Abstract
This paper investigated the characteristics of Cu(II) sorption from aqueous solution by using bioflocculant MBFR10543 and discussed the mechanism during the sorption process. Results have demonstrated that the removal efficiency of Cu(II) reached 96.9 % by adding MBFR10543 in two stages, separately, 1.5 × 10−2 % (w/w) in the 1.0-min rapid mixing (180 rpm) and 2.0 × 10−2 % (w/w) after 2.0-min slow mixing (80 rpm), with pH value fixed at 6.0. Cu(II) sorption process could be described by the pseudo-second-order kinetic model and the Langmuir isotherms model. The negative Gibbs free energy change indicated the spontaneous nature of the sorption. Fourier transform infrared spectra analysis indicated that functional groups, such as –OH, −COOH, C═O, and –NH2, were existed in MBFR10543 molecular chains, which had strong capacity for removing Cu(II). Furthermore, both charge neutralization and bridging being the main mechanisms involved in Cu(II) removal by MBFR10543.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well recognized that heavy metals discharged into the aquatic environment especially surface water, such as Cu(II), Zn(II), Pb(II), Cr(VI), and Cd(II), are hazardous to human and the other living organisms, owing to their accumulation in living tissues throughout the food chain as non-biodegradable pollutants, even if small quantities (Sarı et al. 2007). Thus, the removal of heavy metals from waters and wastewaters is extremely important. Cu(II) exists in the wastewater of many industries, like metal cleaning and plating, electrical industry, chemical catalysis, and the fertilizer industry, is known to be one of the heavy metals most toxic to living organisms, whose pollution is of major concern (Ghaed et al. 2013). The presence of Cu(II) in drinking water in high concentrations can cause several physiological and health problems or even death (Han et al. 2006). Thus, the removal of excess Cu(II) from wastewater is of great significance for protecting human health as well as the environment.
The commonly used processes to remove excessive Cu(II) from wastewater are precipitation, ion exchange, electrolysis, adsorption on activated carbon, and membrane processes (Benaïssa and Elouchdi 2011). For example, the maximum adsorption capacity of the activated carbon with zinc chloride activation at 973 K in nitrogen atmosphere for Cu(II) was found to be 6.6 mg g−1 (Immamuglu and Tekir 2008). Cu(II) removal from lithium bromide refrigerant was successfully achieved by pretreatment with Ba(OH)2 solution followed by electrocoagulation using carbon steel plates as electrodes, and the residual Cu(II) concentration was 2.1 mg L−1 under the optimal electrocoagulation conditions (Cheng 2006). However, these methods are either inefficient or expensive when Cu(II) exist in low concentrations especially less than 100 ppm (Ahluwalia and Goyal 2007). As a result of this problem with the aforementioned solutions, it has in recent years led to a growing interest in the application of biological materials and technology for removal of trace amounts of toxic metals from dilute aqueous wastes, due to their advantages of minimization of chemical and/or biological sludge volumes and high efficiencies of detoxifying effluents (Blázquez et al. 2011).
Biosorption, which utilizes the ability of biological materials to bind and sequester heavy metals from aqueous solutions, is currently considered one of the most promising technologies that can be used in the recovery of precious metals that are mostly regarded as toxic pollutants (Velmurugan et al. 2010). Biological materials including algae, bacteria, fungi, plants, and products derived from these organisms. Some of them such as fungus Aspergillus niger and seaweeds have shown potential for the removal of heavy metal (Vilar et al. 2008). Microbial bioflocculants (MBFs), secreted by microorganisms during their active secretion and cell lysis, is a kind of environment friendly material with the character of harmless and biodegradable, which has been considered as a potential solution to the toxicity to aquatic life and environment pollution in recent years (Liu et al. 2010; Sun et al. 2012). Broadly, due to the special properties (adsorption capability and degradability), MBFs have attracted wide attention in wastewater treatment, drinking water purification and downstream processes in biotechnology, and so on (You et al. 2008). Depending on the chemical composition and structure of the MBFs that are complex mixtures of macromolecular polyelectrolytes, they exhibit ability to bind with metal ions. Therefore, it is interesting to investigate the possible application of MBFs to remove metal ions. For example, removal of Cu(II) from aqueous solutions by Bacillus and Aspergillus species (Ghaed et al. 2013), biosorption of Cu(II) and Cd(II) from aqueous solutions using Botrytis cinerea fungal byproducts (Akar et al. 2005).
Based on the aforementioned facts, the bioflocculant MBFR10543, a product secreted by Rhodococcus erythropolis using alkaline-thermal (ALT) pretreated sludge as nutrients, was applied in the Cu(II) removal from aqueous solution. The main objective was to investigate the effectiveness of MBFR10543 in Cu(II) sorption and to explore the mechanism involved. Cu(II) removal potential was evaluated as functions of MBFR10543 dose, CaCl2 dose, solution pH, and contact time. Following the Cu(II) sorption experiment, kinetics, isotherms, and thermodynamics were described, and the interactions between MBFR10543 and Cu(II) were investigated by Fourier transform infrared spectra (FT-IR) and environmental scanning electron microscope (ESEM) analysis to determine the sorption characteristics. Bonding mechanism was detected by adding 20 mL EDTA (3.0 mol L−1) or CH4N2O (3.0 mol L−1) into the stable Cu(II)-loaded MBFR10543 systems. Further, the zeta potential during the sorption process was also monitored to propose the sorption mechanism.
