Abstract
Isolate B17 from Kombucha was estimated to be an efficient producer of bacterial cellulose (BC). The isolate was deposited under the number P 1463 and identified as Komagataeibacter rhaeticus by comparing a generated amplified fragment length polymorphism (AFLP™) DNA fingerprint against a reference database. Static cultivation of the K. rhaeticus strain P 1463 in Hestrin and Schramm (HS) medium resulted in 4.40 ± 0.22 g/L BC being produced, corresponding to a BC yield from glucose of 25.30 ± 1.78 %, when the inoculum was made with a modified HS medium containing 10 g/L glucose. Fermentations for 5 days using media containing apple juice with analogous carbon source concentrations resulted in 4.77 ± 0.24 g/L BC being synthesised, corresponding to a yield from the consumed sugars (glucose, fructose and sucrose) of 37.00 ± 2.61 %. The capacity of K. rhaeticus strain P 1463 to synthesise BC was found to be much higher than that of two reference strains for cellulose production, Komagataeibacter xylinus DSM 46604 and Komagataeibacter hansenii DSM 5602T, and was also considerably higher than that of K. hansenii strain B22, isolated from another Kombucha sample. The BC synthesised by K. rhaeticus strain P 1463 after 40 days of cultivation in HS medium with additional glucose supplemented to the cell culture during cultivation was shown to have a degree of polymerization of 3300.0 ± 122.1 glucose units, a tensile strength of 65.50 ± 3.27 MPa and a length at break of 16.50 ± 0.83 km. For the other strains, these properties did not exceed 25.60 ± 1.28 MPa and 15.20 ± 0.76 km.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC) is sometimes called nanocellulose, because of its crystallinity and the nanodimensions of its fibres, which are several micrometres long with diameters ranging from 25 to 100 nm, being about 100 times thinner than the fibres of plant cellulose (Lee et al. 2014). BC has some specific features, such as an excellent water absorption capacity (up to 99 % moisture content) and an ability to form a very strong and flexible hydrogel. BC has a wide range of applications and is in demand on the world market. By using specific bacteria and cultivation techniques, and by controlling the BC fibre formation, it is possible to purposefully regulate the three-dimensional network of BC and synthesise BC in the form of geometric structures (Klemm et al. 2013). Applications such as bone implants and vascular replacements are possible because BC is compatible with human body tissues, since it does not trigger antibody formation and is not degraded in the human body (Gama et al. 2013). In addition, transparent BC gels with good air permeability containing large amounts of absorbed water can be used in medicine as wound dressings and as prolonged drug dosage materials (Czaja et al. 2006). The strength of BC is also unique. Dried BC has been reported to be as strong as steel (Klemm et al. 2011), and BC has been recognised to be the strongest naturally synthesised biological material (Lee et al. 2014).
Although BC is a product with a great deal of market potential, extensive commercial use is hampered by its high production costs. Slow cellulose synthesis by bacterial cells is one of the reasons for this and may be due to the low growth rate of cellulose-producing bacteria and the low rate of cellulose crystallization by the cells (Benziman et al. 1980). In addition, BC production under large-scale fermentation conditions is limited by the fact that it is not possible to obtain BC with the necessary physical properties using a medium-mixing mode (Raspor and Goranovic 2008). High-quality BC is currently synthesised predominantly under static cultivation conditions, but this process increases the costs because of the large surface area that is then required for production.
Attempts to increase BC synthesis by its producing bacterial strains, using genetic engineering techniques have not been successful to date (Gama et al. 2013). Therefore, for the time being, attempts to reduce the costs of BC synthesis are based mainly on the isolation of more productive bacterial strains, optimization and targeted control of its cultivation conditions and the use of cheap substrates (Sahena and Brown 2013). In this study, the aim was to isolate bacteria that produce BC more efficiently.
Bacteria of the family Acetobacteraceae are most commonly used for BC production, mainly bacteria from the genus Komagataeibacter, and usually strains of the species Komagataeibacter xylinus and Komagataeibacter hansenii (previously known as Gluconacetobacter xylinus and Gluconacetobacter hansenii). However, other species of this genus have also been reported to harbour cellulose-producing strains, such as Komagataeibacter swingsii, Komagataeibacter rhaeticus and Komagataeibacter medellinensis (Castro et al. 2013; Dos Santos et al. 2014).
One of the typical niches of BC-producing acetic acid bacteria is Kombucha, a beverage obtained through the fermentation of sugared tea with a symbiotic culture of yeast and acetic acid bacteria. In this study, four different commercially available Kombucha beverages were used as the isolation source. Potential suitable isolates were screened for BC production under static cultivation conditions using different sugars (glucose, fructose, sucrose, galactose and ribose) and the sugar-alcohol mannitol as carbon sources, as well as apple juice and cheese whey as low-cost substrates. Increasing BC synthesis through the selection of optimal cultivation conditions was also tried. BC synthesis by potential suitable isolates was compared with BC synthesis by two reference strains for cellulose production, the K. xylinus strain DSM 46604 and K. hansenii strain DSM 5602T. Finally, the physical and mechanical properties of the synthesised cellulose were investigated.
