Abstract
Pathogenic Escherichia coli (E. coli) is an important infectious Gram-negative bacterium causing millions of death every year. Outer membrane protein A (OmpA) has been suggested as a potential vaccine candidate for conferring protection against bacterial infection. In this study, a universal vaccine candidate for E. coli infection was developed and evaluated. Bioinformatics analysis revealed the OmpA protein from E. coli shares 96~100 %, 90~94 %, and 45 % identity with Shigella, Salmonella, and Pseudomonas strains, respectively. The ompA gene was cloned from the genomic DNA of E. coli, and then the OmpA protein was expressed in BL21 (DE3) using the auto-induction method. The recombinant OmpA (rOmpA) protein had an average molecular weight of 36 kDa with the purity of 93.5 %. Immunological analysis indicated that the titers of anti-rOmpA sera against rOmpA and whole cells were 1:642,000 and 1:140,000, respectively. Moreover, rOmpA not only conferred a high level of immunogenicity to protect mice against the challenge of E. coli, but also generated cross-protection against Shigella and Salmonella. The anti-rOmpA sera could enhance the phagocytic activity of neutrophils against E. coli. The survive ratios of mice immunized with rOmpA and PBS were 50 % and 20 % after 48 h post-challenge, indicating mice were protected from E. coli infection after immunization with rOmpA. All these results clearly indicate that rOmpA may be a promising candidate for the development of a subunit vaccine to prevent E. coli infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli (E. coli), a facultative and extracellular Gram-negative bacterium, is the predominate organism in intestinal tract of human and other homoiothermal animal. Although most E. coli are harmless, some strains can cause diverse gastrointestinal tract diseases, such as diarrhea and emesis, and even colorectal cancer (CRC) in human and other animals (Guiral et al. 2011; Levine and Edelman 1984; Pakalniskiene et al. 2009; Arthur et al. 2012). This opportunistic pathogens entering into internal environment can also cause hematologic malignancies, such as hemolytic–uremic syndrome, early onset neonatal sepsis, and so on (Borgatta et al. 2012; Rasko et al. 2011; Stoll et al. 2011). There are 1.4~2.5 million deaths due to pathogenic E. coli each year (Murray et al. 2013). Moreover, some extra intestinal pathogenic E. coli strains are also the cause of significant economic losses in animal farms, especially in the poultry industry (Ron 2006).
Presently, the disease caused by E. coli is mainly controlled by the use of antibiotics or vaccines. However, abuse of antibiotics has led to an increasing number of emergences of drug resistance to E. coli and destruction of intestinal flora micro-ecology balance (Ronzio 2003; Johnson et al. 2010; Perez et al. 2014; Zhu et al. 2013). In comparison, vaccination is a more effective way of preventing infectious diseases than antibiotics. Vaccines, including inactivated vaccine and subunit vaccine, against E. coli strains have been studied closely in recent years. Inactivated vaccine could induce remarkable immune response associated with protection against pathogenic bacteria. Savarino et al. reported inactivated enterotoxigenic E. coli (ETEC) plus cholera toxin B has been proved to be safe and immunogenic in 2- to 12-year-old children as oral vaccine (Savarino et al. 1999).
The number of E. coli serotypes is very high, 50,000~100,000 or more (Ørskov and Ørskov 1992). E. coli infection is commonly caused by multiple serotypes, so it is quite expensive and time-consuming to develop vaccines against each serotype of pathogenic E. coli strains. In order to effectively resolve this problem, the subunit vaccines are often administered along with adjuvants and substances, which are able to increase the immunogenicity of antigens. Güereña-Burgueño et al. (2002) reported that a vaccine against ETEC could induce significant systemic immune responses in the presence of an adjuvant such as heat-labile enterotoxin (Güereña-Burgueño et al. 2002). Several studies revealed that type III secreted proteins vaccines significantly reduce fecal shedding in cattle, and prevalence of E. coli O157:H7 in a clinical trial was significantly reduced (Potter et al. 2004; Snedeker et al. 2012). These few vaccines available against E. coli provide only limited protection against homologous challenge. Therefore, there is a need for a more universal effective vaccine against E. coli.
Outer membrane protein A (OmpA), a major surface protein in E. coli, plays an important role in biofilm formation, host cell invasion, pore-forming, and multidrug resistance (Ma and Wood 2009; Martinez et al. 2014; Zakharian and Reusch 2005; Smani et al. 2014). Moreover, OmpA is also an important factor of adhesion in E. coli O157:H7 and other strains, which is of vital importance to pathogenicity of bacteria (Nair and Venkitanarayanan 2007; Smith et al. 2007). OmpA has been revealed to be highly immunogenic proteins and may represent a good candidate for vaccine development against bacterial infection (Li et al. 2014). Hu et al. (2013) reported that more than 30 % of the animals immunized with OmpA survived after lethal challenge, which demonstrated that OmpA significantly protect against E. coli, Klebsiella pneumonia, and Shigella flexneri. Yan et al. (2010) found that three recombinant OmpA-like proteins generated strong immune responses, enhanced survival, and reduced the severity of histopathological lesions, which indicated that these three OmpA-like proteins may serve as novel vaccine candidates for leptospirosis (Yan et al. 2010). OmpA in other Gram-negative bacteria has also been reported to be a potential vaccine candidate in other bacteria (Dabo et al. 2008; Maiti et al. 2011; Yan et al. 2010).
