 to stimulate CTLL-2 proliferation was determined to be about 0.1261 ng/mL, close to that of the reference (Nellis et al. 2012).
During the purification, one concern was rhIL-15’s tendency to aggregate and degrade. Zbasic–∆I-CM–IL-15 fusion was found to aggregate both in solution and on column, reducing the step recovery of SP FF chromatography. We screened Tween 20, glycerol, and l-arginine as cosolvent (data not shown) and found that 0.5 M l-arginine was effective to reduce the aggregation and improve the recovery of SP FF chromatography. Another concern was the pre-mature cleavage during the fermentation induction stage. Along with the fusion protein accumulating with time, some of it would cleave under suboptimal temperature(s) and intracellular pH. To reduce the pre-mature cleavage, we induced the fusion protein at a lower temperature (20 °C) and with shorter duration (4 h). Another strategy to improve fusion protein recovery is to raise pH of LB media during induction. As we observed, higher pH 8 can preserve obviously more fusion protein than lower pH 6 (Fig. S2). Engineering of the contiguous intein into a split intein can also avoid the pre-mature cleavage. According to Shi et al., ∆I-CM could be split into a 110-amino acid N-terminal fragment (I N) and a 58-amino acid C-terminal fragment (I C). The cleaving activity of the truncated intein was tightly repressed during induction and restored when I N and I C recombined during purification (Shi et al. 2013). It is possible to utilize Zbasic and split ∆I-CM to achieve higher yield of rhIL-15.
According to our study, screening and selection of the optimal N-terminal purification tag are essential in the C-terminal cleaving intein-based system, since the cleavage activity of intein is highly influenced by its N- and C-exteins. However, to evaluate performances of all the tags are labor and cost consuming. There are already X-ray or NMR structure information of many inteins; computational simulation can potentially be used to evaluate the intein activity when fused to different tags and target proteins. Our success on rhIL-15 soluble expression and purification suggested that the intein-based system is of great potential in large-scale biopharmaceutical production.
References
Amitai G, Callahan BP, Stanger MJ, Belfort G, Belfort M (2009) Modulation of intein activity by its neighboring extein substrates. Proc Natl Acad Sci USA 106(27):11005–11010
Ashley RL, Militoni J, Lee F, Nahmias A, Corey L (1988) Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 26(4):662–667
Bailey JEO (1986) D. F. Biochemical engineering fundamentals. McGraw-Hill Book Company, New York
Béhar G, Solé V, Defontaine A, Maillasson M, Quéméner A, Jacques Y, Tellier C (2011) Evolution of interleukin-15 for higher E. coli expression and solubility. Protein Eng Des Sel 24(3):283–290
Böcker JK, Friedel K, Matern JC, Bachmann AL, Mootz HD (2015) Generation of a genetically encoded, photoactivatable intein for the controlled production of cyclic peptides. Angew Chem Int Ed 54(7):2116–2120
Brincks EL, Woodland DL (2010) Novel roles for IL-15 in T cell survival. F1000 Biol Rep 2(1):67–67
Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, Beach RK, Lifson JD, Felber BK, Pavlakis GN (2013) Characterization and favorable in vivo properties of heterodimeric soluble IL—15Ralpha cytokine compared to IL-15 monomer. J Biol Chem 288(25):18093–18103
Engler C, Marillonnet S (2014) Golden gate cloning. Methods Mol Biol 1116:119–131
Esipov RS, Stepanenko VN, Chupova LA, Boyarskikh UA, Filipenko ML, Miroshnikov AI (2008) Production of recombinant human epidermal growth factor using Ssp dnaB mini-intein system. Protein Expr Purif 61(1):1–6
