Abstract
Effects of high ZnSO4·7H2O supplementation on cell growth and monoclonal antibody (mAb) production in chemically defined suspension cultures of recombinant Chinese hamster ovary (rCHO) DG44 cells were examined. The supplementation of ZnSO4·7H2O up to 120 μM gradually increased specific mAb production rate of rCHO DG44 cells in the early growth phase (0–4 days of culture). The ZnSO4·7H2O concentration for enhancing mAb production without any cytotoxic effects on cell growth was 30–60 μM. In addition of 60 μM ZnSO4·7H2O to in-house protein-free medium and in-house chemically defined medium, mAb production was increased 2.0-fold and 6.5-fold, respectively. Moreover, addition of ZnSO4·7H2O to three kinds of commercial chemically defined media yielded a greater than 1.2-fold enhancement of mAb production. These data indicate that simple supplementation of a relatively high zinc ion concentration to cell culture media without significant changes of rCHO DG44 cell culture process can be useful for achieving high production of mAb.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese hamster ovary (CHO) DG44 cell line is widely used for the commercial production of therapeutic antibodies because this cell line exhibits the efficient DHFR selection and amplification system. DHFR− mutation in this cell line is stable and irreversible (Urlaub et al. 1983; Lee and Hwang 2004). Chemically defined media (CDM) that support high cell densities and high production rates have generally been preferred in large-scale monoclonal antibody (mAb) production because of economic reason and safety concerns related to transmissible spongiform encephalopathy and other contaminants. However, no universal chemically defined medium suitable for all recombinant Chinese hamster ovary (rCHO) DG44 cell lines has been identified. Different cell lines might have different nutrient requirements. Optimization of cell culture media for each rCHO DG44 cell line is a lengthy and labour-intensive process.
Developments of simple applicable methods for improving mAb production such as media additives supplementation have been preferred. For instance, the addition of butyrate, propionate, dimethyl sulfoxide, and pentanoic acid to rCHO culture media have all been investigated, with the aim of improving recombinant protein production such as erythropoietin, follicle-stimulating hormone, mAb, and fusion protein (Yoon and Ahn 2007; Choi et al. 2007; Mimura et al. 2001; Liu et al. 2001). Although positive effects were observed on recombinant protein production, the use of these compounds at high concentrations for maximising protein production is limited by their cytotoxic effects on rCHO cell lines such as rCHO DUKX-B11, rCHO K1, and rCHO NTHU-108 (Yoon and Ahn 2007; Choi et al. 2007; Mimura et al. 2001; Liu et al. 2001).
Addition of trace elements is important for optimal growth of CHO cells. However, which component among trace elements acts essentially on CHO cell growth is still not clear. Hamilton and Ham (1977) developed the protein-free media (PFM) containing more than seven kinds of trace elements for growth of CHO cell lines such as CHOP, CHO K1, and CHO C14. When trace metals (such as Fe2+, SeO3 2−, Li+, Zn2+, and Cu2+) with foetal bovine serum were fed to the culture periodically, the maximum viable cell density, culture span, and final mAb production were increased (Xie and Wang 1996). The authors, however, did not report which component was effective for cell growth and mAb production (Xie and Wang 1996). In fed-batch suspension cultures of rCHO DG44 cell lines, trace metal enrichment in the feed medium has been shown to significantly increase mAb production by promoting cell longevity (Huang et al. 2010). The composition of the concentrated feed medium was, however, unknown. For clarifying effects of trace elements in rCHO DG44 cell suspension culture, further studies are needed in a quantitative manner.
In this study, we selected six trace elements such as copper, selenium, vanadium, zinc, manganese, and molybdenum and used the combination of these six elements as a control. The objectives of this study were (i) to identify the trace element concentration that best enhances mAb production by batch suspension cultures of rCHO DG44 cell line, (ii) to identify the individual trace elements that mediate enhanced mAb production, (iii) to determine the concentrations of zinc ion which was the most effective component identified in (ii) that yield maximum mAb production in PFM and in CDM, and (iv) to determine the effects of the concentration identified in (iii) on mAb production in various commercial CDMs.
