Abstract
Many filamentous fungi produce only conidia for dispersal and survival in vitro or in vivo. Here, we show that the developmental regulator WetA and the velvet protein VosA are not only required for conidial maturation but indispensable for conidiation in Beauveria bassiana, a filamentous entomopathogen. Deletion of wetA or vosA resulted in more than 90 % transcriptional depression of brlA and abaA, two activator genes in the central developmental pathway, during the critical period of conidiophore development and conidiation. Consequently, ΔwetA and ΔvosA strains lost 98 % in and 88 % of their conidiation capacities under optimal culture conditions, respectively. The conidia of ΔwetA showed more defective features than those of ΔvosA, including smaller size, lesser density, lower hydrophobicity, and impaired cell walls although intracellular trehalose content decreased more in the aging culture of ΔvosA than of ΔwetA. As a result, conidial sensitivity to cell wall perturbation was elevated in ΔwetA but unaffected in ΔvosA, which produced conidia more sensitive to the oxidant menadione and the wet-heat stress at 45 °C. Both deletion mutants showed similar defects in conidial tolerance to high osmolarity or UV-B irradiation but no change in conidial sensitivity to the other oxidant H2O2 or the fungicide carbendazim. Moreover, ΔwetA lost more virulence to Galleria mellonella larvae than ΔvosA. All these phenotypical changes were restored by either wetA or vosA complementation. Taken together, WetA and VosA are indispensable for asexual development and contribute differentially to conidial quality and hence the biological control potential of B. bassiana against insect pests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The entomopathogenic fungus Beauveria bassiana produces aerial conidia to infect the broadest spectrum of insects as far as known to date (Vega et al. 2012; Wang and Feng 2014). Such conidia can be massively produced as active ingredients of fungal insecticides on inexpensive solid substrates, such as small grains (Ye et al. 2006). Thus, both conidiation capacity and conidial quality are crucial for the biological control potential of the filamentous fungal pathogen against arthropod pests.
Fungal conidiation is an event that is precisely timed and genetically programmed by a central developmental pathway in response to internal and external cues. In Aspergillus, the central pathway comprises the developmental regulators BrlA, AbaA, and WetA that activate the expression of downstream conidiation-specific genes in a hierarchical manner during conidiophore development, conidiation, and conidial maturation (Mirabito et al. 1989; Yu et al. 2006). Of those, WetA functions in the late phase of condiophore development to activate the expression of proteins/enzymes involved in the synthesis of spore wall components (Sewall et al. 1990a; Marshall and Timberlake 1991; Son et al. 2014) and hence is essential for conidiation and conidial maturation (Park and Yu 2012). This hints to a close link of WetA to the conidial quality that largely determines the biological control potential of a filamentous fungal insect pathogen. The maturation process also requires the activation of the velvet protein VosA to depress the brlA expression for termination of its control cycle and to facilitate trehalose biogenesis and accumulation in newly formed conidia (Ni and Yu 2007). The family of velvet proteins share a velvet domain that is highly conserved in filamentous fungi (Ni and Yu 2007; Bayram and Braus 2012). The velvet domain has been shown to be a novel DNA-binding motif that specifically recognizes an 11-nucleotide consensus sequence consisting of two motifs in the promoters of key developmental regulatory genes (Ahmed et al. 2013). Therefore, VosA is not only required for conidial maturation but likely involved in a transcriptional relationship with the regulators in the central pathway due to the DNA-binding motif existing in its velvet domain.
The roles of these developmental regulators have been intensively studied in Aspergillus (Adams et al. 1988; Sewall et al. 1990a, b; Marshall and Timberlake 1991; Ogawa et al. 2010; Tao and Yu 2011) and Penicillium (Borneman et al. 2000; Andrianopoulos 2002; Sigl et al. 2011; Wang et al. 2015) and summarized in several reviews (Adams et al. 1998; Etxebeste et al. 2010; Park and Yu 2012; Alkhayyat et al. 2015). However, none of these regulators has been functionally explored in filamentous entomopathogens, such as B. bassiana and Metarhizium anisopliae sensu lato, which are widely applied for the biological control of global arthropod pests (Wang and Feng 2014). During asexual development, B. bassiana lacking sexual stage develops very short, zigzag, or denticulate rachises (conidiophores) from hyphal cells and produces several subspherical conidia of 1–2 μm on each denticula, forming spore balls with all conidia surrounding the rachises. This asexual developmental pattern is largely different from that seen in Aspergillus species, which develop dozens of phialides (conidiophores) from a single foot cell and form a chain of conidia on each phialide (Park and Yu 2012; Alkhayyat et al. 2015). For B. bassiana, cultivation of conidia on a standard medium requires a period of less than 24 h for full germination. After 1 or 2 days of hyphal growth, conidiophore development and low level, but measurable, conidiation usually occur on day 3, followed by increasing conidiation until a peak of conidial yield is achieved approximately on day 7. The sequenced genome of B. bassiana (Xiao et al. 2012) harbors the orthologs of all the developmental activators well characterized in Aspergillus. This study seeks to elucidate how WetA and VosA regulate conidiation compacity and conidial quality in B. bassiana and hence contribute to the fungal potential against arthropod pests. Our phenotypic and transcriptional analyses of single-gene deletion mutants show that both WetA and VosA are indispensable for conidiation as well as conidial maturation in B. bassiana due to their interactions with the central activators BrlA and AbaA at transcriptional level.
