Abstract
Cinnamic acid (CA) is the chemical basis for bulk production of flavoring reagents and chemical intermediates, and it can be fermented from biomass. Phenylalanine ammonia lyase (PAL) has been used exclusively in the bacterial fermentation of sugar biomass in which the fermentation intermediate phenylalanine is deaminated to CA. Here, we designed an alternative metabolic pathway for fermenting glucose to CA. An Escherichia coli strain that generates phenylalanine in this pathway also produces Wickerhamia fluorescens phenylpyruvate reductase and ferments glucose to d-phenyllactate (d-PhLA) (Fujita et al. Appl Microbiol Biotechnol 97: 8887–8894, 2013). Thereafter, phenyllactate dehydratase encoded by fldABCI genes in Clostridium sporogenes converts the resulting d-PhLA into CA. The phenyllactate dehydratase expressed by fldABCI in the d-PhLA-producing bacterium fermented glucose to CA, but d-PhLA fermentation and phenyllactate dehydration were aerobic and anaerobic processes, respectively, which disrupted high-yield CA fermentation in single batch cultures. We overcame this disruption by sequentially culturing the two strains under aerobic and anaerobic conditions. We optimized the incubation periods of the respective aeration steps to produce 1.7 g/L CA from glucose, which exceeded the yield from PAL-dependent glucose fermentation to CA 11-fold. This process is a novel, efficient alternative to conventional PAL-dependent CA production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cinnamic acid (CA) and its derivatives are in great demand by a broad range of industries as they are used together with their esters as materials for preparing fragrances for cosmetics, flavoring agents for foods, synthetic indigo and thermoplastics (Kaneko et al. 2006; Roscoe 1881; Surburg and Panten 2006). They are also used as components of antitumor, antioxidant and antibiotic pharmaceuticals (Sova 2012) and CA derivatives that absorb ultraviolet light are included in cosmetic skin-firming agents. Commercial amounts of CA in the petroleum chemistry are prepared from reactions between benzaldehyde and malonic acid, acetic anhydride and other organic acids (Carmichael et al. 1999; Johnson 2011; Jones 2011; Thiemann 2007). However, public pressure is driving a need to replace petroleum-derived CA with that produced by natural means. Thus, enzymatic and microbial processes have been constructed to convert natural phenylalanine (Phe) to CA (Cui et al. 2014). Microorganisms can ferment CA from glucose derived from sugar biomass (Vargas-Tah and Gosset 2015).
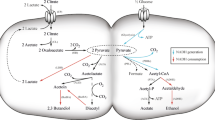
Cinnamic acid and its derivatives are the major naturally occurring phenolic compounds in plants (Humphreys and Chapple 2002) that supply building blocks for the synthesis of lignin and of diverse secondary metabolites including flavonoids, stilbenoids and alkaloids (Chong et al. 2009). The biosynthesis of CA depends on the shikimate metabolic pathway, which is a major source of cellular aromatic compounds (Herrmann 1995). The amino acid Phe is produced de novo through the pathway and deaminated to CA by Phe ammonia lyase (PAL) (Zhang and Liu 2015) (Fig. 1). Metabolic engineering technology has led to Phe yields in the order of 40 g/L (Sprenger 2007), which in combination with PAL reactions enables microbial CA production from glucose. Currently, recombinant Escherichia coli (Vannelli et al. 2007; Vargas-Tah et al. 2015), Pseudomonas putida (Nijkamp et al. 2005) and Streptomyces lividans (Noda et al. 2012) that produce various origins of PAL ferment 5 to 740 mg/L of CA. These amounts are far below those of de novo Phe production, suggesting that Phe deamination by PAL limits CA production. One way to increase the production of biomass-derived CA would be to improve of the PAL reaction in the bacteria or to create an alternative biosynthetic route that does not involve the PAL reaction. Some bacteria and fungi produce PAL like plants and transform Phe to CA and CA-related compounds (Xiang and Moore 2005), whereas Clostridium bacteria possess a unique enzyme system that dehydrates d-phenyllactate (d-PhLA) to CA (Dickert et al. 2000, 2002; Pitsch and Simon 1982). The enzyme, phenyllactate dehydratase (FLD), comprises four proteins encoded by the fldABCI genes and is a potential alternative route of microbial CA production that bypasses Phe deamination (Fig. 1a). The FLD reaction proceeds with CoA-transfer from cinnamoyl-CoA to d-PhLA to yield not only CA, but also phenyllactyl-CoA, which is subsequently dehydrated to cinnamoyl-CoA (Fig. 1b) (Dickert et al. 2000, 2002). The cinnamoyl-CoA:phenyllactate CoA-transferase (FldA) and a dehydratase containing an iron sulfur cluster [4Fe-4S]-(FldBC) forms a heterotrimeric enzyme complex and catalyzes each step of the FLD reaction. The initiator protein (FldI) contains the [4Fe-4S] cluster and has ATPase activity, which is required for activating FldABC dehydration (Fig. 1b). The entire FLD reaction is sensitive to oxygen because the [4Fe-4S] cluster of FldI is sensitive to oxygen. Here, we describe the ability of recombinant E. coli expressing Clostridium sporogenes fldABCI to convert PhLA to CA. Coupled with a bacterium that produces d-PhLA (Fujita et al. 2013), the strain generated CA from glucose as the raw material. Sequential aerobic/anaerobic reaction processes were designed that optimized the efficiency of glucose conversion to CA. This process can serve an alternative to conventional PAL-dependent routes for more efficient, petroleum-free CA production.