Materials and methods
Cellulose-producing strains, isolation and identification
Four different commercially available Kombucha beverages were screened for BC-producing bacteria. Isolates were obtained after the inoculation of decimal dilutions of Kombucha in physiological water (NaCl, 9.0 g/L) on Petri dishes with Hestrin and Schramm (HS) agar medium with an initial pH value of 6.2 ± 0.2, containing the following (g/L): d-glucose 20.0, peptone 5.0, yeast extract 5.0, Na2HPO4 2.7, citric acid 1.15 and agar 15.0 (Hestrin and Schramm 1954). The medium also contained cycloheximide (0.1 g/L) in order to prevent the growth of the yeast cells in the symbiotic cultures. Bacteria were cultured at 30 °C for 2–3 days. Potential cellulose-producing bacteria (light, cream-coloured colonies with a weakly rough surface, not forming a brown water-soluble pigment) were retrieved for cultivation in flasks or tubes with liquid HS medium under static conditions at 30 °C. BC-producing isolates were identified by film formation on the surface of the liquid medium after 3 days of cultivation. The best BC producers were maintained in liquid HS medium containing 15 % glycerol at a temperature of −20 °C, and two of them (B17 = P 1463 and B22) were selected for further investigation.
Two reference strains for cellulose production, K. xylinus strain DSM 46604 and K. hansenii strain DSM 5602T, were obtained from DSMZ, the German Collection of Microorganisms and Cell Cultures, and used as control strains in BC production tests.
The two selected BC-producing isolates were identified by the identification service of the BCCM/LMG Bacteria collection, Laboratory of Microbiology, Faculty of Sciences, Ghent University (Belgium), by amplified fragment length polymorphism (AFLP™) DNA fingerprinting and comparison of the obtained profiles against a reference database, as described previously (Castro et al. 2013).
BC characterization through FT-IR spectroscopy
BC was freeze-dried and crushed into powder form, mixed with KBr, and pressed into a small tablet that was subjected to Fourier transform infrared (FT-IR) spectroscopy using a Spectrum One Perkin Elmer spectrometer (Waltham, USA) in absorption mode. For each sample, 32 scans at a 4 cm−1 resolution at wave numbers ranging from 4000 to 400 cm−1 were collected.
BC production experiments
Bacteria for inoculation were cultivated in 500-mL volume Erlenmeyer flasks filled with 50-mL HS medium containing 20 g/L glucose or a modified HS medium containing 10 g/L glucose, on a shaker (180 rpm) at 30 °C. After 24 h of cultivation, this inoculum was added to the main fermentation medium (5 % of inoculum volume/volume), which was standard HS medium or a modified HS medium, in which the 20 g/L glucose of the HS medium was replaced with 40 g/L glucose or 20 or 40 g/L fructose, or sucrose, galactose, ribose or mannitol at concentrations of 20 g/L. In some experiments, a mixture of 20 g/L sucrose and 5 g/L fructose was used. Apple juice and cheese whey were also used in some experiments as cheap sources of carbon substrates. Average values of glucose, fructose and sucrose concentrations in apple juice were estimated to be 12.1, 49.2 and 18.1 g/L (ratio 1.0:4.0:1.5), respectively, meaning the total sugar concentration in apple juice was 79.4 g/L. Cheese whey for medium preparation was pretreated with β-galactosidase to convert disaccharide lactose via hydrolysis into its monosaccharide components, glucose and galactose. Average concentrations of glucose, galactose and lactose in cheese whey after hydrolysis were estimated to be 57.0, 69.0 and 3.5 g/L (ratio 1.0:1.2:0.06), respectively, meaning the total sugar concentration in the cheese whey after hydrolysis was 129.5 g/L. Mediums with apple juice and cheese whey were prepared to ensure an initial sugar concentration of 20 g/L.l
BC synthesis fermentation experiments were usually performed as “low-volume experiments” by culturing bacteria in 300-mL volume flasks filled with 50 mL of fermentation medium for 5 days; some experiments (if specified) were performed by culturing bacteria for 4, 7 or 14 days. “Large-volume experiments” were carried out by culturing bacteria in 3000-mL glass jars filled with 200 mL of medium for 14 or 40 days. To increase the productivity of BC synthesis, additional amounts of the carbon source were added to the cell culture of large-volume experiments during the fermentation. All fermentation experiments were conducted under static conditions at 30 °C in at least triplicate. The following parameters were analyzed: pH and concentration of sugars and organic acids in the fermentation medium, the amount of synthesised cellulose and the yield of BC from the bacteria-consumed sugar. For the low-volume experiments, the parameters were analyzed after 5 days of cultivation (unless stated otherwise), while for the large-volume experiments, they were analyzed after 14 or 40 days of cultivation.