Material and methods
Microbial strains and culturing conditions
The wild-type strain B. bassiana ARSEF 2860 (BbWT herein) and its mutants were grown in rich SDAY [Sabouraud dextrose agar (4 % glucose, 1 % peptone, and 1.5 % agar) plus 1 % yeast extract] at 25 °C and 12:12 h (light/dark cycle) for standard cultures. Their stress responses were assayed in 1/4 SDAY (amended with 1/4 of each SDAY nutrient) or minimal CZA (Czapek agar: 3 % sucrose, 0.3 % NaNO3, 0.1 % K2HPO4, 0.05 % KCl, 0.05 % MgSO4, and 0.001 % FeSO4). Escherichia coli DH5α and E. coli Top10 from Invitrogen (Shanghai, China) were cultured in Luria-Bertani medium plus kanamycin (100 μg/ml) or ampicillin (100 μg/ml) at 37 °C for plasmid propagation. Agrobacterium tumefaciens AGL-1 used as a T-DNA donor for fungal transformation was cultivated at 28 °C in YEB medium (Fang et al. 2004).
Cloning and analysis of wetA and vosA in B. bassiana
The B. bassiana genome under the NCBI Accession NZ_ADAH00000000 (Xiao et al. 2012) was searched via online BLASTP analysis (http://blast.ncbi.nlm.nih.gov/blast.cgi) using the queries of wetA and vosA homologs in Aspergillus nidulans and Aspergillus fumigatus. The coding sequences of the identified wetA and vosA (tag loci: BBA_06126 and BBA_01023) were amplified from BbWT via PCR with paired primers (Table S1 in the Supplementary Material) and verified by sequencing at Invitrogen. The sequences of the deduced proteins were compared with the counterparts of other filamentous fungi in the NCBI database via online analyses of BLASTP, SMART (http://smart.embl-heidelberg.de/), and PANTHER (http://www.pantherdb.org/), followed by phylogenetic analysis with MEGA5 software (Tamura et al. 2011).
Generation and identification of wetA and vosA mutants
Our backbone plasmids p0380-bar and p0380-sur-gateway (Xie et al. 2012; Wang et al. 2012) were used for targeted gene manipulation via Agrobacterium-mediated transformation (Fang et al. 2004). Either wetA or vosA was deleted from BbWT via homogenous replacement of its partial coding (5′ and 3′ fragments) sequence with a bar-inclusive cassette and rescued by ectopic integration into ΔwetA or ΔvosA of a cassette consisting of full-length coding sequence (with flank regions) and a sur marker. The 5′ and 3′ fragments and the full-length sequences of wetA or vosA were cloned from BbWT with paired primers, digested with appropriate restriction enzyme sites, and inserted into the backbone plasmids with paired primers (Table S1 in the Supplementary Material). Putative mutants were screened in terms of the bar resistance to phosphinothricin (200 μg/ml) or the sur resistance to chlorimuron ethyl ammonium (10 μg/ml) and then identified via PCR and Southern blot analyses with paired primers and amplified probes (Table S1 in the Supplementary Material). Positive deletion mutants were analyzed in conjunction with parental BbWT and rescued mutants (control strains) in triplicate experiments as follows.
Assessing growth rates on different media
Hyphal mass plugs (5 mm diameter) were bored from 3-day-old cultures grown at 25 °C on cellophane-overlaid SDAY (CO-SDAY) plates, which were spread with 100 μl aliquots of 106 conidia/ml suspension to initiate uniform cultures, and attached centrally to the plates of SDAY, CZA, and 18 CZA-derived media with altered carbon/nitrogen sources and availability. The CZA-derived media were prepared by removing 3 % sucrose, 0.3 % NaNO3 or both from CZA, replacing the sole carbon with 3 % of glycerol, ethanol, acetate (NaAc), glucose, trehalose, maltose, fructose, lactose, mannitol, sorbitol, oleic acid, or olive oil, and replacing the sole nitrogen with 0.3 % of NH4Cl, NH4NO3, or NaNO2, respectively. After 7 days of incubation at 25 °C and 12:12 h, each colony diameter was cross-measured as an index of radial growth on a given medium.
Assessing conidiation capacity and conidial viability
Aliquots of 100 μl conidial suspension were evenly spread on CO-SDAY plates and incubated for 7 days at 25 °C and 12:12 h. From day 3 onwards, three plugs (5 mm diameter) were bored daily from each plate culture using a cork borer. Conidia on each plug were released into 1 ml of 0.02 % Tween 80 by ~5 min vibration. The conidial concentration in the suspension was determined with a hemocytometer and converted to the number of conidia per cm2 plate culture. During cultivation, fungal mass samples were stained with calcofluor white (a dye specific to cell wall) and examined under a confocal microscope to reveal possible changes in the abundance of hyphae, conidiophores, and conidia of each deletion mutant versus BbWT.
To assess the viability of the conidia from the 7-day-old cultures, 100 μl aliquots of conidial suspension were evenly spread onto the plates of a germination medium (GM; 2 % sucrose, 0.5 % peptone, and 1.5 % agar), followed by 24 h incubation at 25 °C. Germination percentage was determined at 2 h intervals using three microscopic counts per plate. Time (GT50) required for 50 % germination was estimated by fitting the germination trends to the time of incubation. In addition, viability changes of the conidia in the 10- to 50-day-old cultures were also assessed based on germination percentage within 24 h at the same regime.