Materials and methods
Cell culture
The rCHO DG44 cell line, which expresses a human mAb of IgG1 isotype, was kindly provided by the Green Cross Corp (Kyonggi, Korea). rCHO DG44 cells were maintained in both in-house PFM and in-house CDM. The in-house CDM contains no hydrolysate and is fortified with components including amino acids, vitamins, and glucose. Cells were routinely cultured in a 37 °C incubator and maintained under a 5 % CO2 atmosphere. A solution of trace elements (0.01 μM CuSO4·5H2O (Cu), 3 μM ZnSO4·7H2O (Zn), 0.03 μM Na2SeO3 (Se), 0.01 μM NH4VO3 (V), 0.001 μM MnSO4·H2O (Mn), and 0.01 μM (NH4)6Mo7O24·4H2O (Mo)) was used as a control to supplement both kinds of medium. These chemicals were purchased from Sigma-Aldrich (St. Louis, Mo, USA). PFM and CDM were supplemented with either the trace elements or Zn in various concentrations, which ranged from 1 to 40 times of that in the control. For commercial CDM, Power CHO-2 CD (Lonza, Verviers, Belgium), CDM4CHO (Hyclone, Logan, UT, USA), EXCELL CD CHO (SAFC Bioscience, St. Louis, Mo, USA), and CD Opti CHO (Gibco, Grand Island, NY, USA) formulations were used. The zinc ion concentrations of commercial CDM formulations were also controlled by supplementation with the Zn stock solution that was made by using the corresponding CDM formulation. The effects of Zn on mAb production in commercial CDM were evaluated at the third passage after subculturing in each condition, to minimise carryover effects from the in-house CDM. All experiments were performed by batch mode without pH control in 125-mL Erlenmeyer flasks with screw caps (Corning, Acton, MA, USA) and a working volume of 30 mL. The flasks were placed in a horizontal orbital shaker (Barnstead International, Dubuque, IA, USA) and shaken at 120 rpm with a 1.9-cm rotation diameter. Cells from stock cultures were inoculated into the Erlenmeyer flasks at approximately 4 × 105 cells/mL. Viable and dead cells were counted with a Neubauer haemocytometer, using the trypan blue exclusion method.
Zinc ion quantification
The concentrations of zinc ions in commercial CDMs were quantitated with an inductive coupled plasma-optical emission spectrometer (JY Ultima2C, HORIVA Jobin Yvon, Longjumeau, France) at the Seoul Center, Korea Basic Science Institute.
Enzyme-linked immunosorbent assay for mAb detection
mAb production was quantitated at 4, 6, 8, 10, and 12 days postinoculation of rCHO DG44 cells using a sandwich enzyme-linked immunosorbent assay (ELISA). Anti-human IgG antibodies (Fab-specific) (Sigma) were used as primary antibodies and anti-human IgG (Fc-specific)-horseradish peroxidase antibodies (Sigma) were used for detection. Nonspecific binding sites were blocked with 1 % (w/v) bovine serum albumin/phosphate-buffered saline. 3,3′,5,5′-tetramethylbenzidine (TMB) (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) was used as the substrate for colour development, and reactions were stopped with 2 M H2SO4. Absorbances were measured at 450 nm using a kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Determination of specific growth and production rates
The specific growth rate (μ) and specific mAb production rate (q mAb) were based on data collected during the exponential growth phase and were estimated as previously described (Lee et al. 1991).
Statistical analysis
Statistical analysis for obtained data was performed with Minitab Statistical Software version 17 (MiniTab), and two-sample t test was used to evaluate significant effect with 60 μM Zn supplementation on cell growth and mAb production in various commercial CDMs, with the confidence interval as 95 %.