Bacto agar, peptone and yeast extract were purchased from Becton, Dickinson and Company (USA), the sugars from Sigma-Aldrich chemicals (Taufkirchen, Germany), and β-galactosidase from Chr. Hansen Natural Colors (Hoersholm, Denmark); the other chemicals used were all of analytical grade.
BC quantification
BC pellicles, produced at the air-liquid interface, were withdrawn from the culture media, rinsed with distilled water to remove excess medium and then treated with 0.1 M NaOH solution. After heating for 20 min at 90 °C in a water bath, the BC pellicles were purified by extensive washing in distilled water until a pH of 7.0 was reached. Finally, the purified cellulose was dried overnight at 105 °C before estimating the dry weight gravimetrically. The BC concentration was then calculated as the mass of BC per litre of medium (g/L).
Determination of concentrations of sugars and organic acids
The concentrations of reducing sugars in the medium during cultivations were determined using the Lane-Eynon titration method, a modification of Bertrand’s method (Dziedzic and Kearsley 1995). Concentrations of mannitol and individual sugars in apple juice (glucose, fructose and sucrose) and cheese whey hydrolysate (lactose, glucose and galactose) were determined using a high-performance liquid chromatography (HPLC, model Waters 600 E, Milford, USA), equipped with a refractive index detector (Waters 2410 C). The HPLC was run on an Agilent Zorbax carbohydrate analysis column at 35 °C at a mobile phase (75 % acetonitrile water solution) flow rate of 0.6 mL/min. Concentrations of the individual sugars sucrose, d-fructose and d-glucose in the cultivation media were determined using a commercially available enzymatic assay kit (K-SUFRG). The presence of acetic and gluconic acids in the cultivation media was also followed using specific enzymatic kits (K-ACET and K-GATE, Megazyme Co. Wicklow, Ireland), following the manufacturer’s instructions.
Calculation of the yield of BC and organic acid production
Sugar (S) consumption by the bacterial cells during cultivation was calculated as the difference between the initial sugar concentration (S0) of the cultivation medium and that at selected time points. Using data of the changes in the BC concentration and the ΔS at the respective time points, the yield of BC production (YBC/ΔS, %) was calculated. The yield of organic acid production was calculated in a similar way. All presented data are averages of at least three runs of experiments, performed with three to six replicates of the mean. Standard deviations of the mean were calculated using Microsoft Excel 2013 (Microsoft Corporation, Redmond, USA).
Determination of the degree of cellulose polymerization
Degree of polymerization (DP), characterizing the number of repeated units of d-glucose in cellulose molecule, was estimated according to the intrinsic viscosity of a cellulose sample. Viscosity estimation was carried out according to the ISO 5351-1-2010 standard method. Briefly, a sample of freeze-dried BC (dry weight was calculated from test run values) was suspended in 25 mL of deionised water for 30 min, after which 25 mL of Bis-(ethylenediamine)-copper (II)-hydroxide (final concentration 1.0 M) was added in order to increase the cellulose solubility. The intrinsic viscosity value of the prepared solution was estimated using a Rheotek RPV-1 capillary viscometer (Granger, USA). Based on the estimated value, the DP value of a BC sample was calculated with the help of the software that came with the equipment.
Mechanical properties of BC
BC pellicles were dried using the organic solvent exchange method, removing water from the pellicles by their sequential immersion in methanol, acetone and hexane. The hexane-saturated pellicles were then pressed firmly between two organic glass sheets and air-dried for 24 h. The tensile properties of the BC pellicles were tested according to the standard method (ISO 1924-2:2008) using a Vertical F 81838 computer-controlled tensile tester (AJT Equipment, Brockmoor, UK). Dried BC films with a thickness of about 100 μm were cut into samples with a size of 35 × 15 mm, before being mounted between the upper and lower clamps of the tensile test machine, where force was applied from the upper clamp with a speed of 5 mm/s in tension mode. The thickness of the BC specimens was measured at ten different positions with an F16502 thickness gauge (Frank-PTI, Vorchdorf, Austria). The obtained values were manually entered into the tensile tester software. At least five samples were measured per BC pellicle, and the average result was calculated.
Tensile strength (TS), defined as the load required to break a material, was calculated as the ratio of the tensile load applied to the original cross-sectional area of the sample. Length at break (LB), defined as the length of a hanging sample supporting its own weight before breaking, was calculated according to the following equation: LB = 102 × TS/R, were R is the basis weight of a sample (BC mass per unit of sample surface area, g/m2). Calculations of numeric values of TS (in MPa) and LB (in km) of each sample were carried out with the help of the software program that the tensile tester machine was equipped with.