Examination and assessment of conidial quality
Several methods were used to examine newly formed and mature conidia, collected from the 7- and 15-day-old cultures, respectively, for their quality difference. First, ultrastructural changes on the surfaces of conidia were observed via scanning electron microscopy (SEM). Second, size and density of conidia were determined with the respective readings of forward scatter (FSc) and side scatter (SSc) detectors from the flow cytometry of 2 × 104 conidia per sample (three samples per strain) as described previously (Puttikamonkul et al. 2010; Zhang et al. 2013). Third, carbohydrate epitopes on the surfaces of conidia were determined in different lectin-binding assays (Wanchoo et al. 2009). Briefly, washed conidia were suspended in the binding buffers of the Alexa fluor 488-labeled lectins ConA [i.e., concanavalin A specific to α-N-acetylglucosamine (α-GlcNAc) and α-glucose], WGA (wheat germ agglutinin specific to β-GlcNAc and sialic acids) and GNL (Galanthus nivalis lectin specific to mannose residues) from Molecular Probes-Invitrogen (Eugene, OR, USA) and Vector Laboratories (Burlingame, CA, USA) following the user’s guide. After 1 h labeling in darkness, the unbound lectin was removed by washing in the buffer. The fluorescence intensity for the amount of each lectin bound to conidial surfaces was read through the flow cytometry of 2 × 104 labeled conidia with an argon laser at excitation/emission wavelengths of 488/530 nm. Finally, an index of conidial hydrophobicity, which is important for conidial adhesion to host integument, was assessed using a diphase method as described previously (Holder et al. 2007; Wang et al. 2014).
Assaying cellular responses to chemical and environmental stresses
Conidial responses to different types of chemical stressors were assayed by spreading 100 μl aliquots of conidial suspension onto GM plates alone (control) or supplemented with a sensitive concentration of menadione (0.2 mM), H2O2 (2 mM), SDS (0.2 mg/ml), Congo red (0.8 mg/ml), NaCl (1.2 M), or carbendazim fungicide (1 μg/ml). After 24 h incubation at 25 °C, germination percentage was determined with three microscopic counts per plate.
Conidial thermotolerance and UV-B resistance of each strain were quantified by exposing conidial samples to a hot water bath at 45 °C for 0–120 min and to the irradiation of the weighted UV-B wavelength of 312 nm at the gradient doses of 0–0.6 J/cm2 as described previously (Wang et al. 2012; Xie et al. 2012). Median lethal time (LT50) and median lethal dose (LD50) were estimated as indices of the two parameters by fitting conidial survival (relative germination) trend to the intensities of each stress.
Assessing intracellular trehalose content
Aliquots of 1 g fungal mass (mixtures of mycelia and conidia) from 3-, 5-, 7-, 10- and 15-day-old SDAY cultures were ground in liquid nitrogen and resuspended in 1 ml dd-H2O. Each suspension was boiled in a water bath for 6 h, followed by 30-min centrifugation at 16,000×g. The content of trehalose in the supernatant was determined in an HPLC system as described previously (Liu et al. 2009; Wang et al. 2012) and expressed as mg/g dry mass.
Bioassays for fungal virulence
Conidia from the 7-day-old SDAY cultures of all the strains were bioassayed for virulence to Galleria mellonella larvae (~300 mg each) from a vendor (Da Mai Chong Insectaries, Wuxi, Jiangsu, China). For each strain, briefly, three cohorts of ~35 larvae were inoculated by immersing them for ~10 s in 30-ml aliquots of 1 × 107 conidia/ml suspension for normal cuticular infection or injecting 5 μl of 1 × 105 conidia/ml suspension into the hemocoel of each larva (i.e., 500 conidia per larva) for cuticle-bypassing infection. The same volume of 0.02 % Tween 80 was used as a control for the immersion or injection treatment. All treated cohorts were maintained in large Petri dishes (15 cm diameter) for 8 days at 25 °C and 12:12 h and monitored every 12 h for mortality records. LT50 (no. days) was generated as an index of the fungal kill action by probit analysis of each time-mortality trend.
Transcriptional profiling of conidiation-required genes
The CO-SDAY cultures of all deletion mutants and BbWT were initiated by spreading 100 μl of 107 conidia/ml suspension per plate, followed by 7 days of incubation at 25 °C and 12:12 h. In addition, ΔbrlA and ΔabaA mutants, created with the same protocol as described previously, were grown for 7 days in CO-SDAY by spreading 100 μl of hyphal suspension per plate because none of them produced any conidium. Total RNAs were extracted daily from the cultures with an RNAiso™ Plus Reagent kit (TakaRa, Dalian, China) and reversely transcribed into complementary DNAs (cDNAs) with a PrimeScript® RT reagent kit (TaKaRa). Each cDNA (10-fold dilution) was used as a template to determine transcript levels of brlA, abaA, wetA, and vosA via quantitative real-time PCR (qRT-PCR) with paired primers (Table S1 in the Supplementary Material) using the fungal 18S rRNA as an internal standard. All the qRT-PCR experiments of four cDNA samples per strain were performed under the action of SYBR® Premix Ex Taq™ (TaKaRa). Relative transcript level (RTL) of each gene was computed as the ratio of its transcript in each deletion mutant over that in BbWT on a given day using the 2−ΔΔCt method (Livak and Schmittgen 2001).
The same protocol was used to determine temporal transcription patterns of the four genes in BbWT during 7 days of cultivation at the same regime. The RTL of each gene was estimated as the ratio of its transcript on a given day over that on day 1.
Statistical analysis
All phenotypic observations, measurements, and fitted estimates from the experiments of three replicates were differentiated by one-factor (strain) analysis of variance, followed by Tukey’s honestly significant difference (HSD) test of the means from each experiment.
Results
Features of WetA and VosA in B. bassiana
An online search through the B. bassiana genome with the queries of homologous sequences of A. nidulans and A. fumigatus in the NCBI database resulted in identified wetA (1920 bp) and vosA (926 bp). The identified genes amplified from BbWT encode 639 and 273 amino acids (molecular sizes: 66.96 and 31.17 kDa), respectively. The deduced WetA and VosA are featured with a Esc1/WetA-related protein domain at the C-terminal end (Mi et al. 2010; Son et al. 2014) and a conserved Velvet domain at the C-terminal side (Bayram and Braus 2012) (Fig. S1A in the Supplementary Material) and share 30–74 and 31–91 % sequence identities (Fig. S1B, C) with the counterparts of other 13 fungi in the NCBI database, respectively.