In this study, the ompA gene was cloned from the genomic DNA of E. coli strain CVCC 1515 and expressed by a prokaryotic system. The resultant recombinant OmpA (rOmpA) was used as an antigen to prepare vaccines, and protective efficacy of the rOmpA vaccine was evaluated against E. coli in vivo and in vitro.
Materials and methods
Bacterial strains and mice
E. coli CVCC 1515, E. coli CVCC 195, Salmonella choleraesuis CVCC 503, Salmonella enteritidis CVCC 3377, and Salmonella pullorum CVCC 1802 were purchased from China Veterinary Culture Collection Center (CVCC) (Beijing, China). E. coli CICC 21530 (serotype O157:H7), Salmonella typhimurium CICC 22596, Pseudomonas aeruginosa CICC 10419, CICC 21625, CICC 21636, and CICC 22630 were purchased from China Center of Industrial Culture Collection (CICC) (Beijing, China). Shigella dysenteriae CMCC (B) 51252, Shigella flexneri strain CMCC (B) 51571, and CMCC (B) 50336 were purchased from National Center for Medical Culture Collection (CMCC) (Beijing, China). E. coli competent strains of DH5α and BL21 (DE3) were purchased from TransGen Biotech Co., Ltd. (Beijing, China). All stains were cultured on Luria Bertani (LB) at 37 °C.
The ompA gene sequence of E. coli strain CVCC 1515 was deposited in the National Center for Biotechnology Information (NCBI) GenBank (GenBank accession no. KP031704).
Specific pathogen-free (SPF) female 6∼8-week-old BALB/c mice were purchased from Vital River Laboratories (VRL, Beijing). Mice were housed in appropriate conventional animal care facilities and handled according to international guidelines required for animal experiments.
Bioinformatics analysis of the OmpA protein
Amino acid sequences of OmpA were manually aligned as described previously (Yousef Mohamad et al. 2008). Sequence of OmpA in E. coli strain K12 was used as a reference for blasting. Based on similarities of OmpA, five E. coli strains, two Shigella strains, five Salmonella strains, and four P. aeruginosa strains were used for phylogenetic analysis using the bootstrap method.
Expression and purification of the rOmpA protein
Genomic DNA was extracted from E. coli strain CVCC1515 using a TIANamp Bacteria DNA Kit (Tiangen Biotech, Beijing, China) following the manufacturer’s instructions. The primers, rOmpA F-EcoRI: 5′-GAATTCGCTCCGAAAGATAACACCTGGTACAC-3′ with the EcoRI restriction enzyme site and rOmpA R-NotI: 5′-GCGGCCGCAGCTTGCGGTTGAGTTACTACGTC-3′ with the NotI restriction enzyme site, were designed to amplify the ompA gene. The amplified PCR fragment was inserted into the pMD19 (Simple) T Vector (TaKaRa Biotech. (Dalian) Co., Ltd) and transformed into E. coli DH5α competent cells. The recombinant pMD19-ompA plasmid was isolated using the TIANprep Mini Plasmid Kit (Tiangen Biotech, Beijing, China), and digested with EcoRI/NotI.
The digested DNA fragment was then cloned into the similarly digested pET28a expression vector to generate the plasmid pET28a-ompA. The recombinant plasmids were transformed into E. coli BL21 (DE3) and identified by restriction enzyme digestion and DNA sequencing. The recombinant His-tagged OmpA protein was expressed in BL21 (DE3) using the auto-induction method with some modifications (Studier 2005). Briefly, the transformant cells were cultured in LB medium at 37 °C on a platform shaker at 250 rpm to an optical density at 600 nm (OD600 nm) of 0.40 to 0.60, and then transplanted to ZYM-5052 auto-induction media with 1 % inoculum density. The stains were cultured at 37 °C on a platform shaker with a speed of 300 rpm for 24 h.