Results
Effects of various trace elements concentration on cell growth and mAb production
To determine the effects of various trace elements concentration on rCHO DG44 cells with respect to growth and mAb production, batch suspensions were cultured in triplicate with concentrations of the trace elements supplement ranging from 1 to 40 times of the control. Cell growth was not significantly altered with trace elements concentrations up to 10 times above that of the control (Fig. 1a). However, the maximum viable cell density decreased sharply as the trace elements concentration in the in-house PFM increased from 20 to 40 times of the control (Fig. 1a). The maximum viable cell density obtained at 20× trace elements concentration was about 1.2 × 107 cells/mL, which is approximately 80 % of the control (Fig. 1a). After reaching the maximum viable cell density, the viable cell density in the 20× condition remained higher than the control density throughout the cultivation period (Fig. 1a). In the 20× condition, the viable cell density exceeded 0.9 × 107 cells/mL until the ninth day of culture (Fig. 1a). In contrast, the 40× trace elements condition significantly suppressed cell growth (Fig. 1a). The suitable range of trace elements for mAb production is shown in Fig. 1b. The maximum mAb concentration was gradually increased as the trace elements concentration increased up to 20×. However, further supplementation of trace elements led to a significant decrease in mAb production. These results indicate that a 20× concentration of trace elements compared with the control is the suitable condition for mAb production.
Growth and mAb production of rCHO cells grown in in-house PFM supplemented with various trace elements (TE) concentrations. a Viable cell density. b mAb titer. Control (filled square), 10× TE (filled circle), 20× TE (filled triangle), 30× TE (filled inverted triangle), and 40× TE (filled diamond). The error bars represent the standard deviations calculated from three independent experiments
Identification of the trace elements that influence cell growth and mAb production
To identify the trace elements responsible for this increase in mAb production, rCHO DG44 cells were cultured in the in-house PFM that was supplemented with 20× of each individual component. The viable cell density and mAb concentration were then measured in triplicate for each condition. The cell growth and mAb production obtained with 20× Zn supplementation were similar to those obtained with the 20× trace elements for suspension cultures of rCHO DG44 cells (Fig. 2a, b). None of the other elements tested exerted any significant effect on cell growth or mAb production compared with the control (Fig. 2a, b). These results indicate that Zn is the only trace element that enhances mAb production.
Growth and mAb production of rCHO cells grown in in-house PFM with supplementation of only one TE at a time. a viable cell density. b mAb titer. Control (filled square), 20× Cu (filled circle), 20× Zn (filled triangle), 20× Se (filled inverted triangle), 20× V (open square), 20× Mn (open circle), 20× Mo (open triangle), and 20× TE (open inverted triangle). The error bars represent the standard deviations calculated from three independent experiments
Effects of zinc ion concentration on cell growth and mAb production
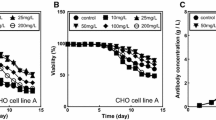
To determine the appropriate concentration of zinc ions for cell growth and mAb production, various Zn concentrations ranging from 3 μM (control) to 120 μM were used to supplement the in-house PFM. The maximum viable cell density exceeded 1.4 × 107 cells/mL with 3–30 μM Zn supplementation, whereas cell density gradually decreased as the Zn concentration increased from 60 to 120 μM (Fig. 3a). After 6 days of culture, the viable cell density remained higher with 30–60 μM Zn supplementation compared with control cells; however, the most of culture cells suddenly died on the seventh day for Zn concentrations higher than 90 μM (Fig. 3a). These toxic effects observed at Zn concentrations more than 90 μM were related to the drop of pH values in the culture medium. The pH values at these conditions were found below 6.4 after 6 days of culture (data not shown). These pH values were known to be detrimental conditions for CHO cell growth (Morita et al. 1992). On the other hand, the pH was maintained above 6.8 for Zn concentrations lower than 60 μM throughout the cultivation period (data not shown). mAb production was significantly increased with 3–60 μM Zn supplementation, while it was decreased for Zn concentrations above 90 μM (Fig. 3b). The maximum mAb concentration obtained with 60 μM Zn supplementation was 360 ± 12 mg/L, which is approximately 2-fold higher than control conditions (Fig. 3b).