Cinnamic acid produced by alternative route via FLD. Typical (gray) and alternative (black) routes for producing cinnamic acid (CA) (a). Mechanism for the dehydration of D-PhLA (b). AT aminotransferase, DAHP 3-deoxy-D-arabinoheptulosonate 7-phosphate, E4P erythrose 4-phosphate, FldABCI phenyllactate dehydratase components, PAL phenylalanine ammonia lyase, PEP phosphoenolpyruvate, PhLA phenyllactate, PprA phenylpyruvate reductase. AroGfbr and PheAfbr are feedback-resistant isozymes of DAHP synthase and chorismate mutase.
Materials and methods
Bacterial strains and reagents
Supplementary Table S1 lists the strains used in this study. Cinnamic acid, p-hydroxy cinnamic acid (4HCA), and D/L-p-hydroxyphenyllactic acid (DL-pHPhLA) were obtained from Wako Pure Chemicals Industries Ltd. (Osaka, Japan). D-PhLA, L-PhLA, D/L-indole-3-lactate(DL-indolelactate) and 3-indoleacrylate were purchased from Tokyo Chemical Industry (Tokyo, Japan). D-p-hydroxyphenyllactate (D-pHPhLA) was prepared from ferments as described (Nguyen et al. 2016). Plasmids were constructed using PrimeSTAR HS DNA polymerase and restriction enzymes (Takara Bio Inc., Shiga, Japan).
Construction of plasmids for fld expression
Supplementary Table S1 lists the plasmids used in this study. Codon-optimized C. sporogenes fldA, fldB, fldC and fldI (GenScript, Piscataway, NJ, USA) (accession numbers; KU754499, KU754500, KU754501, and KU754502) were synthesized and cloned into pUC57 to generate pUC-fldA, pUC-fldB, pUC-fldC and pUC-fldI, respectively. We digested pUC-fldA with BamHI and SalI, and then cloned purified fldA fragments into pRSFDuet-1 (Novagen, Madison, WI, USA) that was digested with the same enzymes to generate pRSFfldA. We digested pUC-fldI with EcoRI and XhoI, and cloned fldI fragments into pRSFfldA to generate pRSFfldAI. We digested pRSFfldAI with BamHI and XhoI, and the resulting purified fldAI fragments were cloned into pETDuet-1 and pCDFDuet-1 (Novagen) that were digested with the same enzymes to generate pETfldAI and pCDFfldAI. We digested pUC-fldB with BamHI and SalI, and cloned fldB fragments into pETDuet-1 to generate pETfldB. We digested pUC-fldC with EcoRI and XhoI, and cloned fldC fragments into pETfldB to generate pETfldBC. We digested pETfldBC with BamHI and XhoI, and resulting fldBC fragments were purified and cloned into pRSFDuet-1 and pCDFDuet-1 that were digested with the same enzymes to generate pRSFfldBC and pCDFfldBC. DNA fragments of pprA (AB621792) were amplified by PCR using pET21pprA (Fujii et al. 2011), pprA-f (5′-GGCCATGGCAAAAAGCCTCAGGTCCTTATAC-3′) and pprA-r (5′-CGGGATCCTCAAACTACAAGATTCATTTCTTC-3′) primers, digested with NcoI and BamHI and cloned into pCDFDuet-1 to generate pCDFppr.
Bacterial cultures
E. coli transformants were rotary-shaken at 120 rpm and 30 °C overnight in 3 mL of LB medium (1 % tryptone, 0.5 % yeast extract, 0.5 % NaCl) and then 2 mL was transferred into 100 mL LB in 500-mL conical flasks that were sealed with a cotton plug and incubated at 30 °C. When the optical density (OD) at 600 nm reached 0.6, 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) was added and the cells were further incubated at 30 °C (aerobic culture). Head space in the flasks was replaced with nitrogen gas by purging the air for 15 min, and then the flasks were sealed with butyl rubber stoppers (anaerobic culture). Sodium ampicillin (100 mg L−1), kanamycin sulfate (40 mg L−1), chloramphenicol (35 mg L−1) and streptomycin (40 mg L−1) were added to maintain the plasmids.