The rOmpA protein was purified and refolded according to Saleem’s protocol with some minor modifications (Saleem et al. 2012). Briefly, after the fusion protein being sufficiently expressed, the cultured cells were harvested by centrifugation at 5000×g for 30 min at 4 °C and resuspended in lysis buffer (50 mM Tris-HCl buffer, pH 7.9, containing 5 mg of lysozyme and 5 μl of DNase I type IV stock per gram of cell paste) (8 ml/g wet weight of cell paste). The cells were sonicated for 5~6 min with an Ultrasonic Crasher Noise Isolating Chamber (SCIENTZ, Ningbo Science Biotechnol Co., Ltd., China) on ice. Inclusion bodies (IBs) were precipitated with centrifugation at 14,000×g for 20 min at 4 °C and washed twice in 50 ml of 50 mM Tris-HCl buffer (pH 7.9) containing 1.5 % (v/v) lauryl dimethyl amine oxide (LDAO) for each 1~1.5 g wet weight. After that, the IBs were precipitated and dissolved in denaturing buffer (10 mM Tris-HCl buffer, pH 7.5, containing 1 mM ethylenediamine tetraacetic acid (EDTA) and 8 M urea). The IBs solution was centrifuged at 14,000×g for 20 min to remove any undissolved material and was added dropwise to rapid stirred refolding buffer (20 mM Tris-HCl buffer, pH 7.9, containing 1 M NaCl and 5 % (v/v) LDAO) to produce a final 1:1 volume ratio. The solution was dialyzed at 4 °C against two changes of 4 l of dialysis buffer (20 mM Tris-HCl buffer, pH 7.9, containing 0.5 M NaCl and 0.1 % (v/v) LDAO) every 6 h for refolding. The fusion protein was purified using a Ni2+-nitriloacetate (NTA) super flow resin column (QIAGEN, Germany) with equilibration buffer (20 mM Tris-HCl buffer, pH 7.9, containing 0.5 M NaCl, 0.1 % (v/v) LDAO and 40 mM imidazole) and elution buffer (20 mM Tris-HCl buffer, pH 7.4, containing 0.5 M NaCl, 0.1 % (v/v) LDAO and 500 mM imidazole) according to the manufacturer’s instructions. Then the eluted recombinant protein was desalted using a HiPrep 26/10 desalting column with desalination buffer (20 mM Tris-HCl buffer, pH 7.4, containing 150 mM NaCl and 0.1 % (v/v) LDAO). All proteins were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The purity of proteins was calculated by the Gel-Pro Analyzer™ version 6.3 (Media Cybernetics). The purified rOmpA protein was then lyophilized with an ALPHA 1-2 LD plus freeze dryer (Christ, Germany) and kept at −20 °C.
Immunization protocols
Five SPF female Balb/c mice, 6∼8 week-old were immunized with the purified rOmpA protein on day 0, day 21, and day 35 as previously reported by Reddy et al. (2010). The lyophilized rOmpA protein was resuspended in sterile-filtered PBS at 1.0 mg/ml. For the primary immunization, 25 μl of the rOmpA solution was mixed with 25 μl of complete Freund’s adjuvant (Sigma-Aldrich, Inc.) and 50 μl of sterile PBS. Mice were hypodermically injected with antigen mixture (100 μl/mouse). The mice in control group were immunized with PBS instead of rOmpA.
Subsequent two intraperitoneal injections consisted of 25 μg of rOmpA emulsified with 25 μl of incomplete Freund’s adjuvant (Sigma-Aldrich, Inc.). All mice were housed in individually ventilated cages (Suzhou Fengshi Laboratory Animal Equipment Co., Ltd, Suzhou) and monitored daily. Cages were changed once per week. The mice were bled from the tail vein on days 0, 5, 25, and 39, and sera were stored at −20 °C until used.
Indirect enzyme-linked immunosorbent (iELISA) assay
rOmpA detection by iELISA
One hundred microliters of rOmpA (0.2 μg/well) in coating buffer (0.015 M sodium carbonate, 0.035 M sodium bicarbonate, pH 9.6) were incubated overnight at 4 °C in 96-well plates. After washing four times with 0.01 M PBST (PBS containing 0.05 % Tween 20), the plates were blocked for 2 h at 37 °C by adding 0.01 M PBST containing 5 % BSA. After washing with PBST four times, the plates were added with serial dilutions of mice serum and incubated for 1.5 h at 37 °C and washed as above. HRP conjugated goat anti-mouse IgG was diluted 1:5000 and added into the plates. The plates were incubated for 30 min at 37 °C. Finally, 3,3′,5,5′-tetramethylbenzidine (TMB) was added to each well and incubated in the dark at room temperature for 20 min. The reaction was stopped by adding 2 mol/l H2SO4 (50 μl/well). The absorbance of each well at 450 nm was read by a microplate reader (Perlong Medical, Beijing). The result was considered as positive when the ratio of the test group and negative control group was greater than 2.1.
Bacterial cell detection by iELISA
The 96-well plates were coated by incubating them for 1 h at 37 °C with 150 μl 0.1 mol/l NaHCO3 containing 2.5 % glutaraldehyde and washed with sterile water four times. Bacterial cells (108 colony-forming unit (CFU)/ml, 100 μl/well) were then added into the plates and incubated at 37 °C for 24 h. The subsequent steps were the same as the procedure given above.
Serum bactericidal assay
Serum bactericidal assay (SBA) was performed by the protocol described previously with some modifications (Marzoa et al. 2012). Briefly, the mid-log phase bacteria were resuspended in PBS (105 CFU/ml). The 96-well cell culture plates were placed on ice until assayed. The volume of each reaction mixture was 100 μl, containing 50 μl of serial twofold mouse serum, 25 μl of the bacterial cells, and 25 μl of baby rabbit complement (Cedarlane, Hornby, ON, Canada). The plates were incubated at 37 °C for 45 and 90 min, and 20 μl of samples from each well were plated onto LB agar. Colony counts were recorded after 0, 45, and 90 min of incubation at 37 °C. The percentage of cells killed at each dilution was computed by using the post-incubation colony counts from the inactive complement control wells as the zero kill reference. The serum dilution giving closest to 50 % kill was reported as the titer.