Materials and methods
Reagents
Cu (NO3)2·4H2O (Tianjin Hengxing Chemical Preparation Co., Ltd., China) was prepared by dilution of 1.0 g L−1 stock solution, and the fresh diluents were used in each experiment. CaCl2 (Tianjin Hengxing Chemical Preparation Co., Ltd., China) was prepared at the concentration of 5.0 g L−1. NaOH and HCl (Shanxi Sanpu Chemicals Reagent Co., Ltd., China) were prepared at the concentration of 1.0 mol L−1. EDTA and CH4N2O (Tianjin Fuchen Chemical Reagent Co., Ltd., China) were prepared at the concentration of 3.0 mol L−1. Unless otherwise stated, all reagents used were analytically pure.
Bacteria strain and bioflocculant MBFR10543
Bioflocculant-production strain, R. erythropolis, was deposited in China Center for Type Culture Collection (CCTCC) (No. ACCC.10543). Bioflocculant MBFR10543, a kind of microbial flocculant, was harvested from alkaline-thermal (ALT) pretreated sludge with suspended sludge solids concentration of 25 g L−1 by R. erythropolis; this sludge was collected from biofiltration unit at a swine wastewater treatment plant located in Fuhua pig farm, Hunan Province, China (Guo et al. 2014). Sludge treatments disintegrated the organic fractions and released soluble carbon into the sludge medium, which further changed bioflocculants secretion pattern and yields. Thus, before bioflocculant production, the sludge suspensions were treated by sterilization (ST), alkaline-thermal (ALT) and acid-thermal (ACT) treatments, respectively. Sterilization was carried out by autoclaving (steam sterilization) at 121 °C for 30 min. In ALT treatment, first, pH value of the sludge was raised to 10 using 1.0 mol L−1 NaOH at room temperature (25 °C) and then autoclaved in the same procedure. In ACT treatment, first, pH value was reduced to 2.0 using 1.0 mol L−1 HCl at room temperature (25 °C) and then autoclaved in the same procedure. After autoclaving, pH value of all the sludge samples were adjusted to 7.0 using 1.0 mol L−1 HCl or NaOH. In our previous study, it is clearly that the three pretreatments could disintegrate the sludge and the effectiveness of alkaline treatment was the maximum, due to the maximum solubilization chemical oxygen demand (SCOD) release (Table 1). Besides this, the suspended solids (SS) and volatile suspended solids (VSS) of sludge were decreased by the treatments and the effectiveness of alkaline treatment was the best and that of acid treatment was the worst (Table 1). Maximum bioflocculant of 2.9, 4.1, and 1.8 g L−1 were harvested from fermented broths of ST, ALT, and ACT sludge, while the control sample (without inoculating) had a very low bioflocculant concentration of less than 0.15 g L−1. Thus, the specific carbon and nutrients content released in the ALT sludge medium was more favorable to bioflocculants secretion as compared to that of ST and ACT sludge, and the bioflocculant from ALT pretreated sludge was utilized directly in the sorption of Cu(II). The thermal and enzymatic stability results indicated that the main backbones of the bioflocculant from ALT pretreated sludge were protein rather than polysaccharide. In addition, chemical analysis of the bioflocculant also revealed that the activity ingredients were protein (Guo et al. 2014).
Flocculation tests
A standard Jar Tester was used for the flocculation tests in Cu(NO3)2 solution dosed with MBFR10543. CaCl2 and MBFR10543 (prepared as liquid of 1.0 g L−1 using the purified MBFR10543) were added into 1.0 L of Cu(II) solution at concentration of 100 mg L−1 in 1.0 L beaker in turn and then fixed on the floc-tester (ET-720, Lovibond, Germany) (Gao et al. 2011; Guo and Yu 2014). The pH of the mixture was adjusted using 1.0 mol L−1 NaOH or HCl. MBFR10543 was added in two stage into the Cu(II) solution, separately, before rapid mixing (first stage) and after 2-min slow mixing (second stage). Flocculation was the first stage, began with 1.0-min rapid mixing at 180 rpm and ended with 2.0-min slow mixing at 80 rpm, and at last, the mixture was allowed to stand for 60 min to establish its equilibrium. The slow mixing after the first stage was the second stage, about sorption. After the two-stage stirring, the mixture was allowed to stand to establish its equilibrium. The influence of the flocculation parameters, including solution pH, doses of MBFR10543, and CaCl2, were investigated by analyzing the removal efficiency of Cu(II) and the zeta potentials of the flocculation systems. The concentrations of Cu(II) were determined by flame atomic absorption spectrometry (Modle Analyst 700, PerkinElmer, USA) after being filtered by 0.45-sμm filter membrane. The experiments were performed at room temperature (25 °C). The removal efficiency (RE) and removal capacity of Cu(II) was calculated as follows:
where C 0 and C e (mg L−1) are the initial and equilibrium Cu(II) concentrations, respectively. V (L) is the volume of the Cu(II) solution and W MBFR10543 (g) is the weight of MBFR10543 used.