Results
Isolation and identification of cellulose producers
Four different Kombucha cell cultures were screened for potential cellulose-producing strains. The best cellulose producers were isolates B17 and B22. These isolates were obtained from various Kombucha samples and selected for further investigation.
Isolates were characterised as Gramme-negative bacteria with coccoid or rod-shaped cells (1.0 μm × 2.0–5.0 μm in size) which occur singly or in pairs, and do not form spores. Bacteria are aerobes that have the ability to perform the incomplete oxidation of carbohydrates, alcohols and sugar alcohols. We found that they could oxidise d-glucose to 2- and 5-ketogluconic acid; they were negative for the oxidase reaction and positive for the catalase reaction. Based on their morphological and biochemical characteristics, as well as information found in the second edition of Bergey’s manual of systematic bacteriology (Sievers and Swings 2005), isolates B17 and B22 were presumed to be members of Gluconacetobacter, a genus of the family Acetobacteraceae, that was later reclassified as genus Komagataeibacter (Yamada et al. 2012).
AFLP™ DNA fingerprinting of isolates B17 and B22, and comparison of the obtained profiles against a reference database with AFLP™ DNA fingerprints of acetic acid bacteria taxa, identified isolate B17 to be K. rhaeticus and isolate B22 to be K. hansenii (Fig. 1). Isolate B17 was deposited in the Microbial Strain Collection of Latvia (MSCL, registered as no. 854 in the World Depositary Collection of Microorganisms) as K. rhaeticus strain P 1463. Isolate B22 was deposited in the Collection of Microorganisms of the Institute of Microbiology and Biotechnology, University of Latvia, as K. hansenii strain B22.
BioNumerics software generated AFLP™ DNA fingerprints and a dendrogram supporting the identifications of isolates B17 (= P 1463) and B22 as K. rhaeticus and K. hansenii, respectively. The species K. kombuchae is considered to be a later heterotypic synonym of K. hansenii (Cleenwerck et al. 2009). Type strains are marked with T
FT-IR spectroscopy characteristics of BC produced by K. rhaeticus strain P 1463 and K. hansenii strain B22
Cellulose synthesised by K. rhaeticus strain P 1463 and K. hansenii strain B22 were found to have identical FT-IR spectra that were similar to cellulose absorption spectra reported for this material (El-Saied et al. 2008). Figure 2 shows the spectrum of BC produced by K. rhaeticus strain P 1463. Only typical peaks could be found in this figure, such as a peak at the wave number 898 cm−1 characterizing a β-1,4-glycosidic bond, the broad bands in the region 3600–3100 cm−1 corresponding to OH-stretching intramolecular hydrogen bonds and others reported for the FT-IR spectrum of cellulose materials (Carillo et al. 2004) showing that the pellicles synthesised by bacterial cells consist of cellulose and do not contain impurities.
Assessment of the cellulose synthesis capacity of K. rhaeticus strain P 1463 and K. hansenii strain B22
In order to test the BC production capacity of K. rhaeticus strain P 1463 and K. hansenii strain B22, preliminary experiments were carried out using HS medium (containing 20 g/L glucose) to prepare the inoculum, and HS medium or modified medium containing 40 g/L glucose or 20 or 40 g/L fructose as the main fermentation medium (Table 1). The amounts of BC produced by K. rhaeticus strain P 1463 and K. hansenii strain B22 after 5 days of cultivation were 1.94 ± 0.09 and 1.60 ± 0.08 g/L, respectively, in HS medium, and 1.83 ± 0.08 and 1.66 ± 0.08 g/L in the modified medium containing 20 g/L fructose. A glucose concentration of 40 g/L instead of 20 g/L in the main fermentation medium resulted in a decrease in BC production by the bacterial cells and in a drastic increase in gluconic acid synthesis of (up to 20 g/L with a yield of 50 %). In contrast, the synthesis of acetic acid under both medium conditions was low (in the range of 0.24–0.33 g/L with a yield from glucose of only 0.8–2.5 %). Therefore, it is plausible that the increased gluconic acid but not acetic acid synthesis causes the pH decrease and decrease in BC production under cultivation conditions with an initial glucose concentration of 40 g/L in the medium.
For the medium containing 40 g/L of fructose, the results were different, revealing not only a slight increase in BC synthesis for strain P1463 but also a significant decrease in the BC yield compared to the values using the medium with 20 g/L of fructose. For all subsequent fermentation experiments, it was decided to use main fermentation medium containing an initial sugar concentration of 20 g/L.
The results of the preliminary experiments further revealed that K. rhaeticus P 1463 produced more cellulose than K. hansenii B22 under similar culture conditions (Table 1). Compared to the reported data for other acetic acid bacteria, K. rhaeticus strain P 1463 produced comparable amounts of cellulose in HS medium under static cultivation conditions, namely 1.89 ± 0.08 g/L (Aydin and Aksoy 2014).