Opsonophagocytosis assay in vitro
As previously described, neutrophils were isolated from mouse peritoneal fluid and adjusted to a concentration of 4 × 106 cells/ml (Luo and Dorf 2001). Briefly, 400 μl of log-phase E. coli CVCC 1515 cells (4 × 104 CFU/ml) was incubated with 100 μl of appropriately diluted immune or nonimmune mouse serum at 37 °C for 30 min. The bacteria were then incubated with 500 μl of neutrophils suspension and 100 μl of baby rabbit complement (Cedarlane, Hornby, ON, Canada) at 30 °C for 1 h. After incubation, neutrophils were lysed by adding sterile water into the mixture. The mixture was then serially diluted for plate count.
Feces E. coli counting
E. coli strain CICC 21530 was cultivated to the logarithmic phase and adjusted to 1011 CFU/ml. Mice were intragastric administrated after an overnight fast. Fecal samples were collected every day for up to 7 days, and viability was measured by the plate colony count.
Challenge assay
Lethal dose of 50 % (LD50) was determined by the method of Reed and Muench (Reed and Muench 1938). Fourteen days after the final immunization, 10 mice from each group were injected intraperitoneally with 100 μl (1011 CFU/ml) logarithmic phase E. coli CVCC 1515 strain. Mortality was recorded each 12 h, and animals were monitored for up to 7 days post-challenge.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0. One-way repeated analysis of variance (ANOVA) Dunkan method and the Mann-Whitney rank test were used to determine the significance of the differences between groups. Differences were considered significant at p < 0.05.
Results
Bioinformatics analysis of the OmpA protein
The hylogenetic analysis result showed that OmpA is highly conserved among the Enterobacteriaceae family (96~100 % identity). OmpA from E. coli shares 96~100 %, 90~94 %, and 45 % identity with that of Shigella, Salmonella, and Pseudomonas strains, respectively (Fig. 1).
Cloning, expression, and purification of the rOmpA protein
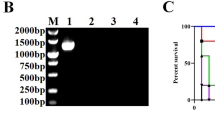
The DNA fragment of ompA with the size of 987 bp was successfully amplified from the genomic DNA of E. coli and cloned into the pMD19 T vector. The digestion fragment of the ompA gene was inserted into the pET28a digested with the same enzymes and resultant the new recombinant pET28a-ompA plasmid. The DNA sequencing and restriction enzyme digestion results showed the pET28a-ompA plasmid was constructed successfully. The expression of rOmpA fused with His tag in E. coli BL21 (DE3) was auto-induced to 24 h at 37 °C. As shown in Fig. 2, the expressed rOmpA protein was present in the IBs. The molecular weight of rOmpA was approximately 36 kDa, which is consistent with theoretical molecular weight. The purification of rOmpA was performed by a Ni2+-NTA affinity chromatography. After purification and refolding, the purity of rOmpA was up to 93.5 %, falling within the 80~98 % purity ranges of other protein antigens which have been shown to be the effective vaccine candidates (Frace et al. 1999; Garcõa-Garcõaa et al. 2000; Zhang and Pan 2005; Cheng et al. 2009).
Expression and purification of rOmpA. Proteins were stained with Coomassie and analyzed by SDS-PAGE. Lane 1, total proteins of BL21; lanes 2, 3, total proteins of BL21 harboring pET28a; lane 4, total proteins of BL21 harboring pET28a-ompA; lanes 5, 6, eluent of the purified rOmpA protein; lane M, molecular weight marker (14~94 kDa)
Immunogenicity of the rOmpA protein in mice
To assess the immunogenic property of the rOmpA protein, sera were collected from mice on days 0, 5, 25, and 39 prior to challenge and tested for reactivity with the purified rOMPs by iELISA. The results shown in Fig. 3a revealed mice immunized with rOmpA exhibited a significant immunogenicity. The initial strong and significant immune responses to rOmpA were first detected at day 25 and continued to increase after the third immunization. The titer of the OmpA sera reached their highest levels of 1:642,000 after the third immunization. Meanwhile, the OmpA sera also have a high affinity to E. coli CVCC 1515 (1:140,000 dilution after the third immunization). The result of sera obtained from PBS vaccinated mice clearly indicated the absence of any rOmpA specific antibody.
Sera antibody titers of rOmpA analyzed by iELISA. Mice were vaccinated with rOmpA or PBS. Serum samples were collected at days 0, 5, 25, and 39, respectively, and antibody titers were measured by iELISA using the purified rOmpA as antigen. The error bars represent the standard deviations of triplicate measures. a Sera antibody titer in different groups post-vaccination. PBS sera-rOmpA, the antibody titer of PBS vaccination group against rOmpA; rOmpA sera-rOmpA, the antibody titer of rOmpA vaccination group against rOmpA; PBS sera-CVCC1515, the antibody titer of PBS vaccination group against E. coli CVCC 1515; rOmpA sera-CVCC1515, the antibody titer rOmpA vaccination group against E. coli CVCC 1515. b Cross-reaction properties of the anti-rOmpA sera (titer of 1:27,000) against different bacteria. Statistical significance (p < 0.05) is indicated by a lowercase letter
Cross-reaction properties of the anti-rOmpA sera
Cross-reaction properties of the anti-rOmpA sera were performed among E. coli, Shigella, Salmonella, and P. aeruginosa stains by the whole cell iELISA assay. Mice sera were serially diluted, and the ratio of test group and negative control group greater than 2.1 was considered as positive. As shown in Fig. 3b, E. coli, Shigella, and Salmonella strains except for P. aeruginosa stains (1:3000~9000) have a high antibody titer (>1:27,000). The results showed that OmpA was highly conserved in E. coli, Shigella, and Salmonella, which is in accordance with the results of bioinformatics analysis.