Following Cu(II) sorption experiments, kinetics, isotherms, and thermodynamics were described, and the bonding mechanism was detected by adding 20 mL EDTA (3.0 mol L−1) or CH4N2O (3.0 mol L−1) into the stable Cu(II)-loaded MBFR10543 systems. The variation of zeta potential during the process of flocculation was monitored by Zetasizer 2000 (Malvern Instruments Ltd., Company, England). Samples were carried out at different time points: Cu(II) solution at pH 6.0, after adding MBFR10543, after adding CaCl2, and during flocculation process. All experiments were performed in triplicates for the mean calculation.
Kinetics, isotherms, and thermodynamics studies
Kinetic models were always used to investigate the characteristics of sorption and its potential rate-controlling steps that include mass transport and chemical reaction processes (Yahaya et al. 2009). In addition to clarifying the sorption kinetics of Cu(II) onto MBFR10543, two kinetic models, pseudo-first-order and pseudo-second-order were applied, and the equations were determined by Eqs. (3) and (4).
where q e and q t (mg g−1) are the equilibrium sorption capacity of Cu(II) and the amount of adsorbed Cu(II) at time t. k 1 (min−1) and k 2 (g mg−1 min−1) are the rate constants of the pseudo-first-order and pseudo-second-order models, respectively.
Kinetic studies were conducted by contacting 1.5 × 10−2 + 2.0 × 10−2 % (w/w) MBFR10543 into 1.0 L Cu(II) solution at different initial concentrations (80, 100, and 120 mg L−1) with the samples stirring for designated time. The experiments were performed at temperature of 25 °C and were carried out in triplicate and the average values were presented (with standard error less than 5 % of the mean).
Isotherm fitting with model equations is a key issue to explore flocculation mechanisms, and the Langmuir and Freundlich models were applied to understand the sorption behavior of Cu(II) during Cu(II) sorption process (Sarı and Tuzen, 2008; Sarı et al. 2008). The Langmuir isotherm assumes that adsorption occurs at specific homogeneous sites within the adsorbent and can be saturated as Eq. (5).
where q m (mg g−1) is the maximum sorption capacity of Cu(II). k L (L mg−1) is the Langmuir constant which is related to the affinity of the binding sites.
The essential feature of the Langmuir isotherm can be expressed by means of a separation factor or equilibrium parameter R L, which is calculated by the Eq. (6). There are four probabilities for the R L value: (i) for favorable exchange, 0 < R L < 1; (ii) for unfavorable exchange, R L > 1; (iii) for linear exchange, R L = 1; and (iv) for irreversible exchange, R L = 0 (Riahi et al. 2009).
The Freundlich isotherm is based on multilayer sorption by assuming that the adsorbent has a heterogeneous surface with nonuniform distribution of sorption sites, and the liner form of the Freundlich isotherm equation is normally given as Eq. (7).
where k f and 1/n represent the Freundlich capacity coefficient and the Freundlich intensity parameter, respectively, and 1/n is also known as the heterogeneity factor.
Isotherm studies were conducted by contacting 1.5 × 10−2 + 2.0 × 10−2 % (w/w) MBFR10543 into 1.0 L Cu(II) solution at concentration of 100 mg L−1 with the solution stirring in two stages. The experiments were performed at different temperatures (25, 35, and 45 °C) by water bath. Sorption isotherms are plots of the equilibrium sorption capacity (q e) (according to Eq. (2)) versus the equilibrium concentration of the residual Cu(II) in the solution (Ce). The equilibrium uptake was calculated by Eqs. (8) and (9).
where C f (mg L−1) is the concentration of Cu(II) after the first stage. W (g) is the weight of adsorbent. All the batch experiments were carried out in triplicate, and the average values were presented (with standard error less than 5 % of the mean).
Effects of sorption temperature on the flocculation of Cu(II) were given from the calculated thermodynamic parameters, and the thermodynamic data such as enthalpy (ΔH°), free energy change (ΔG°), and entropy (ΔS°) can be calculated by Eqs. (10)–(12).
where K is the equilibrium constant (L g−1). R is the universal gas constant (8.314 J mol−1 K−1) and T is the temperature (K).