Bioconversion by resting cells
E. coli BL21 Star (DE3) transformed with the indicated fldAI and fldBC expression plasmids (Table S2) were cultured as described above for 6 h under aerobic or anaerobic conditions. The cells were collected by centrifugation at 5000×g for 5 min, washed with 50 mM potassium phosphate buffer (pH 7.0), and incubated in 1 mL of the same buffer containing D-PhLA, l-PhLA, DL-pHPhLA, D-pHPhLA or DL-indolelactate (1 g L−1 each) at 30 °C and reciprocally shaken at 120 rpm. Test tubes were sealed with cotton plugs for aerobic incubation or air in the head space was purged and replaced with nitrogen gas for 15 min then the tubes were sealed using butyl rubber stoppers for anaerobic incubation.
Fermentation of glucose to cinnamic acid
E. coli NST37 (DE3) harboring pETfldAI, pRSFfldBC, pCDFpprA and pACYC-aroG4 (N3fldppr strain) was pre-cultured in 3 mL of LB, and then 2 mL portions were inoculated into 100 mL volumes of fermentation medium (10 g tryptone, 5 g yeast extract, 24 g Na2HPO4, 12 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 0.5 g MgSO4 7H2O, 15 mg CaCl2, 50 mg thiamine-HCl and 2 mL of trace element solution/L; Fujita et al. 2013) containing 2 % glucose in 500-mL conical flasks. The medium contained a final concentration of 1 mg/L FeSO4·7H2O derived from the trace element solution. The mixtures were rotary-shaken at 120 rpm for 3 h at 30 °C under aerobic conditions. After adding 0.5 mM IPTG, the cells were further incubated under aerobic or anaerobic conditions. Co-cultured E. coli NST37/pMGA1/pHSGpprA and E. coli BL21 Star (DE3) harboring pETfldAI and pRSFfldBC (B2fld strain) were incubated as described above and then 1 mL of each was inoculated into 100 mL of fermentation medium in a single 500-mL conical flask containing 2 % glucose. After culture at 30 °C and 120 rpm for 3 h under aerobic conditions, IPTG was added, and the cells were further incubated for 24 and 48 h under aerobic conditions before subsequent culture under anaerobic conditions.
Sequential reaction to produce cinnamic acid
E. coli NST37/pMGA1/pHSGpprA was cultured in fermentation medium containing 2 % glucose for 3 h, 0.5 mM IPTG was added, and the cells were further cultured under aerobic conditions for 3, 27 and 77 h. After culture in LB (100 mL) under anaerobic conditions, B2fld cells were collected by centrifugation at 5000×g for 5 min, added to the E. coli NST37/pMGA1/pHSGpprA cultures and incubated under anaerobic conditions (cell transfer). Escherichia NST37/pMGA1/pHSGpprA cells in the culture broth were removed by centrifugation at 5000×g for 5 min, the B2fld cells prepared as above were added, and anaerobic incubation proceeded in 500-mL conical flasks (cell exchange).
Determination of metabolites
Ferments were analyzed by high-performance liquid chromatography (HPLC) using a 1200 infinity series (Agilent Technologies, Palo Alto, CA, USA) equipped with a 250 × 4.6-mm Purospher® Star RP-18 end-capped column with a particle size of 5 μm (Millipore-Merck, Billerica, MA, USA). The initial mobile phase of 98:2 solvent A (20 mM potassium phosphate, pH 7.0): solvent B (methanol) was maintained for 7 min. The concentration of solvent B was increased to 50 % for 5 min and maintained for another 5 min. The flow rate was 0.8 mL min−1 and absorption at 210 or 280 nm was monitored.
Results
Functional transfer of C. sporogenes FLD to E. coli
C. sporogenes fldA, fldB, fldC, and fldI encoding FLD subunits were codon-optimized for expression in E. coli. Thereafter, the DNA fragments were synthesized, cloned into pET-duet1, pRSF-duet1 and pCDF-duet1 in various combinations and expressed under the control of the T7lac promoter in E. coli BL21 Star (DE3). Due to the anticipated oxygen sensitivity of the FLD activity (Dickert et al. 2000, 2002), recombinant FldABCI proteins were produced from cultures incubated under anaerobic conditions after adding IPTG. The cells were then harvested for resting cell reactions that convert D-PhLA under anaerobic conditions. The results indicated that cells expressing fldAI and fldBC in all combinations of the expression plasmids efficiently converted D-PhLA to CA (Table S2) whereas cells expressing only either fldAI or fldBC did not (Table S2). Therefore, fldABCI introduced into E. coli produced functional FLD. E. coli BL21 Star (DE3) harboring pET-fldAI and pRSF-fldBC (B2fld strain) produced the most CA (0.85 g L−1) with a 95 % molar conversion yield (vs. D-PhLA; Table S2, Fig. 2a), and was thus used for the following experiments.