Graslund S, Nordlund P, Weigelt J, Hallberg BM, Bray J, Gileadi O, Knapp S, Oppermann U, Arrowsmith C, Hui R, Ming J, dhe-Paganon S, Park HW, Savchenko A, Yee A, Edwards A, Vincentelli R, Cambillau C, Kim R, Kim SH, Rao Z, Shi Y, Terwilliger TC, Kim CY, Hung LW, Waldo GS, Peleg Y, Albeck S, Unger T, Dym O, Prilusky J, Sussman JL, Stevens RC, Lesley SA, Wilson IA, Joachimiak A, Collart F, Dementieva I, Donnelly MI, Eschenfeldt WH, Kim Y, Stols L, Wu R, Zhou M, Burley SK, Emtage JS, Sauder JM, Thompson D, Bain K, Luz J, Gheyi T, Zhang F, Atwell S, Almo SC, Bonanno JB, Fiser A, Swaminathan S, Studier FW, Chance MR, Sali A, Acton TB, Xiao R, Zhao L, Ma LC, Hunt JF, Tong L, Cunningham K, Inouye M, Anderson S, Janjua H, Shastry R, Ho CK, Wang D, Wang H, Jiang M, Montelione GT, Stuart DI, Owens RJ, Daenke S, Schutz A, Heinemann U, Yokoyama S, Bussow K, Gunsalus KC (2008) Protein production and purification. Nat Methods 5(2):135–146
Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz PA, Romero CA, Rhode PR, Wong HC (2011) IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 56(3):804–810
Hedhammar M, Hober S (2007) Z(basic)—a novel purification tag for efficient protein recovery. J Chromatogr A 1161(1–2):22–28
Huang XQ, Hamilton MJ, Li CL, Schmidt C, Ellem KA (2006) An extraordinarily high level of IL-15 expression by a cell line transduced with a modified BMGneo vector displays hypoxic upregulation. Mol Biotechnol 33(1):49–56
Jiang A, Jin W, Zhao F, Tang Y, Sun Z, Liu JN (2015) Split Ssp DnaB mini-intein-mediated production of recombinant human glucagon-like peptide-1/7-36. Biotechnol Appl Biochem 62(3):309–315
Kwong KW, Wong WK (2013) A revolutionary approach facilitating co-expression of authentic human epidermal growth factor and basic fibroblast growth factor in both cytoplasm and culture medium of Escherichia coli. Appl Microbiol Biotechnol 97(20):9071–9080
Liu C-C, Perussia B, Young JD-E (2000) The emerging role of IL-15 in NK-cell development. Immunol Today 21(3):113–116
Liu Z, Frutos S, Bick MJ, Vila-Perelló M, Debelouchina GT, Darst SA, Muir TW (2014) Structure of the branched intermediate in protein splicing. Proc Natl Acad Sci 111(23):8422–8427
Luo G, Yang L, Liang G, Wan X, Chen C, Wang B, Chen J, Zeng K, Zhan S, Chen X (2015) Construction and synergistic effect of recombinant yeast co-expressing pig IL-2/4/6 on immunity of piglets to PRRS vaccination. Procedia Vaccinology 9:66–79
Ma A, Koka R, Burkett P (2006) Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol 24:657–679
Mueller YM, Katsikis PD (2010) IL-15 in HIV infection: pathogenic or therapeutic potential? Eur Cytokine Netw 21(3):219–221
Mujika JI, Lopez X, Mulholland AJ (2012) Mechanism of C-terminal intein cleavage in protein splicing from QM/MM molecular dynamics simulations. Org Biomol Chem 10(6):1207–1218
Nellis DF, Michiel DF, Jiang MS, Esposito D, Davis R, Jiang H, Korrell A, Knapp GC, Lucernoni LE, Nelson RE, Pritt EM, Procter LV, Rogers M, Sumpter TL, Vyas VV, Waybright TJ, Yang X, Zheng AM, Yovandich JL, Gilly JA, Mitra G, Zhu J (2012) Characterization of recombinant human IL-15 deamidation and its practical elimination through substitution of asparagine 77. Pharm Res 29(3):722–738
Pennati A, Deng J, Galipeau J (2014) Maltose-binding protein fusion allows for high level bacterial expression and purification of bioactive mammalian cytokine derivatives. PLoS One 9(9):e106724
Perler FB (2002) InBase: the intein database. Nucleic Acids Res 30(1):383–384
Perler FB, Allewell NM (2014) Evolution, mechanisms, and applications of intein-mediated protein splicing. J Biol Chem 289(21):14488–14489