Comparison of BC synthesis by K. rhaeticus strain P 1463, K. hansenii strain B22 and two reference strains
By using another medium for making the inocula, namely a modified HS medium containing 10 g/L of glucose instead of 20 g/L, an increase in BC synthesis by the strains K. rhaeticus P 1463 and K. hansenii B22 could be achieved (Fig. 3). Using this medium, the pH value of the inocula (pH 4.5) was found to be significantly higher than that of inocula made with HS medium (pH 3.6). These results indicate that the physiological state of the bacteria in the inoculum culture is essential to increasing BC production in the main fermentation.
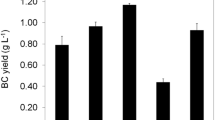
Carbon source utilization (a), BC production (b) and yield of BC production (c) by  K. rhaeticus strain P 1463,
K. rhaeticus strain P 1463,  K. hansenii strain B22 and two reference strains for cellulose production (
K. hansenii strain B22 and two reference strains for cellulose production ( K. xylinus DSM 6513T and
K. xylinus DSM 6513T and  K. hansenii DSM 5602T) after 5 days of cultivation in media containing 20 g/L of different carbon sources (1, glucose; 2, fructose; 3, sucrose; 4, galactose; 5, mannitol; and 6, ribose) inoculated with inocula cultured in modified HS medium containing 10 g/L glucose. Presented data are averages of four to five runs of experiments, performed with three to six replicates of the mean
K. hansenii DSM 5602T) after 5 days of cultivation in media containing 20 g/L of different carbon sources (1, glucose; 2, fructose; 3, sucrose; 4, galactose; 5, mannitol; and 6, ribose) inoculated with inocula cultured in modified HS medium containing 10 g/L glucose. Presented data are averages of four to five runs of experiments, performed with three to six replicates of the mean
Using an inoculum culture made with the modified HS medium containing 10 g/L glucose, a detailed study was carried out of the different carbon-substrate utilization, cellulose synthesis and yield from substrate by the isolated strains (K. rhaeticus P 1463 and K. hansenii B22) compared to two reference strains (K. xylinus DSM 46604 and K. hansenii DSM 5602T). Results of static cultivations of strains showed that the utilization of carbon sources (glucose, fructose, sucrose and galactose) was in many cases quite similar for the isolated strains and the reference strains (Fig. 3a). An exception was ribose utilization, which was only consumed by bacteria of K. rhaeticus strain P 1463.
Despite the similarity in substrate utilization that was estimated in many cases (Fig. 3a), the cellulose synthesis activity of strains differed significantly (Fig. 3b). K. rhaeticus strain P 1463 was shown to produce more BC from glucose, fructose and mannitol than the other strains (except for cellulose production from mannitol by K. hansenii DSM 5602T), and also produced cellulose from ribose. Based on the experimental data shown in Fig. 3b, the cellulose synthesis by K. rhaeticus strain P 1463 was 1.45-, 7.0- and 8.0-fold higher in the glucose-containing medium than the cellulose synthesis by the strains K. hansenii B22, K. xylinus DSM 46604 and K. hansenii DSM 5602T, respectively, and 1.9-, 22.2- and 8.8-fold higher in fructose-containing media. In media containing sucrose and galactose, BC production by K. rhaeticus strain P 1463 was relatively low, similar to the other strains.
The amount of BC synthesised by the strains K. rhaeticus P 1463 and K. hansenii B22 after 5 days of cultivation was 4.40 ± 0.22 and 3.03 ± 0.15 g/L, respectively, in media containing glucose and 3.34 ± 0.16 and 1.80 ± 0.09 g/L in media containing fructose; the amounts of BC synthesised by the reference strains were in the range of 0.30–0.54 g/L in media containing glucose and in the range of 0.15–0.39 g/L in media containing fructose. Also, the yield of BC production from glucose and fructose obtained with the strain K. rhaeticus P 1463 was significantly higher than that obtained with the other strains (Fig. 3c).
In Fig. 3b, it is shown that the BC synthesis in sucrose-containing medium by the strains K. rhaeticus P 1463 and K. hansenii B22 was relatively low, whereas the two reference strains did not produce BC at all. Sucrose utilization was observed for the strains K rhaeticus P 1463, K. hansenii B22 and K. xylinus DSM 46604, but not for K. hansenii DSM 5602T (Fig. 3a). The inability of the latter strain to utilise sucrose has been reported before (Cleenwerck et al. 2009). A possible reason for the low or no sucrose utilization may be that sucrose cannot be transported through the cell membrane of acetic acid bacteria and needs to be hydrolysed to glucose and fructose in the periplasm before it can be used (Velasco-Bedran and Lopez-Isunza 2007).