Bactericidal activity and phagocytosis of the anti-rOmpA sera against E. coli CVCC 1515 in vitro
SBA was performed to evaluate the bactericidal activity of serum. As shown in Fig. 4a, colony counts of other groups except for the control group were found to be raised in a time dependent manner. After incubation for 90 min, group 1 (rOmpA sera + complement) and the control group 2 (PBS) have the maximum and minimum number of colonies, respectively. The results indicated that anti-rOmpA sera had no bactericidal activity against E. coli CVCC 1515 in vitro, nutritional ingredient in sera maybe inversely promote the growth of E. coli.
Bactericidal activity and phagocytosis of the anti-rOmpAsera in vitro. a Bactericidal activity of sera in classic complement pathway. E. coli strain CVCC 1515 was incubated with rOmpA sera and complement (group 1), rOmpA sera and PBS (group 2), PBS and complement (control group 1), and PBS (control group 2), respectively. b Colony counts of different groups in phagocytosis. E. coli strain CVCC 1515 was incubated with antisera of PBS vaccination group (group 1: PBS sera), antisera of OmpA vaccination group (group 2: rOmpA sera) and PBS (control group). Statistical significance (p < 0.05) is indicated by a lowercase letter
E. coli CVCC 1515 cells were incubated with neutrophils and sera from mice immunized with rOmpA (group 2) or PBS (group 1). As shown in Fig. 4b, after incubation for 30 min, the number of viable bacterial cells in group 1 (PBS sera) was decreased by 18 % compared with control group (PBS), which showed that neutrophils have phagocytic activity. Bacterial cells in group 2 (rOmpA sera) was decreased by 37.33 % compared to group 1 (PBS sera), and 59.66 % to control group (PBS) with p value <0.05, showing that the anti-rOmpA sera can enhance the phagocytic activity of neutrophils against E. coli in vitro.
Efficacy of the anti-rOmpA sera against E. coli in vivo
Mice were administered intragastrically with E. coli CICC 21530 (1011 CFU), and feces were collected for plate counting. The shedding of E. coli in feces after the administration of E. coli CICC 21530 is shown in Fig. 5a. There was a dramatic decrease in the first 3 days after administration in both groups. In mice immunized with rOmpA, the shedding of E. coli in feces was significantly (p < 0.05) decreased after 5 days post-administration and was about 107 CFU/g at 7 days post-administration. After 4 days of administration, fecal shedding of E. coli in the control group (PBS) was increased continuously and was up to 108 CFU/g at 7 days post-administration.
Protection efficacy of the anti-rOmpA sera against E. coli in vivo. The mice immunized with rOmpA or PBS (control) were administered intragastrically with E. coli CICC 21530 (109 CFU) and observed for 7 days after challenge. a Viable count of fecal from the mice vaccinated with rOmpA or PBS. b Survival percent of the mice immunized with rOmpA or PBS
Two weeks after the third vaccination, control and rOmpA-immunized mice were challenged with E. coli CVCC1515 by intraperitoneal injection, and the protective efficacy of rOmpA was evaluated in terms of survival number. Survival ratio of mice immunized with rOmpA was higher than that of control mice. After 36 h post-challenge, the survival ratio of rOmpA group was kept to 50 %, but the survival ratio of control group was 20 % after 48 h post-challenge (Fig. 5b). The results indicated that the vaccination with rOmpA protected mice from lethal E. coli infection.
Discussion
OmpA is a major structural protein of the outer membrane of E. coli and a highly immunogenic bacterial component due to their exposed epitopes on the cell surface. Due to the different serotypes of pathogenic E. coli, the development of a versatile vaccine that provides heterologous protection for E. coli has been a growing concern. Previous reports have demonstrated the immunogenic potential of OmpA as novel vaccine candidates against Leptospirosis (Yan et al. 2010), Riemerella anatipestifer (Huang et al. 2002), Edwardsiella tarda (Maiti et al. 2011), and Shigella (Pore et al. 2011). The goal of the present study was to develop a universal vaccine in E. coli strains.
Only approximately 50 % sequence homology of PRRSV virus genotypes can be distinguished by their immunological properties (Dea et al. 2000). Our results suggest that there is a high degree of amino acid sequence conservation (identity >96 %) of OmpA among E. coli and Shigella strains (Fig. 1). The similarities of the OmpA protein from Salmonella and Pseudomonas ranged from 45 to 94 %, which implies that OmpA may be a shared antigen among these strains (Li et al. 2014).