During 7 days of standard cultivation, wetA was transcribed more abundantly in BbWT on days 3–7, a period for the initiation of conidiophore development through full conidiation, than the first 2 days for conidial germination and hyphal growth (Fig. 1a). The vosA transcript was largely increased only on days 5–7, a period for the conidiation after conidiophore development. Interestingly, transcriptional expressions of brlA and abaA began from days 3 and 4, respectively, and maintained at high levels for the following days. Notably, brlA and abaA showed similar maxima of transcript levels 10- to 25-fold higher than those of wetA on days 4 and 5, a critical period for the formation of most conidiophores and a rapid increase of conidiation in B. bassiana. These transcription profiles implicated sequential activation of brlA and abaA during the asexual development of B. bassiana, as documented in Aspergillus (Alkhayyat et al. 2015), but a somewhat difference in wetA, which was activated as early as brlA in B. bassiana rather than after the abaA activation in Aspergillus.
Deleted wetA or vosA affects profoundly the central developmental pathway of B. bassiana. a Relative transcript levels (RTL) of wetA and vosA in wild-type culture grown for 2–7 days in SDAY at 25 °C. The first-day transcript of each gene at the end of conidial germination was used a standard. b–e RTLs of wetA, vosA, brlA, and abaA in ΔwetA and ΔvosA mutants versus wild-type (BbWT) over the days of cultivation in SDAY, respectively. f RTLs of wetA and vosA in ΔbrlA and ΔabaA mutants versus BbWT during cultivation in SDAY. Error bars: standard deviations (SD) from four cDNA samples
Either wetA or vosA deletion alters transcriptional profiles of other developmental activators
The expected recombination events in ΔwetA, ΔvosA, and complementary mutants were confirmed by PCR and Southern blot analyses (Fig. S2 in the Supplementary Material). Consequently, either wetA or vosA was undetectable at transcription level during the 7-day cultivation. Compared with that in BbWT, the vosA transcript in ΔwetA decreased by ~50 % on days 1, 4, and 6 and 88 % on day 5 but increased by 4-fold on day 7 and slightly changed on days 2 and 3 (Fig. 1b). In ΔvosA, the wetA transcript was repressed by 55, 59, 64, and 37 % on days 1 and 3–5, respectively, despite a minor change on other days (Fig. 1c). These suggested a strong interaction between wetA and vosA at transcriptional level. Intriguingly, the brlA transcript in ΔwetA was drastically depressed by 79 % on day 1, 62 % on day 2, and 91–99 % on days 3–6 but upregulated by 12-fold on day 7 (Fig. 1d). The abaA transcript in ΔwetA was also depressed by 37 % on day 1 and 98–99 % on days 4–6 but upregulated by 53-fold on day 7, accompanied with a minor upregulation on days 2 and 3 (Fig. 1e). The vosA deletion resulted in more drastic depression of brlA on days 3–5 (92–99 %) than on days 1 (64 %) and 2 (41 %), and of abaA on days 4 (98 %) and 5 (99 %) than on days 1 (35 %) and 3 (58 %), followed by their sharp upregulation by 33- and 44-fold on day 7. In ΔbrlA, more interestingly, the wetA transcript was reduced by 65 % on day 2, 56 % on day 3, and 84–94 % on days 4–7, and the vosA transcript was reduced by 60 % on day 1 and 85–90 % on days 4–6 (Fig. 1f). The deletion of abaA from BbWT also led to a remarkable down-regulation of wetA by 50–63 % on days 1, 4, and 5 and of vosA by 51–90 % on days 1, 2, 4, and 5 despite minor effects on other days. The temporal transcription patterns of brlA and abaA in ΔwetA or ΔvosA and of wetA and vosA in ΔbrlA or ΔabaA indicated a tight link of each to three others in the central developmental pathway of B. bassiana.
Contributions of WetA and VosA to growth, asexual cycle, and virulence
Compared with BbWT, all the deletion mutants showed different degrees of growth defects in rich SDAY or 1/4 SDAY and minimal CZA or 18 CZA-derived media, which were initiated with small hyphal plugs to initiate fungal colonies. After 7 days of standard incubation, colony sizes were significantly smaller in ΔvosA than the control strains grown on all the rich and minimal media (Tukey’s HSD, P < 0.05), as summarized in Table S2 in the Supplementary Material. The ΔwetA mutant exhibited milder growth defects in 1/4 SDAY and most minimal media.
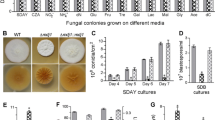
Apart from smaller sizes, the colonies of ΔwetA and ΔvosA were more cottony and thicker than those of the control strains (Fig. 2a) on SDAY, a standard medium for cultivation of fungal entomopathogens (Feng et al. 1994; Roberts and St Leger 2004). Additionally, deeper folds appeared in the colonies of ΔvosA than of ΔwetA. In microscopic examination of fungal masses taken from 5-day-old SDAY cultures, BbWT showed many spore balls on clustered zigzag rachises and hence far more abundant conidia than ΔwetA and ΔvosA (Fig. 2b). However, neither rachis nor conidium was visible in the cultures of ΔbrlA and of ΔabaA at the same time (Fig. S3 in the Supplementary Material). Thus, the latter two mutants were excluded in the following phenotypic experiments.