Sangawa T, Tabata S, Suzuki K, Saheki Y, Tanaka K, Takagi J (2013) A multipurpose fusion tag derived from an unstructured and hyperacidic region of the amyloid precursor protein. Protein Sci 22(6):840–850
Setrerrahmane S, Zhang Y, Dai G, Lv J, Tan S (2014) Efficient production of native lunasin with correct N-terminal processing by using the pH-induced self-cleavable Ssp DnaB mini-intein system in Escherichia coli. Appl Biochem Biotechnol 174(2):612–622
Shi C, Meng Q, Wood DW (2013) A dual ELP-tagged split intein system for non-chromatographic recombinant protein purification. Appl Microbiol Biotechnol 97(2):829–835
Shi C, Tarimala A, Meng Q, Wood DW (2014) A general purification platform for toxic proteins based on intein trans-splicing. Appl Microbiol Biotechnol 98(22):9425–9435
Soman G, Yang X, Jiang H, Giardina S, Vyas V, Mitra G, Yovandich J, Creekmore SP, Waldmann TA, Quinones O, Alvord WG (2009) MTS dye based colorimetric CTLL-2 cell proliferation assay for product release and stability monitoring of interleukin-15: assay qualification, standardization and statistical analysis. J Immunol Methods 348(1–2):83–94
Sun W, Lai Y, Li H, Nie T, Kuang Y, Tang X, Li K, Dunbar PR, Xu A, Li P, Wu D (2016) High level expression and purification of active recombinant human interleukin-15 in Pichia pastoris. J Immunol Methods 428:50–57
Vyas VV, Esposito D, Sumpter TL, Broadt TL, Hartley J, Iv GCK, Wei C, Jiang MS, Roach JM, Yang X (2012) Clinical manufacturing of recombinant human interleukin 15. I. Production cell line development and protein expression in E. coli with stop codon optimization. Biotechnol Prog 28(2):497–507
Ward A, Anderson M, Craggs RI, Maltby J, Grahames C, Davies RA, Finch D, Pattison D, Oakes H, Mallinder PR (2009) E. coli expression and purification of human and cynomolgus IL-15. Protein Expr Purif 68(1):42–48
Wood DW, Camarero JA (2014) Intein applications: from protein purification and labeling to metabolic control methods. J Biol Chem 289(21):14512–14519
Wood DW, Wu W, Belfort G, Derbyshire V, Belfort M (1999) A genetic system yields self-cleaving inteins for bioseparations. Nat Biotechnol 17(9):889–892
Wood DW, Derbyshire V, Wu W, Chartrain M, Belfort M, Belfort G (2000) Optimized single-step affinity purification with a self-cleaving intein applied to human acidic fibroblast growth factor. Biotechnol Prog 16(6):1055–1063
Xie YG, Luan C, Zhang HW, Han FF, Feng J, Choi YJ, Groleau D, Wang YZ (2013) Effects of thioredoxin: SUMO and intein on soluble fusion expression of an antimicrobial peptide OG2 in Escherichia coli. Protein Pept Lett 20(1):54–60
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (Nos. 81273576 and 81473127), the Science and Technology Commission of Shanghai Municipality (Nos. 14DZ2294200 and 15431907000), and the Medical and Engineering Cross Research Foundation of Shanghai Jiao Tong University (No. YG2012MS01). We would also like to thank the BRB/NCI lab Frederick for their kind offer of rhIL-15 drug product used as reference standard in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 81273576, 81473127), the Science and Technology Commission of Shanghai Municipality (No. 14DZ2294200, 15431907000), and the Medical and Engineering Cross Research Foundation of Shanghai Jiao Tong University (No. YG2012MS01).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals experiments.
Electronic supplementary material
ESM 1
(PDF 175 kb)
Rights and permissions
About this article
Cite this article
Shi, S., Chen, H., Jiang, H. et al. A novel self-cleavable tag Zbasic–∆I-CM and its application in the soluble expression of recombinant human interleukin-15 in Escherichia coli . Appl Microbiol Biotechnol 101, 1133–1142 (2017). https://doi.org/10.1007/s00253-016-7848-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7848-2