The OmpA protein, expressed in this study, is a 329-amino acid protein composed of an N-terminal extracellular and transmembrane domain (residues ~22–194) and a C-terminal periplasmic domain (residues ~195–329) (Arora et al. 2001; Koebnik 1999). To increase epitope sequestered or masked in the interior of IBs, rOmpA was further refolded after purification. Meanwhile, a mild detergent LDAO, which dissociated the dimer into monomers, was used in desalination buffer in this study (Kruip et al. 1994). The iELISA result showed that higher affinity antisera against rOmpA (1:642,000 dilution) were observed than against E. coli (1:140,000 dilution) (Fig. 3a), which not only indicated membrane protein immunogenicity of the purified rOmpA protein was maintained, but also that antibodies against the extracellular domain of transmembrane proteins can specifically recognize bacteria. Additionally, it was speculated that other non-extracellular domain of rOmpA may be the epitope for anti-OmpA antibody (Shirai et al. 2012). However, the immunological characteristic of OmpA is yet to be further investigated.
Antigenic cross-reactivity of OMPs has been reported among Gram-negative bacteria (Xu et al. 2005; Lun et al. 2014). In our study, significant antibody responses to Shigella and Salmonella strains were observed in Fig. 3b, suggesting high homology in the amino acid sequence among these strains. However, the anti-rOmpA sera weakly recognize Pseudomonas strains, indicating the lower homology in the amino acid sequence. These results were consistent with similarities of the amino acid sequences (Fig. 1). Additionally, the result further indicated that OmpA has a broad cross-reaction property among Gram-negative bacteria.
So far, it is found that there are three pathways of complement activation, including classical pathway (Cooper 1985), alternate pathway (Götze and Mueller-Eberhard 1971), and mannan-binding lectin (MBL) pathway (Vang Petersen et al. 2001). Complement can be activated by classical pathway during antibody recognizing and eliminating pathogen (Carroll 2004; Cooper 1985). In this study, E. coli could not be killed by the coexistence of antisera and complement (Fig. 4a). On the contrary, multiplication rate of E. coli strains in group 1 (rOmpA sera + complement) was faster than those of other three groups. Two reasons may be suggested for the dissimilar results in the four groups. One reason may be that the classic complement pathway was not be activated by the antibody, and the other one may be that adequate nutrition in sera promoted the growth of E. coli (Morrison and Kline 1977; Nieman 1954). However, opsonophagocytosis assay showed that antibody could significantly (p < 0.05) accelerate the phagocytosis of phagocyte against E. coli (Fig. 4b).
It was known that OmpA is also an important factor of adhesion in O157:H7 and other E. coli strains, and it is of vital importance in pathogenicity of bacteria (Nair and Venkitanarayanan 2007; Smith et al. 2007). In this study, E. coli CICC 21530 was used to investigate the scavenging activity of rOmpA vaccination. The result showed that viable count was decreased in the first 3 days and increased at day 4, revealing that the strains begun to colonize in intestinal tract. However, colony count immune with rOmpA was decreased at day 5, which was significantly lower than that of the control group (p < 0.05) (Fig. 5a). The similar result was also found in a previous report that demonstrated that immunized mice with outer membrane protein (OMP)-VP0802 had significantly more efficient clearance of Vibrio parahaemolyticus than that of control mice (Li et al. 2014). It is apparent that, in an effective vaccine-based therapy, specific antibodies against outer membrane proteins (OMPs) can effectually reduce the adhesion and colonization of bacteria and enhance complement-mediated clearance of circulating bacteria (Li et al. 2014).
To evaluate the potential immune protection of the anti-OmpA sera against E. coli infection, an active protection assay in a mouse model was performed in this study. Our results showed that mice vaccinated with rOmpA were well protected when challenged with E. coli CVCC 1515 (Fig. 5b). Similar results were also found in previous studies that demonstrated that anti-OmpA, OmpL, and other Omp serum had a high bactericidal activity for Escherichia, Salmonella, Klebsiella, Shigella, Edwardsiella, and Leptospira serovars (Kawai et al. 2004; Yan et al. 2010; Hu et al. 2013).
In conclusion, the present study found that the OmpA protein shared a high degree of similarity and distributed widely among Gram-negative strains. The anti-rOmpA sera had a significant cross-reaction capacity against several pathogenic strains of Escherichia, Shigella, and Salmonella. Vaccination with rOmpA can effectively reduce the colonization of E. coli and improve the survival rate of mice against E. coli infection. The results indicated that OmpA is the most promising candidate antigen for the development of a subunit vaccine.