Recombinant E. coli expressing fldABCI bioconverts D-PhLA to cinnamic acid. HPLC chromatograms of bioconversion reactions of B2fld strain and 1 g L−1 D-PhLA. Effects of aeration upon cinnamic acid (CA) production (a). B2fld and N3fld were incubated under aerobic (AE) and anaerobic (AN) conditions during IPTG-induced FldABCI production and D-PhLA conversion (b). Effects of added glucose (10 g/L) and CA (3 mg/L) upon D-PhLA bioconversion reactions (c). Error bars, standard deviation (n = 3)
Effect of oxygen on D-PhLA bioconversion to cinnamic acid by strain B2fld
Bioconversion under anaerobic conditions transformed D-PhLA to CA, whereas strains exposed to air during FLD production produced less CA than those producing FLD under anaerobic conditions (Fig. 2b). Little CA was produced during bioconversion under the aerobic conditions, irrespective of aeration while inducing FLD production by incubating with IPTG (Fig. 2b). These results indicated that the activity of FLD is sensitive to oxygen in E. coli, especially during bioconversion reactions.
Since FldI initiates the FLD reaction through unidirectional electron transfer to FldABC that accompanies ATP consumption (Fig. 1b) (Dickert et al. 2002), we investigated the effect of glucose, which generates cellular ATP, on bioconversion activity. Adding glucose (10 g L−1) to the bioconversion reaction by B2fld produced 3.5-fold more CA than that without added glucose (Fig. 2c). This finding suggested that the cellular ATP concentration limits FLD activity and hence CA production from D-PhLA in the cells during bioconversion. Addition a trace amount of CA (3 mg L−1), which should be sufficient to generate the starter substrate cinnamoyl-CoA for the FLD reaction (Dickert et al. 2000) did not significantly increase CA production (Fig. 2c), indicating that CA does not limit the cellular cinnamoyl-CoA concentration required by the cells to produce FLD activity. The maximum conversion rates of D-PhLA to CA under our conditions were 280 mg L−1 mg dry cells−1 and 12 mg L−1 mg dry cells−1 h−1.
Production of compounds related to cinnamic acid
We investigated the specificity of the bioconversion by the bacteria producing FLD against 3-substituted lactate derivatives other than D-PhLA. Resting B2fld cells preferentially converted D-PhLA as well as some L-PhLA into CA with 53 and 4.7 % molar conversion yields (vs. D-PhLA; Figs. 2a, 3a and Table 1), which was consistent with the specificity of FldA for chiral substrates (Dickert et al. 2000). The cells also converted D/L-p-hydroxyphenyllactic acid (DL-pHPhLA) to yield 108 mg L−1 p-hydroxycinnamic acid (pHCA) with a 12 % molar yield (Fig. 3b and Table 1). Enantioselectivity of FLD towards pHPhLA was not identified, possibly because pHPhLA was not commercially available until we fermented and purified D-pHPhLA (Nguyen et al. 2016). Bioconversion using D-pHPhLA increased the yield of pHCA to 28 % (Table 1). These results indicated that FLD preferentially recognizes D-pHPhLA over L-pHPhLA. The cells also converted D/L-indole-3-lactic acid to produce 60 mg L−1 3-indoleacrylic acid, with a 6.4 % yield (Fig. 3c and Table 1). These results indicated that FLD converts 3-substituted lactate derivatives to the corresponding α,β-unsaturated acids.
Substrate specificity of FLD produced by recombinant E. coli. HPLC chromatograms of reactions of the B2fld strain for 24 h at 30 °C. Substrates (1 g L−1 each) comprised L-PhLA (a), DL-pHPhLA (b), and D/L-indolelactate (DL-ILA) (c). IAA, indoleacrylate. Top right panel in (a), chromatogram at ×200 magnification. FLD, phenyllactate dehydratase
Glucose fermentation to cinnamic acid via FLD-mediated artificial pathway
Wickerhamia fluorescens PPR reduces phenylpyruvate to d-PhLA (Fujii et al. 2011). Its encoded pprA gene was overexpressed in the Phe-producing E. coli NST37 strain that converts glucose to d-PhLA with yields up to 29 g/L (Fujita et al. 2013). Here, we designed a novel fermentation pathway in which FLD mediates the conversion of glucose to CA using fldABCI, pprA, and E. coli NST (DE3) that is λ(DE3)-lysogenized NST37 (Fig. 1). We confirmed that E. coli NST37 (DE3) harboring pET-fldAI and pRSF-fldBC (N3fld strain) produced CA from Phe under anaerobic conditions (Fig. 2b). We introduced the aroG fbr expression plasmid that encodes feedback-resistant DAHP synthase and enhances shikimate pathway metabolism (Kikuchi et al. 1997), and introduced pprA into E. coli NST37 (DE3) to generate the strain, N3fldppr. After culture under aerobic conditions as described in Materials and methods, N3fldppr produced little CA in fermentation medium containing 2 % glucose for 48 h, compared with 0.6 mg L−1 CA under the anaerobic conditions (Fig. 4a). Both cultures produced little pHPhLA and other CA derivatives. The cultures accumulated considerable amounts of d-PhLA both under aerobic (400 mg L−1) and anaerobic (76 mg L−1) conditions (Fig. 4b), indicating that FLD activity limits cellular CA production. Such limitation was more pronounced under aerobic conditions, which reflects the oxygen sensitivity of FLD activity. These results indicate that E. coli fermented glucose to CA in a PAL-independent alternative metabolic pathway.