BC synthesis by strains using media containing apple juice or cheese whey
Further research aimed to determine the efficiency of the isolated strains to synthesise BC using apple juice and cheese whey as carbon sources. Low-volume, short-term (50-mL fermentation medium, 5 days) and large-volume, long-term (200-mL fermentation medium, 14 days) cultivations were carried out for these investigations. It was shown that the BC production of culturing bacteria in media containing cheese whey was lower than the BC production using apple juice and media containing glucose, which did not differ significantly (Tables 2 and 3). However, the yield of BC production was higher for apple juice than for media containing glucose, for both strains. The assimilation of ballast substrate in apple juice, such as polyphenols, that has been reported for the cellulose-producing strains (Keshk and Sameshima 2006) and increased medium buffer capacity, favouring pH regulation by the cell cultures, may explain this. The lower BC production from media containing cheese whey was estimated to be due to the low galactose utilization by the strains (Fig. 3).
By culturing bacteria for a longer time and gradually adding carbon substrates to the cell culture, the amount of substrate assimilated by bacterial cells during cultivations was increased, and relatively large amounts of BC were produced. BC produced in the 200-mL volume, 14-day experiments compared to the 50-mL volume, 5-day experiments (Tables 2 and 3) were for K. rhaeticus strain P 1463: 9.49 ± 0.47 vs. 4.77 ± 0.24 g/L using the medium containing apple juice; 6.55 ± 0.33 vs. 2.86 ± 0.14 g/L using the medium containing cheese whey; and 8.09 ± 0.41 vs. 4.54 ± 0.23 g/L using the medium containing glucose. For K. hansenii strain B22, the amount of synthesised BC using these media was significantly lower: 6.98 ± 0.35 vs. 3.5 ± 0.17 g/L, 5.25 ± 0.26 vs. 2.9 ± 0.15 g/L and 6.29 ± 0.38 vs. 3.36 ± 0.16 g/L, respectively.
By culturing bacteria for a longer time and gradually adding carbon substrates to the cell culture, the amount of substrate assimilated by bacterial cells during cultivations was increased, and relatively large amounts of BC were produced. BC produced in the 200-mL volume, 14-day experiments compared to the 50-mL volume, 5-day experiments (Tables 2 and 3) were for K. rhaeticus strain P 1463 (g/L): 9.49 ± 0.47 vs. 4.77 ± 0.24 using the medium containing apple juice; 6.55 ± 0.33 vs. 2.86 ± 0.14 using the medium containing cheese whey; and 8.09 ± 0.41 vs. 4.54 ± 0.23 using the medium containing glucose. For K. hansenii strain B22, the amount of synthesised BC using these media was significantly lower: 6.98 ± 0.35 vs. 3.5 ± 0.17, 5.25 ± 0.26 vs. 2.9 ± 0.15 and 6.29 ± 0.38 vs. 3.36 ± 0.16 g/L, respectively.
Properties of BC synthesised by K. rhaeticus strain P 1463, K. hansenii strain B22 and two reference strains
To characterise the properties of the cellulose produced by the different strains, one physical property (the DP) and two properties characterizing the mechanical strength of the synthesised cellulose (tensile strength and length at break) were investigated (Table 4).
The DP of cellulose produced after 40 days of fermentation by K. rhaeticus strain P1463 was calculated to be 3300 ± 122.1, for K. hansenii strain B22, 3200.0 ± 131.2; for K. xylinus DSM 46604, 2200.0 ± 86.9; and for K. hansenii DSM 5602T, 1779.0 ± 74.7 glucose units (Table 4). The DP values of cellulose produced after 14 days of fermentation were lower, although for K. rhaeticus strain P1463, they were relatively high compared to K. hansenii strain B22 (2508.0 ± 87.8 vs. 1996.0 ± 79.8 glucose units), which indicates that K. rhaeticus strain P1463 may have an increased rate of cellulose polymerization, and this may favour the use of this strain for cellulose production. The DP values reported in the literature by Wesarg et al. (2010) for cellulose produced by K. xylinus DSM 46604 and K. hansenii DSM 5602T were about 920 and 775 glucose units, respectively, so lower than in our study.
The values of tensile strength and length at break also increased with increasing fermentation times. The highest values for these parameters (65.50 ± 3.27 MPa and 16.50 ± 0.83 km, respectively) were obtained for cellulose produced by the strain K. rhaeticus P 1463 over a fermentation of 40 days. These values were significantly higher than those of cellulose produced over a fermentation of 14 days (25.70 ± 1.28 MPa and 12.30 ± 0.61 km, respectively), or cellulose produced by the other strains over a fermentation of 40 days: 19.60 ± 0.98 MPa and 15.20 ± 0.76 km for K. hansenii B22-cellulose, 25.60 ± 1.28 MPa and 9.80 ± 0.49 km for K. hansenii DSM 5602T-cellulose, and 17.50 ± 0.87 MPa and 9.40 ± 0.47 km for K. xylinus DSM 46604-cellulose.