Fourier transform-infrared spectra and environmental scanning electron microscope analysis
To investigate the characteristics of the flocculation process including the mechanism involved, the FT-IR and ESEM profiles were studied. Fourier transform-infrared spectrometer (EQUINOX 55, Bruker Company, Germany) was employed to examine the interactions between the Cu(II) and functional groups on MBFR10543. Original samples and flocs which MBFR10543 flocculated Cu(II) under the optimal experimental conditions were collected, followed by vacuum freeze-drying. The samples were ground well to make KBr pellets under hydraulic pressure of 400 kg cm2, and spectra were recorded in the range of 400–4000 cm−1. In each scan, the amounts of the sample and KBr were kept constant in order to know the changes in the intensities of characteristic peaks with respect to the structural changes. ESEM (Quanta 200 FEG, FEI, USA) was applied to analyze the surface morphology of original and Cu(II)-loaded MBFR10543 in low vacuum mode at an acceleration potential of 20 keV. Furthermore, microanalysis of the Cu(II)-loaded MBFR10543 was carried out with an energy dispersive spectrometer (EDS) equipped on the Quanta 200.
Results
Removal efficiency and sorption capacity in different experiment conditions
Effects of MBFR10543 and CaCl2 doses on the flocculation behavior
The effects of MBFR10543 dose on the flocculation of Cu(II) was investigated by adding different mass fraction of MBFR10543 and 4.0 × 10−3 % (w/w) CaCl2 into 1.0 L Cu(II) solution (100 mg L−1) with pH value fixed at 6.0. MBFR10543 was added before rapid mixing (first stage) and after 2-min slow mixing (second stage). As can be seen from Fig. 1a, at the first stage, the removal efficiency of Cu(II) increased to 64.7 % when 1.5 × 10−2 % (w/w) MBFR10543 was added and then decreased with increasing addition of MBFR10543. Less dose of MBFR10543 meant less MBFR10543 molecules adsorbed Cu(II), and fewer bridges were developed between them. Further increase of MBFR10543 led to a drastic decrease of the removal efficiency of Cu(II), for the reason that more doses of MBFR10543 would inhibit small flocs to grow into big ones. As a result of stronger repulsion force between flocs, they could be de-flocculated. After the first stage, the residential concentration of Cu(II) was still high (35.3 mg L−1), so MBFR10543 is needed to be added continuously based on the preliminary tests. At the second stage, the removal efficiency of Cu(II) reached about 96.8 % when the addition MBFR10543 was adjusted to 2.0 × 10−2 % (w/w). The removal capacity showed the same tendency to removal efficiency after the second stage, and a maximum value of 276.6 mg g−1 was achieved. Like the sorption of Pb(II), the dose of MBFR10543 should be optimized because more or less dose would be unfavorable to the flocculation (Guo and Yu 2014).
The effect of CaCl2 on the flocculating efficiency of Cu(II) was investigated by adding different volume of CaCl2 and 1.5 × 10−2 + 2.0 × 10−2 % (w/w) MBFR10543 into 1.0 L Cu(II) solution (100 mg L−1) with pH value fixed at 6.0. Figure 1b indicated that the removal efficiency of Cu(II) was low without adding CaCl2. With further addition of CaCl2, the removal efficiency of Cu(II) increased, and the maximum value of 276.9 mg g−1 was achieved after 4.0 × 10−3 % (w/w) CaCl2 added. Thus, during the flocculating process, as a kind of coagulant aid, Ca2+ increased the initial sorption capacity of MBFR10543.
Effect of solution pH on the flocculation behavior
pH value was an important factor on the flocculation of Cu(II) for both solution chemistry of Cu(II) and surface characteristics of biosorbent. Firstly, since metal ions can have different speciation forms at different pH. The distribution diagrams of copper species as a function of pH show that only cationic species (Cu2+, Cu(OH)+) are present in solution over the range of 3.0–6.0. Secondly, the surface of the biosorbent consists of biopolymers with many functional groups, and the net charge on biosorbent, is also pH dependent (Zhu et al. 2008). So the removal of Cu(II) was highly dependent on the pH value of aqueous solution. The pH optimization study of Cu(II) sorption onto MBFR10543 was carried out by adding 4.0 × 10−3 % (w/w) CaCl2 and 1.5 × 10−2 + 2.0 × 10−2 % (w/w) MBFR10543 into 1.0 L Cu(II) solution (100 mg L−1), and the results were presented in Fig. 1c. In order to avoid the precipitation in alkaline condition, the sorption of Cu(II) was carried out in acid condition (pH 2.0–7.0) in this study. As shown in Fig. 1c, the Cu(II) removal is positively correlated with the increase of pH value in the solution, reaching a maximum sorption of 96.9 % (276.9 mg g−1) at pH 6.0. Similar values of optimum pH (5.0) for Cu(II) sorption are reported in the literature using dried activated sludge (Benaïssa and Elouchdi 2011). Additionally, the removal capacity of MBFR10543 for Cu(II) (276.9 mg g−1) was higher than those of some other sorbent that have been reported (2.8–146.3 mg g−1) previously (Table 2). Therefore, it could be noteworthy that MBFR10543 has important potential for the removal of Cu(II) from aqueous solution.