Recombinant E. coli expressing fldABCI and pprA ferments glucose to cinnamic acid and D-PhLA. Concentrations of CA (a) and D-PhLA (b) in culture supernatant of NST37 (DE3) harboring pETfldAI, pRSFfldBC, pCDFppr and pACYC-aroG4 (strain N3fldppr). Strains were cultured in 100-mL fermentation medium at 30 °C for 48 h under aerobic (AE) or anaerobic (AN) conditions. Error bars, standard deviation (n = 3)
Co-cultivation of strains producing D-PhLA and FLD
Although N3fldppr is useful in the simple fermentation of glucose to CA, the amount of generated CA could be improved. B2fld maximized the production of FLD and hence, that of CA, since it converted 2.5-fold more d-PhLA to CA than N3fld (Fig. 2b). Therefore, we investigated the ability of B3fld to transform glucose to CA in anaerobic co-culture with E. coli NST37/pMGA1/pHSGpprA that ferments d-PhLA (Fujita et al. 2013; Fig. 5a). These strains co-cultured under aerobic conditions for 100 h produced <0.01 g L−1 CA while accumulating 2.8 g L−1 D-PhLA (Fig. 5a, b). These findings indicate that D-PhLA was preferentially produced and that FLD activity was inactivated by aeration (Fig. 4). The co-cultures were shifted from aerobic incubation for various periods to anaerobic conditions to circumvent the differing aeration preferences between the two reactions (Fig. 5). Adding IPTG (aerobic culture for 3 h) and then immediately shifting the co-cultures to anaerobic conditions for 122 h yielded 0.4 g L−1 CA (Fig. 5a). Less d-PhLA was generated under these conditions than under any other aerating conditions tested (Fig. 5b). Single N3fldppr cultures produced 250-fold less CA than the co-culture under anaerobic conditions (Fig. 4a), indicating that co-culture conferred an advantage for producing large quantities of CA.
Co-cultured E. coli strains NST37/pMGA1/pHSGpprA and B2fld produce CA from glucose. E. coli NST37/pMGA1/pHSGpprA and B2fld (0.8 mg dry cell weight each) were co-cultured under aerobic conditions throughout experiment (black circle) or for 3 (black diamond), 27 (black square), and 51 (black triangle) h (white arrows) followed by anaerobic incubation (gray arrows) as described in Materials and methods (top panel). Isopropyl-β-d-thiogalactoside was added after culture for 3 h. Cinnamic acid (middle) and D-PhLA (bottom) in culture supernatants determined by HPLC. Vertical arrows, time points for transferring cultures to anaerobic conditions
Sequential reactions improved CA production
We developed stepwise processes for the aerobic production of d-PhLA and its anaerobic conversion to CA to improve the yield of CA. One is a “cell transfer” step through which d-PhLA produced by E. coli is cultivated under aerobic conditions. Thereafter, we added B2fld cells in which FLD activity was induced beforehand, and then d-PhLA was converted to CA under anaerobic conditions (Fig. 6a). Stepwise culture under initial aerobic conditions for 6, 30, and 80 h produced 0.2, 0.9, and 0.4 g L−1 CA, respectively, by the end of the 172-h culture period (Fig. 6a). Therefore, the yield of CA was maximal after 30 h of aerobic culture, with a concentration that was 5.6-fold higher than that produced by co-cultures (Fig. 5). An alternative “cell exchange” approach was designed in which the culture supernatant of the aerobic D-PhLA production was used for the anaerobic conversion of d-PhLA to CA by B2fld cells (Fig. 6b). After the initial 6, 30, and 80 h of aerobic cultivation, this process resulted in the maximal CA production of 0.4, 1.7, and 1.0 g L−1, respectively (Fig. 6b). These findings imply that more CA was produced by cell exchange than cell transfer regardless of the length of the initial aerobic cultivation. The yield of 1.7 g L−1 CA was higher than that achieved by any PAL-dependent process using glucose or any other biomass as a carbon source (Vargas-Tah and Gosset 2015).