Discussion
The results of this study revealed that K. rhaeticus strain P 1463 (Semjonovs et al. 2015), isolated from Kombucha, is the best in terms of cellulose synthesis productivity and yield from sugar, and also cellulose properties. Using an inoculum prepared with modified HS medium containing 10 g/L glucose, this strain produced more cellulose after cultivation for 5 days under stationary conditions than K. hansenii strain B22 (another isolate from Kombucha) and two Komagataeibacter reference strains for cellulose production (K. xylinus DSM 46604 and K. hansenii DSM 5602T), namely 4.40 ± 0.22 g/L in HS medium, being 1.45-, 7.0- and 8.0-fold higher, and 3.34 ± 0.16 g/L in modified HS medium containing 20 g/L fructose, being 1.9-, 22.2- and 8.8-fold higher than was found for the other strains, respectively. The cellulose yield from the amount of consumed sugars for K. rhaeticus strain P 1463 bacteria was about 25.0 % in those experiments.
In the literature, the reported amounts of BC synthesised by other strains isolated from Kombucha after stationary cultivation in HS medium were as follows: about 0.28 g/L for K. xylinus K3 (2.64 g/L using the medium containing mannitol and 3.34 g/L using the medium containing mannitol and corn steep liquor) (Nguyen et al. 2008), 1.50 ± 0.33 g/L for K. hansenii UAC09 (Rani and Appaiah 2013) and 1.89 ± 0.08 g/L for K. hansenii P2A (3.25 ± 0.11 g/L under agitated conditions, Aydin and Aksoy 2014). When K. hansenii UAC09 was cultivated in a medium with coffee cherry husk at a carbon substrate concentration of 35 g/L, 5.6 g/L BC was produced and 8.2 g/L when corn steep liquor was added as a supplement to this medium (Rani and Appaiah 2013). After recalculating these values for a carbon source concentration of 20 g/L, the amounts of BC produced by this strain would be about 3.2 and 4.7 g/L.
When K. rhaeticus strain P 1463 was cultivated for 5 days under stationary conditions in the medium containing apple juice with a carbon substrate concentration of 20 g/L, 4.77 ± 0.22 g/L BC was obtained. When the fermentation time was increased to 14 days and the medium was gradually supplemented with additional carbon substrate, the amount of BC produced increased to 9.49 ± 0.47 g/L.
Another advantage of K. rhaeticus strain P 1463 was that the synthesised cellulose also had good physical and mechanical properties. A degree of cellulose polymerization of 3300 ± 122.1 glucose units could be achieved after 40 days of cultivation of bacterial cells under static conditions, which is close to the maximum DP reported for BC (Gama et al. 2013). Moreover, this cellulose had a tensile strength of 65.50 ± 3.27 MPa and a length at break of 16.50 ± 0.83 km, which are also high. The maximum TS values reported for K. hansenii UAC09-cellulose are about 42.3 MPa (Rani and Appaiah 2013), and about 60 MPa for K. xylinus CGMCC 2955 (Zhong et al. 2013).
The results presented in this study clearly indicate that K. rhaeticus strain P 1463 may be an outstanding BC producer. Using static culture conditions, this strain synthesised significantly greater quantities of BC from a variety of sugars, compared to K. hansenii strain B22, and in particular, the reference strains K. xylinus DSM 46604 and K. hansenii DSM 5602T. The amounts of synthesised BC are comparable to the best values reported in the literature.
References
Aydin Y, Aksoy N (2014) Isolation and characterization of an efficient bacterial cellulose producer strain in agitated culture: Gluconacetobacter hansenii P2A. Appl Microbiol Biotechnol 98:1065–1075
Benziman M, Haigler CH, Brown MR, White AR, Cooper KM (1980) Cellulose biogenesis: polymerization and crystallization are coupled processes in Acetobacter xylinum. Proc Natl Acad Sci U S A 77:6678–6682
Carrillo F, Colom X, Sunol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibers. Eur Polym J 40:2229–2234
Castro C, Cleenwerck I, Trček J, Zuluaga R, De Vos P, Caro G, Aguirre R, Putaux JL, Gañán P (2013) Gluconacetobacter medellinensis sp. nov., cellulose- and non-cellulose-producing acetic acid bacteria isolated from vinegar. Int J Syst Bacteriol 63:1119–1125
Cleenwerck I, De Wachter M, González A, De Vuyst L, De Vos P (2009) Differentiation of species of the family of Acetobacteriaceae by AFLP DNA fingerprinting: Gluconacetobacter kombuchae is a later heterotypic synonym of Gluconacetobacter hansenii. Int J Syst Evol Microbiol 59:1771–1786