References
Arora A, Abildgaard F, Bushweller JH, Tamm LK (2001) Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Mol Biol 8(4):334–338
Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338(6103):120–123
Borgatta B, Kmet-Lunaček N, Rello J (2012) E. coli O104:H4 outbreak and haemolytic–uraemic syndrome. Med Intensiva (Engl Ed) 36(8):576–583
Carroll MC (2004) The complement system in regulation of adaptive immunity. Nat Immunol 5(10):981–986
Cheng Y, Feng YJ, Luo P, Gu J, Yu S, Zhang WJ, Liu YQ, Wang QX, Zou QM, Mao XH (2009) Fusion expression and immunogenicity of EHEC EspA-Stx2A1 protein: implications for the vaccine development. J Microbiol 47(4):498–505
Cooper NR (1985) The classical complement pathway: activation and regulation of the first complement component. Adv Immunol 37:151–216
Dabo SM, Confer A, Montelongo M, York P, Wyckoff JH III (2008) Vaccination with Pasteurella multocida recombinant OmpA induces strong but non-protective and deleterious Th2-type immune response in mice. Vaccine 26(34):4345–4351
Dea S, Wilson L, Therrien D, Cornaglia E (2000) Competitive ELISA for detection of antibodies to porcine reproductive and respiratory syndrome virus using recombinant E. coli-expressed nucleocapsid protein as antigen. J Virol Methods 87(1–2):109–122
Frace AM, Klimov AI, Rowe T, Black RA, Katz JM (1999) Modified M2 proteins produce heterotypic immunity against influenza A virus. Vaccine 17(18):2237–2244
Garcõa-Garcõaa JC, Monteroa C, Redondoa M, Vargas M, Canales M, Boue O, Rodríguez M, Joglar M, Machado H, González IL, Valdés M, Méndez L, de la Fuente J (2000) Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine 18(21):2275–2287
Götze O, Mueller-Eberhard HJ (1971) The C3-activator system: an alternate pathway of complement activation. J Exp Med 134(3):90–108
Güereña-Burgueño F, Hall ER, Taylor DN, Cassels FJ, Scott DA, Wolf MK, Roberts ZJ, Nesterova GV, Alving CR, Glenn GM (2002) Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect Immun 70(4):1874–1880
Guiral E, Mendez-Arancibia E, Soto SM, Salvador P, Fàbrega A, Gascón J, Vila J (2011) CTX-M-15–producing enteroaggregative Escherichia coli as cause of travelers’ diarrhea. Emerg Infect Dis 17(10):1950
Hu R, Fan ZY, Zhang H, Tong CY, Chi JQ, Wang N, Li RT, Chen L, Ding ZF, Chen LX, Tang W, Zhou X, Pu LJ, Zhu ZB, Cui YD (2013) Outer membrane protein A (OmpA) conferred immunoprotection against Enterobacteriaceae infection in mice. Israel J Vet Med 68(1):48–55
Huang B, Subramaniam S, Frey J, Loh H, Tan H-M, Fernandez CJ, Kwang J, Chua K-L (2002) Vaccination of ducks with recombinant outer membrane protein (OmpA) and a 41 kDa partial protein (P45N′) of Riemerella anatipestifer. Vet Microbiol 84(3):219–230
Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M (2010) Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 51(3):286–294
Kawai K, Liu Y, Ohnishi K, Oshima S (2004) A conserved 37 kDa outer membrane protein of Edwardsiella tarda is an effective vaccine candidate. Vaccine 22(25–26):3411–3418
Koebnik R (1999) Structural and functional roles of the surface-exposed loops of the β-barrel membrane protein OmpA from Escherichia coli. J Bacteriol 181(12):3688–3694
Kruip J, Bald D, Boekema E, Rögner M (1994) Evidence for the existence of trimeric and monomeric Photosystem I complexes in thylakoid membranes from cyanobacteria. Photosynth Res 40(3):279–286
Levine MM, Edelman R (1984) Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev 6(1):31–51
Li C, Ye Z, Wen L, Chen R, Tian L, Zhao F, Pan J (2014) Identification of a novel vaccine candidate by immunogenic screening of Vibrio parahaemolyticus outer membrane proteins. Vaccine 32(46):6115–6121
Lun JS, Xia CY, Yuan CF, Zhang YL, Zhong MQ, Huang TW, Hu Z (2014) The outer membrane protein, LamB (maltoporin), is a versatile vaccine candidate among the Vibrio species. Vaccine 32(7):809–815
Luo Y, Dorf ME (2001) Isolation of mouse neutrophils. Curr Protoc Immunol Chapter 3, Unit 3.20
Ma Q, Wood TK (2009) OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol 11(10):2735–2746
Maiti B, Shetty M, Shekar M, Karunasagar I, Karunasagar I (2011) Recombinant outer membrane protein A (OmpA) of Edwardsiella tarda, a potential vaccine candidate for fish, common carp. Microbiol Res 167(1):1–7
Martinez E, Cantet F, Fava L, Norville I, Bonazzi M (2014) Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog 10(3):e1004013
Marzoa J, Sanchez S, Costoya L, Dieguez-Casal E, Freixeiro P, Brookes C, Allen L, Taylor S, Gorringe AR, Ferreiros CM, Criado MT (2012) Induction of immune responses by purified outer membrane protein complexes from Neisseria meningitidis. Vaccine 30(13):2387–2395
Morrison DC, Kline LF (1977) Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol 118(1):362–368
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S (2013) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2197–2223
Nair MKM, Venkitanarayanan K (2007) Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr Res 62(6):664–669
Nieman C (1954) Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev 18(2):147–163
Ørskov F, Ørskov I (1992) Escherichia coli serotyping and disease in man and animals. Can J Microbiol 38(7):699–704