Sequential culture of E. coli strains NST37/pMGA1/pHSGpprA and B2fld produce CA from glucose. Cell transfer (a). E. coli NST37/pMGA1/pHSGpprA (dry cell weight, 0.8 mg) was cultured under aerobic conditions for 6 (■□), 30 (▲△), and 80 (●○) h (white arrows), then B2fld cells (dry cell weight, 40 mg) were added and incubated under anaerobic conditions (gray arrows) as described in Materials and methods. Isopropyl-β-d-thiogalactoside was added after culture for 3 h. Cinnamic acid (●▲■) and D-PhLA (○△□) in culture supernatants quantified by HPLC. Vertical arrows, time points when cultures were transferred to anaerobic conditions. Cell exchange (b). Cells were cultured as described in (a) except E. coli NST37/pMGA1/pHSGpprA cells were removed before adding B2fld cells. Cinnamic acid (●▲■) and D-PhLA (○△□) in culture supernatants quantified by HPLC. Vertical arrows, time points when cultures were transferred to anaerobic conditions
Rates of CA production were compared within 20 h of adding B2fld cells in the cell exchange experiments. The rate was 5-fold for the culture shifted to anaerobic conditions at 30, than at 80 h (0.058 vs. 0.012 g L−1 h−1). The latter accumulated 1.5-fold more D-PhLA than the former, indicating that the lower rate was not caused by a deficiency of D-PhLA for the FLD reaction. Long-term (80 h) D-PhLA fermentation is likely to produce compounds that would inhibit the cellular conversion of D-PhLA to CA. These results showed that length of the aerobic culture for D-PhLA production should be considered to optimize CA production.
Discussion
Cinnamic acid is produced from biomass exclusively via the PAL reaction that deaminates Phe. Here, we created an alternative pathway that bypasses the PAL reaction to ferment glucose to CA using FLD produced by the Clostridiaceae family of bacteria. Metabolically engineered E. coli in this pathway facilitates production of the fermentation intermediate of Phe, phenylpyruvate (Sprenger 2007), that is subsequently reduced to D-PhLA by PPR produced ectopically (Fujita et al. 2013). Another E. coli strain that produces FLD converts D-PhLA to CA (Fig. 1). In addition to FLD, the high-yield fermentation of D-PhLA (∼29 g/L) that we previously achieved (Fujita et al. 2013) could be key to the production of CA from the simple sugar, glucose. The stepwise aerobic/anaerobic culture of strains that ferment D-PhLA and produce FLD was an innovation that maximized CA production. The resulting yield of 1.7 g L−1 CA was 11-fold greater than the current maximum for PAL-dependent CA production by the same bacterium (Vargas-Tah et al. 2015), and it exceeded that of other bacteria (Nijkamp et al. 2005; Noda et al. 2012). Conversion that is dependent on FLD combined with simultaneous saccharification and fermentation systems of lignocellulosic biomass to produce D-PhLA (Kawaguchi et al. 2014, 2015) could generate CA from lignocellulosic biomass in the future.
The dissimilar CA production efficiency of the FLD-and PAL-dependent pathways could be due to the reaction properties of their phenylpyruvate conversion to CA since the two pathways respectively share phenylpyruvate and CA as a precursor and a product for synthesizing CA, (Fig. 1). Their net reactions are described as follows by focusing on direct counter-reaction compounds.
In the PAL-dependent pathway, AT transaminates phenylpyruvate via glutamate to generate Phe, which evokes a link between this pathway and glutamate metabolism. Ammonium, which generates cellular glutamate, is critical for the AT reaction in the PAL pathway. This apparently differs from the FLD pathway that involves the NADH-dependent reduction of phenylpyruvate and no glutamate metabolism, and where ammonia added to culture media increases cellular glutamate production and shifts the metabolic flow of phenylpyruvate to intrinsic AT, and hence decreases the cellular transformation of phenylpyruvate to D-PhLA (Fujii et al. 2011). Thus, the two pathways differ in terms of their ammonium requirements. Another notable difference is that the AT reaction is reversible and under equilibrium between phenylpyruvate and glutamate, whereas PPR is irreversible (Fujii et al. 2011). This avoids complicated regulation of the fermentation process designed for maximal CA production through the FLD pathway.
The FLD family is related to proteins with >90 % identical amino acid sequences in Clostridium, Acidaminococcus, and Fusobacterium bacteria. Proteins with lower (15∼20 %) but significant amino acid identity to FldABC also include 2-hydroxyglutarate dehydratase HgdABC and GctAB (Hans et al. 1999, 2000; Mack et al. 1994), as well as E. coli carnitine dehydratase CaiB, which is similar to FldBC (Dickert et al. 2002). These enzymes share the dehydration activity of 2-hydroxy acid with FLD as well as oxygen-sensitive activity. The stepwise aerobic/anaerobic processes developed herein with this family of proteins could be combined with aerobic pathways to construct other biological processes. For, example, GctAB has been applied to engineer E. coli that produces glutaconate, a potential monomer for biodegradable plastics, under anaerobic conditions (Djurdjevic et al. 2011). The substrate for glutaconate production is 2-oxoglutarate, which E. coli generates from glucose at high yield under aerobic conditions (Perrenoud and Sauer 2005). This indicates that novel fermentation processes of glucose to glutaconate could be engineered by combining these metabolic reactions using the stepwise aerobic/anaerobic procedure.