Czaja W, Krystynowicz A, Bielecki S, Brown RM Jr (2006) Microbial cellulose- the natural power to heal wounds. Biomaterials 27:145–151
Dziedzic SZ, Kearsley MW (1995) Handbook of starch hydrolysis products and their derivatives. Blackie Academic and Professional, London
Dos Santos RAC, Berretta AA, Barud H, Ribeiro SJL, Gonzalez-Garcia LN, Zucchi TD, Goldman GH, Riano-Pachon DM (2014) Draft genome sequence of Komagataeibacter rhaeticus strain AF1, a high producer of cellulose, isolated from Kombucha tea. Genome Announc 2(4):e00731–e00714
El-Saied H, El-Diwany AI, Basta AH, Atwa NA, El-Ghwas DE (2008) Production and characterization of economical bacterial cellulose. Bioresources 3(4):1196–1217
Gama M, Gatenholm P, Klemm D (2013) Bacterial nanocellulose: a sophistical multifunctional material. CRC Press, Tokyo and French Group, Boca Raton, London, New York
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58:345–352
ISO 1924-2:2008 Paper and broad. Determination of tensile properties. ISO copyright office, Geneva, Switzerland
ISO 5351-1:2010 Pulps-Determination of limiting viscosity number in cupriethylenediamine (CED) solution. ISO copyright office, Geneva, Switzerland
Keshk S, Sameshima K (2006) The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl Mirobiol Biotechnol 72:291–296
Klemm D, Ahrem H, Kramer F, Fried W, Wippermann J, Kinne RW (2013) Bacterial nanocellulose hydrogels designed as bioartificial medical implants. In: Gama M, Gatenholm P, Klemm D (eds) Bacterial nanocellulose: a sophistical multifunctional material. CRC Press, Tokyo and French Group, Boca Raton, London, New York, pp. 175–196
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A (2011) Nanocellulose: a new family of nature-based material. Angew Chem Int Ed 50:5438–5466
Lee KY, Buldum G, Mantalaris A, Bismarck A (2014) More than meets the eye in bacterial cellulose: biosynthesis, bioprocessing and applications in advanced fiber composites. Macromol Biosci 14:10–32
Nguyen VT, Flanagan B, Gidley MJ, Dykes GA (2008) Characterization of cellulose production by a Gluconacetobacter xylinus strain from Kombucha. Curr Microbiol 57(5):449–453. doi:10.1007/s00284-008-9228-3
Rani MU, Appaiah KAA (2013) Production of bacterial cellulose by Gluconacetobacter hansenii UAC09, using coffee cherry husk. J Food Sci Technol 50:755–762
Raspor P, Goranovic D (2008) Biotechnological application of acetic acid bacteria. Crit Rev Biotechnol 28:101–124
Sahena IM, Brown RM (2013) Biosynthesis of bacterial cellulose. In: Gama M, Gatenholm P, Klemm D (eds) Bacterial nanocellulose: a sophistical multifunctional material, CRC press. Tokyo and French group, Boca Raton, London, New York, pp. 2–18
Semjonovs P, Ruklisha M, Paegle M, Saka M, Treimane R, Patetko A, Linde R (2015) Komagataeibacter rhaeticus P 1463, producer of bacterial cellulose. Patent: EP 15177983.2, EU
Sievers M, Swings J (2005) Family II. Acetobacteraceae. In: Brenner DJ, Krieg NR, Staley JT (eds) Bergey’s manual of systematic bacteriology, 2nd edn, Springer, New York, vol. 2, pp. 41–96
Velasco-Bedran H, Lopez-Isunza F (2007) The unified metabolism of Gluconacetobacter entanii in continuous and batch processes. Proc Biochem 42:1180–1190
Wesarg F, Heßler N, Kralisch D, Klemm D, Fried W, Müller FA (2010) Structural and mechanical properties of bacterial nanocellulose produced by different Gluconacetobacter strains. Abstract of 239th American Chemical Society National Meeting, March 23–29, San Francisco, CA, USA, Cell-133
Yamada Y, Yukphan P, Vu H, Muramatsu Y, Ochaikul D, Tanasupawat S, Nakagawa Y (2012) Description of Komagataeibacter gen. Nov., with proposals of new combinations (Acetobacteraceae). J Gen Appl Microbiol 58:397–404
Zhong C, Zhong GC, Liu M, Zhong XT, Han PP, Jia SR (2013) Metabolic flux analysis of Gluconacetobacter xylinus for bacterial cellulose production. Appl Microbiol Biotechnol 99:1181–1190. doi:10.1007/s00253-013-4908-8
Acknowledgments
We express our gratitude to M. biol. Madara Saka for the unselfish voluntary assistance in this study. This study was financially supported by the ERDF project no. 2014/0034/2DP/2.1.1.1.0/14/APIA/VIAA/097. The BCCM/LMG collection is supported by the Federal Public Planning Service - Science Policy, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Semjonovs, P., Ruklisha, M., Paegle, L. et al. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl Microbiol Biotechnol 101, 1003–1012 (2017). https://doi.org/10.1007/s00253-016-7761-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7761-8