Deleted wetA or vosA affects vegetative growth and conidiation of B. bassiana. a Top (row 1), bottom (row 2), and side (row 3) views of 10-day-old SDAY colonies initiated with hyphal mass plugs. b Microscopic views of fungal mass samples taken from 5-day-old SDAY cultures and stained with calcofluor white. Arrows indicate only a very few conidia visible in ΔwetA and ΔvosA, contrasting to abundant conidia in BbWT. Scale bars: 5 μm
Quantification of conidiation over 7 days of standard culivation demonstrated a drastic reduction of conidial yield in each deletion mutant (Fig. 3a). Compared with BbWT, ΔwetA and ΔvosA suffered conidial yield losses of 88 and 56 % on day 3, 98–99 % and 95–98 % on days 4–6, and 98 and 88 % on day 7, respectively. Conidia from the 7-day-old culture of BbWT germinated significantly faster than those from the cultures of ΔwetA and ΔvosA (Tukey’s HSD, P < 0.01), whose GT50s were prolonged by 4.4 and 1.2 h under optimal culture conditions, respectively (Fig. 3b).
Deleted wetA or vosA affects asexual development, conidial germination, and virulence of B. bassiana. a Conidial yields quantified from SDAY cultures initiated with 100 μl of conidial suspension per plate. b GT50 (h) for the time required to achieve 50 % germination of conidia. c LT50 (no. days) for conidial virulence to G. mellonella larvae inoculated via topical application (immersed) and hemocoel injection. Asterisked bars in each group differ significantly from those unmarked (Tukey’s HSD, P < 0.05). Error bars: SD from three replicates
In the bioassay of G. mellonella larvae, hemocoel injection and topical application of conidial suspension resulted in the LT50s of ΔwetA 3 and 0.8 days longer than those of BbWT, respectively (Fig. 3c). The ΔvosA mutant also suffered a significant, but minor, LT50 delay irrespective of topical application (Tukey’s HSD, P = 0.0117) or injection (Tukey’s HSD, P = 0.0035).
All the changes were restored by either wetA or vosA complementation. Apparently, ΔwetA suffered more severe defects in conidiation than ΔvosA under normal culture conditions. In addition, the conidia produced by ΔwetA not only germinated slower but were less virulent than those produced by ΔvosA.
Different effects of WetA and VosA on conidial maturation and quality
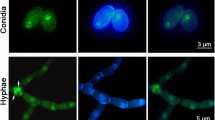
Since full conidiation in BbWT was achieved at the end of 7-day cultivation in SDAY, newly formed and mature conidia from the 7- and 15-day-old cultures were examined to reveal the effects of wetA and vosA on conidial maturation and quality, which are indicated by morphology, viability, and surface features. As demonstrated by SEM, newly formed conidia produced by BbWT showed an ultrastructural coat of well-defined fascicles or bundles (Fig. 4a, upper row), presumably composed of hydrophobin rodlets (Holder et al. 2007). Such bundles became larger and less compact in ΔvosA while most of them were poorly defined in ΔwetA. The bundles of the mature conidia humped more in BbWT than in ΔvosA (Fig. 4a, lower row). In contrast, the mature conidia of ΔwetA lacked conspicuously humping bundles and were readily deformed in the process of pre-treatment for SEM.
Deleted wetA or vosA affects conidial features indicative of maturation and quality in B. bassiana. a SEM images of newly formed and mature conidia from the 7- and 15-day-old SDAY cultures. Note a difference between BbWT and ΔwetA or ΔvosA in the bundles of conidial hydrophobin rodlets (scale bars: 200 nm). b, c Size and density of newly formed and mature conidia indicated by the respective FSc and SSc readings from the flow cytometry (FCM) of 2 × 104 conidia per sample. d, e Relative fluorescence intensities (FCM readings) in 2 × 104 newly formed and mature conidia (per sample) labeled with the fluorescent lectins ConA, WGA, and GNL, respectively. f Hydrophobicity indices of newly formed and mature conidia. Asterisked bars in each group differ significantly from those unmarked (Tukey’s HSD, P < 0.05). Error bars: SD from three independent samples
Moreover, size and density of newly formed conidia were reduced by 58 and 40 % in ΔwetA compared with BbWT but not significantly affected in ΔvosA, as indicated by the respective FSc and SSc readings from the flow cytometry of large conidial samples (Fig. 4b). The FSc and SSc readings from the mature conidia (Fig. 4c) were 48 and 4 % smaller in ΔwetA than in BbWT, respectively. In ΔvosA, mature conidia suffered a significant, but minor, decrease (9.3 %) in density and little change in size.
In fluorescent lectin-binding assays through flow cytometry, ΔwetA and ΔvosA displayed significant alterations of carbohydrate epitopes (Tukey’s HSD, P < 0.05) on the surfaces of newly formed and mature conidia labeled with the Alexa fluor 488-labeled lectins ConA, WGA, and GNL. The amount of ConA bound to the surfaces of newly formed conidia was reduced by 57 % in ΔwetA and 18 % in ΔvosA compared with BbWT (Fig. 4d); the relative reduction of ConA bound to the surfaces of mature conidia changed to 54 % in ΔwetA and 24 % in ΔvosA (Fig. 4e). In contrast, both WGA and GNL were much more bound to conidial surfaces of both ΔwetA and ΔvosA. The amounts of bound WGA and GNL increased from 92 % and 1.4-fold in newly formed conidia to 7.7- and 8-fold in the mature conidia of ΔwetA, respectively. The same estimates increased from 7 and 78 to 98 % and 1.5-fold in ΔvosA. Additionally, newly formed and mature conidia produced by ΔwetA were significantly less (6.4 and 7.4 %) hydrophobic than the BbWT counterparts (Fig. 4f) whereas ΔvosA showed an insignificant change in conidial hydrophobicity (Tukey’s HSD, P > 0.05). All these changes were restored in the complementary rescued mutants.