The strain producing FLD transformed D-pHPhLA to pHCA (Fig. 3). We previously constructed a D-pHPhLA fermentation system that uses glucose as raw material (Nguyen et al. 2016). Combining D-pHPhLA fermentation with strains producing FLD is a promising alternative to pHCA fermentation and distinct from that mediated by tyrosine (Vargas-Tah and Gosset 2015). Both pHCA and CA are intermediates for the biosynthesis of known plant phenylpropanoids that include both commercial and predicted pharmaceuticals. Genetically manipulated bacteria have been applied in many efforts to produce these compounds (Limem et al. 2008). Thus, the novel pHCA process should be a major contributor to the construction of production platforms for such compounds. Because monomeric pHCA is also a raw material for aromatic engineered plastics (Kaneko et al. 2006), its fermentation will result in the replacement of petroleum-derived with biomass-derived aromatic plastics.
References
Carmichael AJ, Earle MJ, Holbrey JD, McCormac PB, Seddon KR (1999) The heck reaction in ionic liquids: a multiphasic catalyst system. Org Lett 1:997–1000. doi:10.1021/ol9907771
Chong J, Poutaraud A, Hugueney P (2009) Metabolism and roles of stilbenes in plants. Plant Sci 177:143–155. doi:10.1016/j.plantsci.2009.05.012
Cui JD, Qiu JQ, Fan XW, Jia SR, Tan ZL (2014) Biotechnological production and applications of microbial phenylalanine ammonia lyase: a recent review. Crit Rev Biotechnol 34:258–268. doi:10.3109/07388551.2013.791660
Dickert S, Pierik AJ, Linder D, Buckel W (2000) The involvement of coenzyme A esters in the dehydration of (R)-phenyllactate to (E)-cinnamate by Clostridium sporogenes. Eur J Biochem 267:3874–3884. doi:10.1046/j.1432-1327.2000.01427.x
Dickert S, Pierik AJ, Buckel W (2002) Molecular characterization of phenyllactate dehydratase and its initiator from Clostridium sporogenes. Mol Microbiol 44:49–60. doi:10.1046/j.1365-2958.2002.02867.x
Djurdjevic I, Zelder O, Buckel W (2011) Production of glutaconic acid in a recombinant Escherichia coli strain. Appl Environ Microbiol 77:320–322. doi:10.1128/aem.02049-10
Fujii T, Shimizu M, Doi Y, Fujita T, Ito T, Miura D, Wariishi H, Takaya N (2011) Novel fungal phenylpyruvate reductase belongs to D-isomer-specific 2-hydroxyacid dehydrogenase family. Biochim Biophys Acta 1814:1669–1676. doi:10.1016/j.bbapap.2011.05.024
Fujita T, Nguyen HD, Ito T, Zhou S, Osada L, Tateyama S, Kaneko T, Takaya N (2013) Microbial monomers custom-synthesized to build true bio-derived aromatic polymers. Appl Microbiol Biotechnol 97:8887–8894. doi:10.1007/s00253-013-5078-4
Hans M, Sievers J, Muller U, Bill E, Vorholt JA, Linder D, Buckel W (1999) 2-hydroxyglutaryl-CoA dehydratase from Clostridium symbiosum. Eur J Biochem 265:404–414. doi:10.1046/j.1432-1327.1999.00748.x
Hans M, Buckel W, Bill E (2000) The iron-sulfur clusters in 2-hydroxyglutaryl-CoA dehydratase from Acidaminococcus fermentans. Biochemical and spectroscopic investigations. Eur J Biochem 267:7082–7093. doi:10.1046/j.1432-1327.2000.01809.x
Herrmann KM (1995) The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell 7:907–919. doi:10.1105/tpc.7.7.907
Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5:224–229. doi:10.1016/S1369-5266(02)00257-1
Johnson JR (2011) The Perkin reaction and related reactions. Org React 1:210–265. doi:10.1002/0471264180.or001.08
Jones G (2011) The Knoevenagel condensation. Org React 15:204–599. doi:10.1002/0471264180.or015.02
Kaneko T, Thi TH, Shi DJ, Akashi M (2006) Environmentally degradable, high-performance thermoplastics from phenolic phytomonomers. Nat Mater 5:966–970. doi:10.1038/nmat1778
Kawaguchi H, Uematsu K, Ogino C, Teramura H, Niimi-Nakamura S, Tsuge Y, Hasunuma T, Oinuma K, Takaya N, Kondo A (2014) Simultaneous saccharification and fermentation of kraft pulp by recombinant Escherichia coli for phenyllactic acid production. Biochem Eng J 88:188–194. doi:10.1016/j.bej.2014.04.014
Kawaguchi H, Teramura H, Uematsu K, Hara KY, Hasunuma T, Hirano K, Sazuka T, Kitano H, Tsuge Y, Kahar P, Niimi-Nakamura S, Oinuma K, Takaya N, Kasuga S, Ogino C, Kondo A (2015) Phenyllactic acid production by simultaneous saccharification and fermentation of pretreated sorghum bagasse. Bioresour Technol 182:169–178. doi:10.1016/j.biortech.2015.01.097