Growth and mAb production of rCHO cells grown in in-house PFM supplemented with various Zn concentrations. a Viable cell density. (b) mAb titer. Control (3 μM Zn, filled square), 30 μM Zn (filled circle), 60 μM Zn (filled triangle), 90 μM Zn (filled inverted triangle), and 120 μM Zn (filled diamond). The error bars represent the standard deviations calculated from three independent experiments
Our in-house PFM formulation includes a yeast-derived hydrolysate as a complex component. A CDM that supports suspension growth of rCHO DG44 cells is highly desirable for large-scale mAb production. Therefore, we also tested the various Zn concentrations in the in-house CDM, which does not contain any complex components. Similar effects on cell growth and mAb production were observed with the in-house CDM as for the in-house PFM (Fig. 4a, b). The maximum viable cell density obtained in the range of 30 to 60 μM Zn exceeded 2.0 × 107 cells/mL, which is 1.2 times higher than the control density (Fig. 4a). With 60 μM Zn supplementation, the viable cell density after 5 days of culture was maintained above 1.0 × 107 cells/mL throughout the rest of the cultivation period, while the viable cell density of control cells decreased suddenly at 5 days (Fig. 4a). The abrupt decline of cell growth on the fifth day for the control and the 120 μM Zn supplementation might be associated with the drop of pH values in the culture medium. The pH values at these conditions were found below 6.3 after 5 days of culture (data not shown). On the other hand, the pH values of the culture medium with 30–90 μM Zn supplementation were maintained above 6.7 until 8 days of culture (data not shown). The maximum mAb concentration obtained with 60 μM Zn supplementation was 444 ± 13 mg/L, which is 6.5-fold higher than the control amount (Fig. 4b). Moreover, increasing the Zn concentration from 3 to 60 μM in suspension culture of a different rCHO DG44 cell line using the in-house CDM improved the maximum mAb concentration up to 1.3-fold without any cytotoxic effects (data not shown). Taking into account the in-house PFM and the in-house CDM results, 30–60 μM Zn appears to be the suitable concentration for cell growth and mAb production.
Growth and mAb production of rCHO cells grown in in-house CDM supplemented with various Zn concentrations. a Viable cell density. b mAb titer. Control (3 μM Zn, filled square), 30 μM Zn (filled circle), 60 μM Zn (filled triangle), 90 μM Zn (filled inverted triangle), and 120 μM Zn (filled diamond). The error bars represent the standard deviations calculated from two independent experiments
Effects of 60 μM zinc ion supplementation in various CDMs
To evaluate the influence of 60 μM Zn supplementation on cell growth and mAb production in various CDM formulations, four different kinds of commercial CDM were used. The rCHO DG44 cell cultures in commercial CDM formulations were conducted under the same batch culture condition as the in-house CDM. These CDM formulations have zinc ion concentrations ranging from 2 to 9 μM. The maximum viable cell densities with 60 μM Zn supplementation of CDM-1, CDM-2, and CDM-3 were similar to those of control CDMs (Fig. 5a). Moreover, the maximum mAb concentrations were increased 1.2-fold, 1.5-fold, and 1.5-fold, respectively (Fig. 5b). While the growth of rCHO DG44 cells ceased at the second passage in CDM-4 without the addition of Zn, growth could be maintained over three consecutive passages with 60 μM Zn supplementation (data not shown).