Pakalniskiene J, Falkenhorst G, Lisby M, Madsen S, Olsen K, Nielsen E, Mygh A, Boel J, Mølbak K (2009) A foodborne outbreak of enterotoxigenic E. coli and Salmonella Anatum infection after a high-school dinner in Denmark, November 2006. Epidemiol Infect 137(3):396–401
Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM (2014) Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69(3):216–225
Pore D, Mahata N, Pal A, Chakrabarti MK (2011) Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS One 6(7):e22663
Potter AA, Klashinsky S, Yl L, Frey E, Townsend H, Rogan D, Erickson G, Hinkley S, Klopfenstein T, Moxley RA, Smith DR, Finlay BB (2004) Decreased shedding of Escherichia coli O157: H7 by cattle following vaccination with type III secreted proteins. Vaccine 22(3–4):362–369
Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin C-S, Iliopoulos D (2011) Origins of the E. coli strain causing an outbreak of hemolytic–uremic syndrome in Germany. N Engl J Med 365(8):709–717
Reddy ST, Ge X, Miklos AE, Hughes RA, Kang SH, Hoi KH, Chrysostomou C, Hunicke-Smith SP, Iverson BL, Tucker PW (2010) Monoclonal antibodies isolated without screening by analyzing the variable-gene repertoire of plasma cells. Nat Biotechnol 28(9):965–969
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Hyg 27(3):493–497
Ron EZ (2006) Host specificity of septicemic Escherichia coli: human and avian pathogens. Curr Opin Microbiol 9(1):28–32
Ronzio RA (2003) The encyclopedia of nutrition and good health. Facts On File Inc, New York
Saleem M, Moore J, Derrick JP (2012) Expression, purification, and crystallization of neisserial outer membrane proteins. Neisseria meningitidis (ed by Myron Christodoulies). Methods Mol Biol 799(Chapter 6):91–106
Savarino SJ, Hall ER, Bassily S, Brown FM, Youssef F, Wierzba TF, Peruski L, El-Masry NA, Safwat M, Rao M (1999) Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. J Infect Dis 179(1):107–114
Shirai T, Fujii H, Ono M, Nakamura K, Watanabe R, Tajima Y, Takasawa N, Ishii T, Harigae H (2012) A novel autoantibody against fibronectin leucine-rich transmembrane protein 2 expressed on the endothelial cell surface identified by retroviralvector system in systemic lupus erythematosus. Arthritis Res Ther 14(4):R157
Smani Y, Fabrega A, Roca I, Sanchez-Encinales V, Vila J, Pachon J (2014) Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob Agents Chemother 58(3):1806–1808
Smith SG, Mahon V, Lambert MA, Fagan RP (2007) A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett 273(1):1–11
Snedeker KG, Campbell M, Sargeant JM (2012) A systematic review of vaccinations to reduce the shedding of Escherichia coli O157 in the faeces of domestic ruminants. Zoonoses Public Health 59(2):126–138
Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID, Hale EC (2011) Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 127(5):817–826
Studier FW (2005) Protein production by auto-induction in high-density shaking cultures. Protein Expr Purif 41(1):207–234
Vang Petersen S, Thiel S, Jensenius JC (2001) The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol 38(2–3):133–149
Xu CX, Wang SY, Zhang ZX, Peng XX (2005) Immunogenic cross-reaction among outer membrane proteins of Gram-negative bacteria. Int Immunopharmacol 5(7–8):1151–1163
Yan W, Faisal SM, McDonough SP, Chang C-F, Pan M-J, Akey B, Chang Y-F (2010) Identification and characterization of OmpA-like proteins as novel vaccine candidates for leptospirosis. Vaccine 28(11):2277–2283
Yousef Mohamad K, Roche SM, Myers G, Bavoil PM, Laroucau K, Magnino S, Laurent S, Rasschaert D, Rodolakis A (2008) Preliminary phylogenetic identification of virulent Chlamydophila pecorum strains. Infect Genet Evol 8(6):764–771
Zakharian E, Reusch R (2005) Kinetics of folding of Escherichia coli OmpA from narrow to large pore conformation in a planar bilayer. Biochemistry 44(17):6701–6707
Zhang D, Pan W (2005) Evaluation of three Pichia pastoris-expressed Plasmodium falciparum merozoite proteins as a combination vaccine against infection with blood-stage parasites. Infect Immun 73(10):6530–6536
Zhu Y-G, Johnson TA, Su J-Q, Qiao M, Guo G-X, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci U S A 110(9):3435–3440
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31372346 and No. 31302004), the Project of the National Support Program for Science and Technology in China (No. 2013BAD10B02 and No. 2011BAD26B02), the Special Fund for Agro-scientific Research in the Public Interest in China (No. 201403047), and the AMP Direction of National Innovation Program of Agricultural Science and Technology in CAAS (2013~2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qingfeng Guan, Xiao Wang, and Xiumin Wang are equal contributors to this paper.
Rights and permissions
About this article
Cite this article
Guan, Q., Wang, X., Wang, X. et al. Recombinant outer membrane protein A induces a protective immune response against Escherichia coli infection in mice. Appl Microbiol Biotechnol 99, 5451–5460 (2015). https://doi.org/10.1007/s00253-014-6339-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6339-6