Kikuchi Y, Tsujimoto K, Kurahashi O (1997) Mutational analysis of the feedback sites of phenylalanine-sensitive 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. Appl Environ Microbiol 63:761–762
Limem I, Guedon E, Hehn A, Bourgaud F, Chekir Ghedira L, Engasser JM, Ghoul M (2008) Production of phenylpropanoid compounds by recombinant microorganisms expressing plant-specific biosynthesis genes. Process Biochem 43:463–479. doi:10.1016/j.procbio.2008.02.001
Mack M, Bendrat K, Zelder O, Eckel E, Linder D, Buckel W (1994) Location of the two genes encoding glutaconate coenzyme A-transferase at the beginning of the hydroxyglutarate operon in Acidaminococcus fermentans. Eur J Biochem 226:41–51. doi:10.1111/j.1432-1033.1994.00t41.x
Nguyen HD, Kaneko T, Takaya N, Fujita T, Ito T (2016) Fermentation of aromatic lactate monomer and its polymerization to produce highly thermoresistant bioplastics. Polym J 48:81–89. doi:10.1038/pj.2015.80
Nijkamp K, van Luijk N, de Bont JA, Wery J (2005) The solvent-tolerant Pseudomonas putida S12 as host for the production of cinnamic acid from glucose. Appl Microbiol Biotechnol 69:170–177. doi:10.1007/s00253-005-1973-7
Noda S, Miyazaki T, Tanaka T, Ogino C, Kondo A (2012) Production of Streptoverticillium cinnamoneum transglutaminase and cinnamic acid by recombinant Streptomyces lividans cultured on biomass-derived carbon sources. Bioresour Technol 104:648–651. doi:10.1016/j.biortech.2011.10.045
Perrenoud A, Sauer U (2005) Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli. J Bacteriol 187:3171–3179. doi:10.1128/JB.187.9.3171-3179.2005
Pitsch C, Simon H (1982) The stereochemical course of the water elimination from (2R)-phenyllactate in the amino acid fermentation of Clostridium sporogenes. Hoppe Seyler’s Z Physiol Chem 363:1253–1257
Roscoe EH (1881) Indigo and its artificial production. Nature 24:227–231
Sova M (2012) Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev Med Chem 12:749–767. doi:10.2174/138955712801264792#sthash.yoWU70ge.dpuf
Sprenger GA (2007) From scratch to value: engineering Escherichia coli wild type cells to the production of L-phenylalanine and other fine chemicals derived from chorismate. Appl Microbiol Biotechnol 75:739–749. doi:10.1007/s00253-007-0931-y
Surburg H, Panten J (2006) Common fragrance and flavor materials: preparation, properties and uses. WILEY-VCH, Weinheim
Thiemann T (2007) Solventless Wittig olefination with fluorinated benzaldehydes. J Chem Res 2007:336–341. doi:10.3184/030823407X225464
Vannelli T, Wei Qi W, Sweigard J, Gatenby AA, Sariaslani FS (2007) Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab Eng 9:142–151. doi:10.1016/j.ymben.2006.11.001
Vargas-Tah A, Gosset G (2015) Production of cinnamic and p-hydroxycinnamic acids in engineered microbes. Front Bioeng Biotechnol 3:116. doi:10.3389/fbioe.2015.00116
Vargas-Tah A, Martínez LM, Hernández-Chávez G, Rocha M, Martínez A, Bolívar F, Gosset G (2015) Production of cinnamic and p-hydroxycinnamic acid from sugar mixtures with engineered Escherichia coli. Microb Cell Factories 14:1–12. doi:10.1186/s12934-014-0185-1
Xiang L, Moore BS (2005) Biochemical characterization of a prokaryotic phenylalanine ammonia lyase. J Bacteriol 187:4286–4289. doi:10.1128/JB.187.12.4286-4289.2005
Zhang X, Liu CJ (2015) Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol Plant 8:17–27. doi:10.1016/j.molp.2014.11.001
Acknowledgments
We thank Norma Foster for critical reading of the manuscript. This study was supported by the Advanced Low Carbon Technology Research and Development Program (5100270) from the Japan Science and Technology Agency [JST-ALCA].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Shunsuke Masuo and Yuta Kobayashi contributed equally to this work as first authors.
Electronic supplementary material
ESM 1
(PDF 71 kb)
Rights and permissions
About this article
Cite this article
Masuo, S., Kobayashi, Y., Oinuma, KI. et al. Alternative fermentation pathway of cinnamic acid production via phenyllactic acid. Appl Microbiol Biotechnol 100, 8701–8709 (2016). https://doi.org/10.1007/s00253-016-7623-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7623-4