Maximum viable cell densities and mAb concentrations in commercial CDM formulations supplemented with 60 μM Zn. a Maximum viable cell density. b maximum mAb concentration. Control (filled bar) and 60 μM Zn (lattice bar). Power CHO-2 CD medium (CDM-1), CDM4CHO medium (CDM-2), EXCELL CD CHO medium (CDM-3), and CD Opti CHO medium (CDM-4). The error bars represent the standard deviations calculated from two independent experiments. *Results were obtained with the first passage of rCHO cells grown in CDM-4
Two-sample t test of the maximum mAb concentrations at 60 μM Zn supplementation of CDM-1, CDM-2, and CDM-3 showed there were significant differences (P values 0.019 < 0.050 (CDM-1), 0.002 < 0.050 (CDM-2), and 0.005 < 0.050 (CDM-3)), while the maximum viable cell densities showed no significant differences at 60 μM Zn supplementation of each CDM (P values 0.143 > 0.050 (CDM-1), 0.060 > 0.050 (CDM-2), and 0.476 > 0.050 (CDM-3)). These results indicate that 60 μM Zn can improve mAb production without any cytotoxic effects on cell growth in chemically defined suspension cultures of rCHO DG44 cells.
Effects on specific production rate, growth rate, and cell longevity
To achieve maximum mAb production, the highest q mAb possible must be maintained. In the early growth phase, the q mAb at 60 μM Zn was 9.4 pg/cell/day (pcd) in the in-house PFM, which is approximately 1.9 times higher than the control value (Table 1). With 60 μM Zn, the q mAb values were 2.1-fold higher than the control value in the late phase of growth (4–8 days of culture). The q mAb values for in-house CDM were similar to those in the in-house PFM (Table 1). In addition, the cell longevities for in-house PFM and in-house CDM were both more than 1.4-fold higher than the control values (Table 1). Moreover, the μ value was not significantly changed by the presence of 60 μM Zn (Table 1). These results indicate that 60 μM Zn is an effective concentration to improve mAb production without cytotoxic effects because this condition maintained higher q mAb and cell viability than that of 3 μM Zn throughout the cultivation period in both PFM and CDM.
Discussion
Zinc ion is a trace element that is required for over 300 key cellular processes such as DNA and protein synthesis, mitosis, cell proliferation, apoptosis, energy transformation, and intracellular signaling. Zinc ion has been used as a medium component in the range of 0.1 to 3 μM for adherent growth of Chinese hamster cell lines from 1960s (Ham 1963; Ham 1965). Zinc ion (5 μM) has been found as an effective insulin replacement for suspension growth of mammalian cells such as hybridoma, myeloma, and CHO-K1 (Wong et al. 2004). Zinc ion has been shown to improve interferon-β production by adherent rCHO K1 cells grown in medium with serum although the higher than 50 μM supplementation of zinc ion in the serum medium has an deleterious effect for cell viability (Zuqueli et al. 2006). These zinc effects were only analysed on confluent cells by replacing their medium with zinc ion-containing medium without a reflection for the effects on cell growth. Zinc ion has also been reported to increase recombinant human growth hormone (hGH) in the adherent rCHO cell culture (Van DyK et al. 2003). The rCHO cell line, however, is established by using the metallothionein promoter-hGH expression system which is an inducible and promotable gene expression by zinc ion in a dose-dependent fashion. Despite the importance of zinc ions in various Chinese hamster cell lines cultures, no study has yet comprehensively investigated the effects of zinc ions on rCHO DG44 cell growth and mAb production as a quantitative manner in chemically defined suspension culture.
In this study, we investigated the effects of high Zn concentration on cell growth and mAb production by suspension cultures of rCHO DG44 cells grown in the in-house PFM and the in-house CDM. We found that supplementation of cell culture media with 60 μM Zn dramatically improves mAb production in batch suspension cultures of rCHO DG44 cells. This result proceeded from the increased prolonged cell longevity and q mAb in high zinc ion supplementation. In case of cell longevity, the viable cell density maintained higher than the control condition after 6 days of culture in the range of 30–60 μM Zn supplementation. In the calculated values from the data of 0–4 days of culture in Figs. 3 and 4, the q mAb in the in-house PFM was linearly increased from 4.99 to 11.2 pcd with linearity correlations of R 2 = 0.94 ± 0.01 and the q mAb in-house CDM was also increased from 3.07 to 7.18 pcd with linearity correlations of R 2 = 0.98 ± 0.01 as the Zn concentration increased from 3 to 120 μM. Although zinc ion has been widely employed as one of the essential trace elements in CHO cell culture media, our data indicate that high Zn supplementation itself is essential for achieving maximum mAb production.