Additionally, a slight decrease in the pH value (ΔpH = pH0 − pHe = 0.22 unit) of the solution between the initial and equilibrium time was observed during Cu(II) sorption process by MBFR10543. In order to investigate the reason, experiments were performed with MBFR10543 tested in distilled water under the same conditions in absence of Cu(II), and the pH value of solution exhibited an increase (ΔpH = pHe − pH0 = 0.64 unit) that can be interpreted as a possible fixation of H3O+ by the negative groups present on the surface of MBFR10543. Concerning the decrease in the pH value in the presence of Cu(II), it can be interpreted as a possible release of H3O+ into the solution due to Cu(II) sorption. The same tendencies were observed with other sorbent-metal ions system (Benaïssa 2006).
Effects of contact time on the flocculation behavior
Figure 1d showed the effects of contact time on the sorption of Cu(II) onto the bioflocculant MBFR10543 by adding 4.0 × 10−3 % (w/w) CaCl2 and 1.5 × 10−2 + 2.0 × 10−2 % (w/w) MBFR10543 into 1.0 L Cu(II) solution (100 mg L−1) with pH value fixed at 6.0. It can be seen that the sorption yield of Cu(II) increased with rise in contact time up to 60 min within the studied temperature range (25, 35, and 45 °C). Maximum sorption of 96.9 % was attained when the contact time was up to 60 min at 25 °C, and afterwards, there was almost no significant increase in Cu(II) sorption. Thus, this time value was selected as optimum contact time for Cu(II) sorption from the aqueous solution by the bioflocculant MBFR10543. The almost same contact time was reported in earlier works which related with the sorption of metal ions on various biomasses (Sarı et al. 2007; Saygideger et al. 2005).
Kinetics, isotherms, and thermodynamics parameters
The model rate constants, correlation coefficients, and calculated q e values of the two kinetics models for the sorption of Cu(II) onto MBFR10543 at various initial concentrations were tabulated in Table 3. It was found that correlation coefficients values of pseudo-first-order equation were relatively low within the studied Cu(II) concentrations’ range, indicating the bad linearization, yet these of pseudo-second-order equation were extremely high and all greater than 0.99. Besides, the calculated q e values of pseudo-second-order equation matched very well with the experimental data (Table 3). These results suggested that the sorption of Cu(II) onto MBFR10543 followed well the pseudo-second-order model. Thus, experiment results supported the assumption that the rate limiting step in sorption of heavy metals are chemisorption involving valence forces through the sharing or exchange of electrons between adsorbent and metal ions (Feng et al. 2013). Additionally, it is worthwhile to note that k 1 and k 2 have higher values at a higher initial Cu(II) concentration, which leads to a higher driving force for the sorption of Cu(II) hence a higher sorption capacity.
The isotherms data were obtained by linear regression method and were summarized in Table 4. Within the studied temperature range (25, 35, and 45 °C), it appears that the Langmuir model acceptably fits the experimental results over the experimental range with good coefficients of regression (based on the higher correlation coefficient, i.e., R 2 values). From Langmuir model, the q m values decreased with the rising temperature; this suggested the affinity between the active sites of the MBFR10543 and Cu(II), and also between the adjacent molecules of the adsorbed phase would be stronger at higher temperature in comparison to lower temperature (Feng et al. 2013; Ghorai et al., 2012). Additionally, all R L values obtained were between 0 and 1 indicated the removal of Cu(II) by MBFR10543 was propitious; this implied that the Langmuir equation was able to properly describe the equilibrium isotherm of Cu(II) adsorbed onto MBFR10543. The fact that the data did not fit well with the Freundlich isotherm indicates that Cu(II) removal is more like a monolayer surface sorption process with finite number of identical sites, which were homogeneously distributed on MBFR10543 surface (Guo and Yu 2014).
The effects of temperature on the sorption of Cu(II) onto MBFR10543 was given from the calculated thermodynamic parameters (Table 5). It is clearly that the values of ΔG° were negative at various temperatures (−9.08, −9.38, and −10.53 kJ mol−1 at 25, 35, and 45 °C, respectively) with Cu(II) concentration of 100 mg L−1, confirming that the sorption of Cu(II) onto MBFR10543 was spontaneous in nature and thermodynamically favorable, and no energy input to the system is required. It is worth noting that ΔG° values became more negative as temperature increased, the increase in ΔG° values with increase temperature showed an increase in driving force for Cu(II) sorption at higher temperature. The positive values of ΔH° indicated that the sorption was an endothermic process in the temperature range from 25 to 45 °C. The positive values of ΔS° confirmed the increased randomness at solid/solution interface during the flocculation process (Feng et al. 2013; Guo and Yu 2014).