Contributions of WetA and VosA to intracellular trehalose accumulation, conidial viability, and multi-stress responses
The level of intracellular trehalose accumulation important for fungal stress response (Elbein et al. 2003) fluctuated greatly in each strain during 15 days of standard cultivation in SDAY. Compared with that in BbWT, trehalose content in ΔwetA increased by 2.5-fold on day 3 and 32 % on day 5 but decreased to 53, 56, and 67 % of those in BbWT on days 7, 10, and 15 (Fig. 5a), respectively. In ΔvosA, trehalose content increased by 5-fold on day 3, 93 % on day 5, and 61 % on day 10 but rapidly decreased to only 61 and 31 % of those in BbWT on days 10 and 15, respectively.
Effects of deleted wetA or vosA on intracellular trehalose accumulation, conidial viability, and multi-stress tolerance in B. bassiana. a Trehalose contents in SDAY cultures grown for 3–15 days under normal conditions. b Germination percentages of the conidia collected from 20-, 30-, 40-, and 50-day-old SDAY cultures after 24 h of normatin incubation. c Relative germination (%) of the conidia collected from 7-day-old cultures and incubated for 24 h at 25 °C on a germination medium supplemented with menadione (0.2 mM), H2O2 (2 mM), Congo red (0.8 mg/ml), SDS (0.2 mg/ml), NaCl (1.2 M), and carbendazim (1 μg/ml), respectively. An unstressed control was used as a standard for the estimates of each strain. d LT50 (min) and LD50 (J/cm2) estimated as indices of conidial tolerance to 45 °C wet-heat stress and UV-B irradiation. Asterisked bars in each group differ significantly from those unmarked (Tukey’s HSD, P < 0.05). Error bars: SD from three replicates
We examined more phenotypes indicative of conidial quality from different strains. Germination percentages of the conidia from less than 20-day-old SDAY cultures did not differ significantly between each deletion mutant and the control strains at the end of 24 h incubation (data not shown). However, ΔwetA and ΔvosA lost conidial viability much more rapidly with the aging of cultures than the control strains (Fig. 5b). Compared with those in BbWT, germination percentages of the conidia from the 30-, 40-, and 50-day-old cultures were decreased by 75, 85, and 82 % in ΔvosA, and by 10, 49, and 61 % in ΔwetA, respectively.
In stress assays, the conidia of ΔwetA and ΔvosA were equally more sensitive to a high osmolarity of NaCl (1.2 M) than those of BbWT (Fig. 5c). Conidial sensitivity to the oxidant menadione increased only in ΔvosA. Only did the ΔwetA conidia become more sensitive to cell wall perturbation by Congo red or SDS. However, neither ΔwetA nor ΔvosA showed a significant change in sensitivity to H2O2 or carbendazim (Tukey’s HSD, P > 0.05).
Conidial thermotolerance and UV-B resistance were quantified as LT50s after exposure to wet-heat stress at 45 °C and LD50s after exposure to UV-B irradiation (Fig. 5d). Compared with BbWT, ΔwetA and ΔvosA lost 25 and 19 % of thermotolerance, and 13 and 10 % of UV-B resistance, respectively.
Discussion
As presented above, WetA and VosA are not only indispensable for conidiation of B. bassiana but responsible for maturation and quality of aerial conidia. The indispensability is largely dependent upon transcriptional interactions of either WetA or VosA with the crucial regulators BrlA and AbaA in the central development pathway, as discussed below.
First of all, WetA and VosA are required for conidiation due to their contributing to 98 and 88 % of conidiation capacity in B. bassiana. The drastic severity of conidiation defects attributed to either wetA or vosA deletion in B. bassiana has not been reported from other filamentous fungi. We also tried to create a double deletion mutant of wetA and vosA but failed in repeated attempts perhaps due to a lethality of the double deletion, which has not been disclosed in reported studies. Previously, deletion of wetA resulted in only 35 % reduction of conidial yield in Fusarium graminearum (Son et al. 2014) but unaffected conidiation accompanied with immature or colorless conidia in Aspergillus (Sewall et al. 1990a; Tao and Yu 2011) and Penicillium (Wang et al. 2015). However, a previous study on the ΔvosA mutant of A. nidulans (Ni and Yu 2007) focused on conidial maturation instead on conidiation level, not showing an effect of the vosA deletion on the fungal conidiation. The severe conidiation defects observed in this study are apparently attributable to the central pathway blocked by either wetA or vosA deletion, which led to almost all transcriptional depression of brlA on days 3–5 or abaA on days 4 and 5. This is because BrlA and AbaA are highly conserved activators of asexual development in filamentous fungi (Etxebeste et al. 2010; Park and Yu 2012). The absence of wetA in A. fumigatus has been shown to increase brlA and abaA mRNA accumulation, suggesting a negative feedback control of conidiation involving wetA (Tao and Yu 2011). In another study, the deletion of vosA in A. nidulans caused uncontrolled activation of asexual development, whereas the enhanced expression of vosA led to blocked sporulation, suggesting that VosA may also function in a negative feedback regulation of sporogenesis (Ni and Yu 2007). In our study, the wetA or vosA deletion did not elevate transcript levels of brlA and abaA during the standard cultivation until full conidiation was achieved on the last day. Instead, the two gene transcripts were largely reduced in our ΔwetA and ΔvosA mutants during the critical period of conidiophore development and conidiation. In B. bassiana, therefore, both wetA and vosA could interact with brlA and abaA at transcriptional level in a way very different from the negative feedback control revealed in Aspergillus. This inference is also evident with the inhibitory effect of either brlA or abaA deletion on the wetA and vosA transcripts.