In the rCHO DG44 cell culture, the in-house media might be preferred than other commercial CDMs because this cell line was fully adapted in the in-house media which were developed by fortifying amino acids, vitamins, and glucose for prevention of depletion of these components during the batch culture of the rCHO DG44 cells. Therefore, the maximum cell density and mAb concentration in the in-house media could be obtained higher than those of the commercial CDMs. The commercial CDMs supplemented with 60 μM Zn showed variable effects for cell growth and mAb production. Although compositions of the commercial CDMs were not known, it might be that the compositions of the commercial media were different with each other. Therefore, the variable effects of Zn could relate to the media components beside Zn which could affect the cell growth and mAb production.
The mechanism by which zinc ion enhances mAb production is still not clear. Future studies will investigate the cellular mechanisms by which zinc ion exerts these effects. Interestingly, zinc ion is known to increase mRNA stability and to exert antiapoptotic and antioxidant effects, all of which may explain the mechanism of zinc ion-mediated mAb production enhancement. For instance, zinc ion has been reported to increase mRNA stability by preventing the binding of destabilising proteins to mRNA (Cao 2004) and also by inhibiting ribonucleases responsible for the degradation of labile mRNA (Hartmann et al. 2001). These effects could contribute to the mechanism by which zinc ion enhances recombinant protein production by cultured mammalian cells. Regarding apoptosis, inhibition of caspase-3 activity through bcl-2 overexpression in rCHO DUKX-B11 cells and deletion of Bak and BaX in rCHO K1 cells has been shown to increase the maximum mAb concentration (Kim and Lee 2000; Cost et al. 2010). This finding is interesting in the context of our results, because zinc ion has also been reported to inhibit caspase-3 activity and to increase the Bcl-2/Bax ratio (Perry et al. 1997; Fukamachi et al. 1998). Regarding redox status, the specific secretion rate of EPO from CHO cells has been shown to be increased by reducing agents (Yoon et al. 1998). Moreover, zinc ion (50 μM) has been shown to act as an antioxidant by inhibiting the generation of reactive oxygen species and increasing the amount of intracellular thiol groups in HeLa-tat cells (Faure et al. 2005).
In conclusion, our results demonstrate that high zinc ion supplementation is important for achieving high mAb production in chemically defined suspension cultures of rCHO DG44 cells. We found that the q mAb in the 0–4 days of culture was gradually increased as the Zn increased from 3 to 120 μM and viable cell density maintained higher than other conditions after reaching the maximum cell density in the range of 30 to 60 μM Zn. Our data indicate that the addition of more than 30 μM Zn to cell culture media is a useful method for improving mAb production in suspension cultures of rCHO DG44 cells.