Flocculation mechanism
Fourier transform-infrared spectra analysis
The studies so far indicated that MBFR10543 can be successfully used for the removal of Cu(II) from aqueous solution. An effort was made to identify the components of the MBFR10543 that are responsible for Cu(II) removal by Fourier transform-infrared spectra analysis. The FT-IR spectrum of Cu(II)-free form showed several distinct and sharp peaks at 3382 cm−1 (indicative of –OH and –NH2 functional groups), 2933 cm−1 (indicative of C–H groups), 1651 cm−1 (indicative of –C=O groups), 1459 and 1370 cm−1 (indicative of the bending of –CH3), 1268 cm−1 (indicative of C–N groups in amide band of the protein peptide bond), 1130 cm−1 (indicative of C–O–C groups), 1056 cm−1 (indicative of C–O groups) (Feng et al. 2013; Gao et al. 2011). In conclusion, the FT-IR spectrum of the Cu(II)-free MBFR10543 showed the presence of hydroxyl, carboxyl, acetyl, and amine groups which played important roles in Cu(II) removal. The significant shifts of the wave number and intensity of these specific peaks after Cu(II) sorption from the spectra of Cu(II)-loaded MBFR10543, suggesting the aforementioned functional groups were mainly involved in the sorption of Cu(II) onto MBFR10543 (Sarı et al. 2007). After loading Cu(II), the significantly enhanced peak intensity at 3382 cm−1 after the Cu(II) sorption suggested that chemical interactions between the Cu(II) and –OH and/or –NH2 occurred on the MBFR10543 surface. The reduced peak intensity between 1267 and 1022 cm−1 after the Cu(II) sorption suggested the reduction of the electron density of the oxygen-containing functional groups, further changed their vibration frequency and intensity. The shifts of C═O from 1651 to 1655 cm−1 in the Cu(II)-loaded MBFR10543 can be assigned to the carboxylate ion (−COO−). The shifts of C–N and the emerging of the peak at 1550 cm−1 (C═O) implied the involvement of amide groups in the flocculation process of Cu(II). All the conspicuous change in the FT-IR spectrum demonstrated that the chemical interactions, between Cu(II) and the amine, carboxyl, and hydroxyl groups of MBFR10543, were mainly involved in the sorption process of Cu(II). The results were in good agreement with those obtained by other researchers (Yahya et al. 2012), which all came to the conclusion that functional groups responsible for Cu(II) removal were mainly hydroxyl, carboxyl, acetyl, and amine groups.
Environmental scanning electron microscope analysis
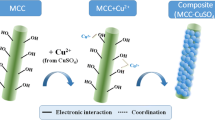
To investigate the flocculation process further, the original and Cu(II)-loaded MBFR10543 were observed under the ESEM (Fig. 2). Micrographs showed that the surface and the texture of Cu(II)-loaded MBFR10543 became rougher and more compact compared with the Cu(II)-free MBFR10543 prepared by vacuum freeze drying, which characterized a stronger sorption capacity. Additionally, the surface of Cu(II)-free MBFR10543 is of the irregular shape and size, which was suggested to be an appropriate structure for metal ion adsorption. The additional bright spots seen in the Cu(II)-loaded MBFR10543 are adsorbed Cu(II), which were verified by the EDX results. EDS analysis provided the evidence that there were C, O, S, and K in the Cu(II)-free MBFR10543, but with no Cu(II). Content of 48.7 % in the detection area of Cu(II)-loaded MBFR10543 and appearance of Cu(II) signal at about 1.8, 3.6, and 8.1 keV presented in Table 6 clearly demonstrated the presence of Cu(II) due to the sorption onto MBFR10543 and a strong coordination linkage between Cu(II) and functional groups involved in MBFR10543.
Bonding and flocculation mechanism
MBFR10543 participates in the flocculation mainly through hydroxyl, carboxyl, and amine groups which induce very high binding capacity. In general, addition of EDTA and CH4N2O in the stable Cu(II)-MBFR10543-Ca2+ systems could destroy the ionic bonds or hydrogen bonds in the system, respectively. In this study, after addition of EDTA (3.0 mol L−1) into the stable system, the flocs dissolved gradually and became cloudy. While there was nothing happened by adding CH4N2O (3.0 mol L−1). The results indicated that ionic bond combination was the main way of combining between MBFR10543 and Cu(II), whereas the hydrogen bonding interaction was not obvious.
Zeta potential and flocculation mechanism
The removal efficiency and zeta potential of Cu(II) during flocculation process were shown in Fig. 3. At the first stage, the removal efficiency of Cu(II) increased rapidly, and then it increased steadily and reached equilibrium (96.9 %) after the second stage. The pH played an important role in the process. MBFR10543 had a linear long chain molecular structure and appeared to have a molecular weight of 4.21 × 105 Da (Guo et al. 2014). With the increasing pH value of below 7.0, the MBFR10543 molecules stretch form a linear structure so that more of the active binding sites emerged, and the removal capacity of Cu(II) was higher. It was found that the zeta potential of Cu(II) solution was about 37.8 mV at pH 6.0 without Ca2+ and MBFR10543 additions, which turned to be 18.4 mV after adding 1.5 × 10−2 % MBFR10543 alone (without Ca2+) and then 11.2 mV when 4.0 × 10−3 % CaCl2 was added. As shown in Fig. 3, after the first stage, the zeta potential appeared to be 9.5 mV. The addition of MBFR10543 aroused a big reduction of about 24.7 mV in the zeta potential of Cu(II) solution after the 1-min stirring indicated that the charge neutralization would not be the main Cu(II) removal mechanism in this stage. Ca2+ reduced the thickness of the diffuse double layer of adjacent colloids, thus reducing the inter-particle distance and making MBFR10543 attract more Cu(II) around its surface and small flocs formed. The main Cu(II) removal mechanism involved in the second stage was sorption, in which bridging occurred after Cu(II) and Ca2+ adsorbed onto MBFR10543. The fact that bridging played a major role during the second stage was confirmed by the flocculation happened at a zeta potential of about −22.6 mV. Many flocs could be adsorbed onto a long molecular chain; simultaneously, they could be absorbed by other polymer chains. Thus, flocs formed three-dimensional structure with a better settling capacity.