Apart from the most severe defects in conidiation, our ΔwetA and ΔvosA mutants exhibited different degrees of phenotypic defects tightly associated with conidial maturity and quality. These defects include smaller size, lesser density, slower germination, more impaired cell walls, and lower hydrophobicity in the conidia of ΔwetA than of ΔvosA. These results confirm that WetA plays more important role than VosA in the conidial maturation of B. bassiana perhaps by activating sets of possible proteins or enzymes involved in the synthesis and assembly of conidial wall components, as revealed in other fungi (Sewall et al. 1990a; Marshall and Timberlake 1991; Tao and Yu 2011; Son et al. 2014). A more significant role of VosA in the conidial maturation of A. nidulans has been shown to contribute to trehalose accumulation in conidia (Ni and Yu 2007). In this study, the level of the multi-functional trehalose (Elbein et al. 2003) largely increased in the ΔwetA and ΔvosA cultures grown for 3, 5, and 7 days except for a significant reduction in ΔwetA on day 7, when full conidiation was achieved in the BbWT culture with a very low level of hyphal debris. From then on, intracellular trehalose accumulation was decreased more rapidly in ΔvosA than in ΔwetA. We also tried to quantify conidial trehalose contents but failed in several attempts because the conidiation of either ΔwetA or ΔvosA was too poor to produce a sufficient amount of conidia for the purpose. Thus, our data simply indicate greater contribution of VosA than WetA to intracellular trehalose content in the aging culture of B. bassiana. More rapid loss of conidial viability during the culture aging hints to lower maturity of conidia in the aging culture of ΔvosA than of ΔwetA.
Moreover, the wetA or vosA deletion exerted profound effect on the biological control potential of B. bassiana. The two deletion mutants produced the conidia impaired in quality aside from a drastic loss of their conidiation capacity discussed above. The impaired quality was featured not only with cell wall damage, slower germination, and rapid viability loss but with attenuated virulence and reduced stress tolerance. These changes implicate that the mutant conidia are less capable of infecting insect pests and tolerating environmental stresses, which are often encountered in the field where a fungal insecticide containing the active ingredients of normal conidia is applied (Wang and Feng 2014). Some of the changes indicative of the impaired quality have also been shown in other fungal ΔwetA mutants with their conidia suffering reduced viability and survival rates (Tao and Yu 2011; Son et al. 2014). The increased sensitivity of our ΔwetA conidia to cell wall perturbation and wet-heat stress coincides well with their cell walls more impaired than those of the ΔvosA conidia, which were not sensitive to the cell wall stress. Interestingly, both deletion mutants showed a similar reduction in conidial osmotolerance or UV-B resistance and an insignificant change in conidial sensitivity to H2O2 or carbendazim. These phenotypic changes are largely, if not all, consistent with increased conidial sensitivity to thermal and/or osmotic stress in A. fumigatus ΔwetA (Tao and Yu 2011) and A. nidulans ΔvosA (Ni and Yu 2007). However, either the ΔwetA or ΔvosA mutant was highly sensitive to the oxidant H2O2 in Aspergillus but not in B. bassiana, in which only ΔvosA showed increased conidial sensitivity to the other oxidant menadione. In Penicillium digitatum, the ΔwetA conidia were extremely sensitive to menadione but showed increased tolerance to H2O2 (Wang et al. 2015). Thus, antioxidant responses attributed to WetA may vary with fungal species. We consider that the differential change of each stress tolerance in the B. bassiana ΔwetA and ΔvosA was attributable to not only impaired cell walls but likely reduced trehalose content, which has been shown in the previous studies (Ni and Yu 2007; Tao and Yu 2011) but failed to be quantified in this study due to their too poor conidiation. In addition, ΔwetA was much less virulent to the susceptible insect than ΔvosA irrespective of cuticular or cuticle-bypassing infection while ΔvosA was more sensitive to nutritional stress and less capable of utilizing environmental carbon and nitrogen sources for growth. All the defects in conidial virulence and responses to nutritional and abiotic stresses indicate that either WetA or VosA plays an important role in the fungal adaptation to host insect and environment.
Taken together, WetA and VosA are crucial regulators of both conidiation capacity and conidial quality in B. bassiana despite their differential roles in conidial maturation. WetA is more involved in the synthesis and assembly of conidial wall components to ensure cell wall integrity while VosA is more responsible for the synthesis and accumulation of intracellular trehalose. Therefore, both of them are required for conidiation capacity and conidial quality, thereby contributing to the biological control potential of B. bassiana. This also implicates that both WetA and VosA could likely be exploited to improve the fungal potential against arthropod pests via overexpression, warranting a further study.