References
Cao H (2004) Expression, purification, and biochemical characterization of the anti-inflammatory tristetraprolin: a zinc-dependent mRNA binding protein affected by posttranslational modifications. Biochemistry 43:13724–13738
Choi YS, Lee DY, Kim IY, Kim HJ, Park HW, Choe TB, Kim IH (2007) Enhancement of erythropoietin production in recombinant Chinese hamster ovary cells by sodium lactate addition. Biotechnol Bioprocess Eng 12:60–72
Cost GJ, Freyvert Y, Vafiadis A, Santiago Y, Miller JC, Reber E, Collingwood TN, Snowden A, Gregory PD (2010) BAK and BAX deletion using zinc-finger nucleaes yields apoptosis-resistant CHO cells. Biotechnol Bioeng 105:330–340
Faure P, Bouvard S, Roucard C, Favier A, Halimi S (2005) Zinc protects HeLa-tat cells against free radical cytotoxicity induced by glucose. J Trace Elem Med Biol 18:269–276
Fukamachi Y, Karasaki Y, Sugiura T, Itoh H, Abe T, Yamamura K, Higashi K (1998) Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio. Biochem Biophys Res Commun 246:364–369
Ham RG (1963) An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res 29:515–526
Ham RG (1965) Clonal growth of mammalian cells in a chemically defined, synthetic medium. Proc Nat Acad Sci 53:288–293
Hamilton WG, Ham RG (1977) Clonal growth of Chinese hamster cell lines in protein-free media. In Vitro 13:537–547
Hartmann R, Walko G, Justesen J (2001) Inhibition of 2′-5′ oligoadenylate synthetase by divalent metal ions. FEBS Lett 507:54–58
Huang YM, Hu W, Rustandi E, Chang K, Yusuf-Makagiansar H, Ryll T (2010) Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol Prog 26:1400–1410
Kim NS, Lee GM (2000) Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnol Bioeng 71:184–193
Lee GM, Hwang SO (2004) Heterologous expression of genes in Chinese hamster ovary cells. In: Baneyx F (ed) Protein expression technologies: current status and future trends. Horizon Bioscience, Norfolk, pp. 427–460
Lee GM, Varma A, Palsson BO (1991) Production of monoclonal antibody using free-suspended and immobilized hybridoma cells: effect of serum. Biotechnol Bioeng 38:821–830
Liu CH, Chu IM, Hwang SM (2001) Enhanced expression of various exogenous genes in recombinant Chinese hamster ovary cells in presence of dimethyl sulfoxide. Biotechnol Lett 23:1641–1645
Mimura Y, Lund J, Church S, Dong S, Li J, Goodall M, Jefferis R (2001) Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. J Immunol Methods 247:205–216
Morita T, Nagaki T, Fukuda I, Okumura K (1992) Clastogenicity of low pH to various cultured mammalian cells. Mutat Res 268:297–305
Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3: a novel target for zinc in the inhibition of apoptosis. J Bio Chem 272:18530–18533
Urlaub G, Käs E, Carothers AM, Chasin LA (1983) Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell 33:405–412
Van DyK DD, Misztal DR, Wilkins MR, Mackintosh JA, Poljak A, Varnai JC, Teber E, Walsh BJ, Gray PP (2003) Identification of cellular changes associated with increased production of human growth hormone in a recombinant Chinese hamster ovary cell line. Proteomics 3:147–156
Wong VVT, Ho KW, Yab MGS (2004) Evaluation of insulin-mimetic trace metals as insulin replacements in mammalian cell cultures. Cytotechnology 45:107–115
Xie L, Wang DIC (1996) High cell density and high monoclonal antibody production through medium design and rational control in a bioreactor. Biotechnol Bioeng 51:725–729
Yoon SK, Ahn YH (2007) Application of sodium propionate to the suspension culture of Chinese hamster ovary cells for enhanced production of follicle-stimulating hormone. Biotechnol Bioprocess Eng 12:497–501
Yoon SK, Ahn YH, Kwon IC, Han K, Song JY (1998) Influence of reducing agents on the secretion rate of recombinant erythropoietin from CHO cells. Biotechnol Lett 20:101–104
Zuqueli R, Prieto C, Etcheverrigaray M, Kratje R (2006) Effect of sodium butyrate and zinc sulphate supplementation on recombinant human IFN-β production by mammalian cell culture. Lat Am Appl Res 36:321–327
Acknowledgments
The authors thank Green Cross Corp. (Kyonggi, Korea) for providing rCHO cell lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kim, B.G., Park, H.W. High zinc ion supplementation of more than 30 μM can increase monoclonal antibody production in recombinant Chinese hamster ovary DG44 cell culture. Appl Microbiol Biotechnol 100, 2163–2170 (2016). https://doi.org/10.1007/s00253-015-7096-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7096-x