Discussion
The MBFR10543 appears as an effective and alternative microbial flocculant to be used for the removal of Cu(II) from wastewaters, and the maximum sorption capacity of 276.9 mg g−1 can be reached when MBFR10543 was added in two stages, separately. And the maximum sorption was attained when the contact time was up to 60 min. For Cu(II) sorption by the bioflocculant MBFR10543, Ca2+ could increase the sorption capacity by decreasing the negative charge on the polymer. It has also been reported to develop bridges between anionic polyelectrolytes and negatively charged colloidal particles, thereby enhancing particle flocculation (Lee et al. 2012). These two mechanisms have been detected in our previous studies, flocculating activity of kaolin clay suspension was enhanced to 94.5 % by adding 20 mg of bioflocculant in presence of 0.5 g Ca2+, while a flocculating activity of 44.7 % was achieved when 20 mg of the bioflocculant was added into kaolin suspension (1.0 L) alone and only 17.2 % reached when 0.5 g Ca2+ was added alone (Guo et al. 2014).
The behavior of Cu(II) as a function of water’s pH can be explained by considering the change in density of hydrogen ions (H+) and the surface charge of MBFR10543. At pH values lower than 6.0, the sorption of Cu(II) was in the range of 62.8–96.9 % and decreased with the decreasing solution pH value, which may be attributed to the increased density of H+ corresponding to the decreasing pH value that could strongly compete with Cu(II) for the active sites, resulting in less sorption. With increasing pH from 3.0 to 6.0, electrostatic repulsions between Cu(II) and surface sites and the competing effect of hydrogen ions decrease, and consequently, the Cu(II) sorption increases. Furthermore, the activity of the functional groups present in the MBFR10543 that are responsible for Cu(II) sorption. Cu(II) removal was inhibited because the functional groups would be protonated at high acidic pH (low solution pH), thereby the strong resistance against Cu(II) binding. In contrast, when pH increases, more functional groups would be exposed (the active sites in MBFR10543) and efficiently release their protons into the solution to carry negative charges to enhance the sorption capacity for Cu(II). Beyond pH 6.0, insoluble Cu(OH)2 starts precipitating resulting in lower amount of Cu(II) sorbed at equilibrium. Other authors have found the same trend for Cu(II) sorption by other sorbent materials (Ofomaja and Naidoo 2011). The effects of pH can also be explained in terms of pHpzc of the MBFR10543 (Yuvaraja et al. 2014). The pHpzc is an important characteristic for adsorbents to determine the pH at which the surface has net electrical neutrality. Surface charge of MBFR10543 is positive which results in low Cu(II) removal at water pH lower than pHpzc, and it is negative at water pH greater than pHpzc for attachment of Cu(II) to the surface to a greater extent. The pHpzc value of the MBFR10543 is found to be 2.5. Meanwhile, the surface charge of MBFR10543 determined by measuring its zeta potential at different pH values showed that zeta potential decreased from +3.93 to −27.80 mV, which was corresponding to the increase in pH from 2.0 to 7.0. This clearly indicated that at low pH values, surface being positively charged would not be favorable for the attachment of positively charged Cu(II) due to electrostatic repulsion. With the increase of pH values, the cell surface became more negatively charged, favorable for Cu(II) removal. That is, at low pH values, increased positive charge (protons) density on the sites of the bioflocculant surfaces restricted the approach of Cu(II) as a result of repulsive force. In contrast, as the pH values increased, bioflocculant surfaces were more negatively charged and subsequently, sorption of the Cu(II) with positive charge was reached maximum around pH 5.0–7.0 (Karthikeyan et al. 2007).