References
Adams TH, Boylan MT, Timberlake WE (1988) brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353–362
Adams TH, Wieser JK, Yu JH (1998) Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev 62:35–54
Ahmed YL, Gerke J, Park HS, Bayram Ö, Neumann P, Ni M, Dickmanns A, Kim SC, Yu JH, Braus GH (2013) The velvet family of fungal regulators contains a DNA-binding domain structurally similar to NF-kappa B. PLoS Biol 11:e1001750
Alkhayyat F, Kim SC, Yu JH (2015) Genetic control of asexual development in Aspergillus fumigatus. Adv Appl Microbiol 90:93–107
Andrianopoulos A (2002) Control of morphogenesis in the human fungal pathogen Penicillium marneffei. Int J Med Microbiol 292:331–347
Bayram O, Braus GH (2012) Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev 36:1–24
Borneman AR, Hynes MJ, Andrianopoulos A (2000) The abaA homologue of Penicillium marneffei participates in two developmental programmes: conidiation and dimorphic growth. Mol Microbiol 38:1034–1047
Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13:17R–27R
Etxebeste O, Garzia A, Espeso EA, Ugalde U (2010) Aspergillus nidulans asexual development: making the most of cellular modules. Trends Microbiol 18:569–576
Fang WG, Zhang YJ, Yang XY, Zheng XL, Duan H, Li Y, Pei Y (2004) Agrobacterium tumefaciens mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J Invertebr Pathol 85:18–24
Feng MG, Poprawski TJ, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Tech 4:3–34
Holder DJ, Kirkland BH, Lewis MW, Keyhani NO (2007) Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiol-SGM 153:3448–3457
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Liu Q, Ying SH, Feng MG, Jiang XH (2009) Physiological implication of intracellular trehalose and mannitol changes in response of entomopathogenic fungus Beauveria bassiana to thermal stress. Antonie Van Leeuwenhoek 95:65–75
Marshall MA, Timberlake WE (1991) Aspergillus nidulans wetA activates spore-specific gene expression. Mol Cell Biol 11:55–62
Mi H, Dong Q, Muruganujan A, Gaudet P, Lewis S, Thomas PD (2010) PANTHER version 7: improved phylogenetic trees, orthologs and collaboration with the gene ontology consortium. Nucleic Acids Res 38:204–210
Mirabito PM, Adams TH, Timberlake WE (1989) Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell 57:859–868
Ni M, Yu JH (2007) A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2:e970
Ogawa M, Tokuoka M, Jin FJ, Takahashi T, Koyama Y (2010) Genetic analysis of conidiation regulatory pathways in koji-mold Aspergillus oryzae. Fungal Genet Biol 47:10–18
Park HS, Yu JH (2012) Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15:669–677
Puttikamonkul S, Willger SD, Grahl N, Perfect JR, Movahed N, Bothner B, Park S, Paderu P, Perlin DS, Cramer Jr RA (2010) Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol Microbiol 77:891–911
Roberts DW, St Leger RJ (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54:1–70
Sewall TC, Mims CW, Timberlake WE (1990a) Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev Biol 138:499–508
Sewall TC, Mims CW, Timberlake WE (1990b) abaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 2:731–739
Sigl C, Haas H, Specht T, Pfaller K, Kürnsteiner H, Zadra I (2011) Among developmental regulators, StuA but not BrlA is essential for penicillin V production in Penicillium chrysogenum. Appl Environ Microbiol 77:972–982
Son H, Kim MG, Min K, Lim JY, Choi GJ, Kim JC, Chae SK, Lee YW (2014) WetA is required for conidiogenesis and conidium maturation in the ascomycete fungus Fusarium graminearum. Eukaryot Cell 13:87–98
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tao L, Yu JH (2011) AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiol-SGM 157:313–326
Vega FE, Meyling NV, Luangsa-Ard JJ, Blackwell M (2012) Fungal entomopathogens. In: Vega F, Kaya HK (eds) Insect pathology, 2nd edn. Academic Press, San Diego, pp. 171–220
Wanchoo A, Lewis MW, Keyhani NO (2009) Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiol-SGM 155:3121–3133
Wang CS, Feng MG (2014) Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol Control 68:129–135
Wang JJ, Qiu L, Chu ZJ, Ying SH, Feng MG (2014) The connection of protein O-mannosyltransferase family to the biocontrol potential of Beauveria bassiana, a fungal entomopathogen. Glycobiology 24:638–648
Wang MS, Sun XP, Zhu Cy XQ, Ruan RX, Yu DL, Hy L (2015) PdbrlA, PdabaA and PdwetA control distinct stages of conidiogenesis in Penicillium digitatum. Res Microbiol 166:56–65
Wang ZL, Lu JD, Feng MG (2012) Primary roles of two dehydrogenases in the mannitol metabolism and multi-stress tolerance of entomopathogenic fungus Beauveria bassiana. Environ Microbiol 14:2139–2150
Xiao GH, Ying SH, Zheng P, Wang ZL, Zhang SW, Xie XQ, Shang YF, Zheng HJ, Zhou Y, St Leger RJ, Zhao GP, Wang CS, Feng MG (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep 2:483
Xie XQ, Li F, Ying SH, Feng MG (2012) Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS One 7:e30298
Ye SD, Ying SH, Chen C, Feng MG (2006) New solid-state fermentation chamber for bulk production of aerial conidia of fungal biocontrol agents on rice. Biotechnol Lett 28:799–804
Yu JH, Mah JH, Seo JA (2006) Growth and developmental control in the model and pathogenic aspergilli. Eukaryot Cell 5:1577–1584
Zhang L, Wang J, Xie XQ, Keyhani NO, Feng MG, Ying SH (2013) The autophagy gene BbATG5, involved in the formation of the autophagosome, contributes to cell differentiation and growth but is dispensable for pathogenesis in the entomopathogenic fungus Beauveria bassiana. Microbiol-SGM 159:243–252
Acknowledgments
We thank Ying-Ying Huang (Core Facilities, School of Medicine, Zhejiang University) and Jun-Ying Li (Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University) for technical assistance with flow cytometry and electron microscopy, respectively. This work was financially supported by the National Natural Science Foundation of China (Grant number 31270537) and the Ministry of Science and Technology, P.R. China (Grant number 2011AA10A204). The authors declare no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 282 kb)
Rights and permissions
About this article
Cite this article
Li, F., Shi, HQ., Ying, SH. et al. WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen. Appl Microbiol Biotechnol 99, 10069–10081 (2015). https://doi.org/10.1007/s00253-015-6823-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6823-7