To further investigate the reason for pH dependence of removal of Cu(II) by MBFR10543, FT-IR studies on the basis of the surface charge density at different pH values were performed for the original (Cu(II)-free form) and Cu(II)-loaded MBFR10543. Results have demonstrated that carboxyl, hydroxyl, and amine groups were mainly responsible for Cu(II) removal. The negative charge between pH 3.0 and 4.0 could develop due to the carboxyl group, whereas hydroxyl group was responsible for negative charges at pH values of 4.0–7.0. The positive charge at low pH (<3.0) might be attributed to the amine group on the MBFR10543. As the acidity decreased in the solution, the deprotonation of acid functional groups are strengthened, and in consequence, the numbers of attractions will increase between negative charge on MBFR10543 and Cu(II). Dissociation of the hydroxyl and carboxylic acid groups was at pKa of 8.0–12.0 for hydroxyl and 3.8–5.0 for carboxylic groups (Pehlivan et al. 2009). Protonation of NH2 to form NH3 + was at lower pH. At pH greater than 4.0, the carboxylic acid group is converted to the –COO− and Cu(II) is adsorbed. The involvement of carboxylic acid groups in the flocculation process explains the effect of pH on Cu(II) removal (Section “Effect of solution pH on the flocculation behavior”). With the decreasing pH of below 4.0, majority of the carboxylate ions are converted to carboxylic acid groups (−COOH) and hence the removal efficiency decreases (Gupta and Rastogi 2008). As more NH2 groups were protonated to NH3 + form, there were only fewer NH2 binding sites on the surface of MBFR10543 for Cu(II) sorption (Hao et al. 2010). The functional groups such as –OH, −COOH, and –NH2 groups interaction with Cu(II) are shown as follows (Gao et al. 2011; Guo and Yu 2014):
According to the studies of kinetics, isotherms, and thermodynamics, Cu(II) sorption process could be described by the pseudo-second-order kinetic model and the Langmuir isotherms model based on the higher correlation coefficient, i.e., R 2 values. Moreover, the negative Gibbs free energy change indicated the spontaneous nature of the sorption, and the increase in ΔG° values with increase temperature showed an increase in driving force for Cu(II) sorption at higher temperature.
EDTA (3.0 mol L−1) should be able to compress the electrical double layer of the colloidal particles, and bridging occurred between EDTA and MBFR10543, and thereby, the cloudy Cu(II)-MBFR10543-Ca2+ systems could be restabilized. However, the experiment results showed that the cloudy systems did not settle in 30 min, indicating Ca2+ played a binding role of the bridging in the flocculation process. After addition of the EDTA, the ionic bonds in the bridging were destroyed and the stable system became cloudy due to the strong coordination between EDTA and Ca2+ (complex stability constant logβ 1 as high as 11.0). If there were hydrogen bonds in the bridging, they could be destroyed in the CH4N2O solution, and new hydrogen bonds formed, and thereby, the stable system became cloudy. After addition of 3.0 mol L−1 CH4N2O, there was no significant de-flocculation phenomenon, indicating that the hydrogen bonding interactions was not obvious.
All in all, the MBFR10543 appears as an effective and alternative microbial flocculant to be used for the removal of Cu(II) from wastewaters by adding in two stages, separately, and the maximum Cu(II) sorption capacity of 276.9 mg g−1 can be reached. The flocculation of Cu(II) was completed by charge neutralization and bridging mechanism, in which MBFR10543 attract Cu(II) around its surface firstly by reducing the thickness of the diffuse double layer of adjacent colloids and thus reducing inter-particle distance by addition of Ca2+. And then slow mixing allowed the aggregates to combine two or more flocs to form larger floc particles, and precipitate as evidenced in the jar tests. Further, the FT-IR analysis illustrated that chemical reaction with carboxyl, hydroxyl, and amine functional groups also happened during flocculation process. Based on the results, MBFR10543 could be used as an efficient source of biomaterial to solve the serious problems caused by heavy metal ions pollution.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257
Akar T, Tunali S, Kiran I (2005) Botrytis cinerea as a new fungal biosorbent for removal of Pb(II) from aqueous solution. Biochem Eng J 25:227–235
Ang XW, Sethu VS, Andresen JM, Sivakumar M (2013) Copper(II) ion removal from aqueous solutions using biosorption technology: thermodynamic and SEM-EDX studies. Clean Technol Envir 15:401–407
Benaïssa H (2006) Screening of new sorbent materials for cadmium removal from aqueous solutions. J Hazard Mater B132:189–195
Benaïssa H, Elouchdi MA (2011) Biosorption of copper (II) ions from synthetic aqueous solutions by drying bed activated sludge. J Hazard Mater 194:69–78
Blázquez G, Mart MA, Dionisio-Ruiz E, Tenorio G, Calero M (2011) Evaluation and comparison of the biosorption process of copper ions onto olive stone and pine bark. J Ind and Eng Chem 17:824–833
Cheng HF (2006) Cu(II) removal from lithium bromide refrigerant by chemical precipitation and electrocoagulation. Sep Purif Technol 52:191–195
Feng J, Yang ZH, Zeng GM, Huang J, Xu HY, Zhang YY, Wei SM, Wang LK (2013) The adsorption behavior and mechanism investigation of Pb(II) removal by flocculation using microbial flocculant GA1. Bioresour Technol 148:414–421
Gao WC, Yang ZH, Huang J, Deng JH, Xu HY, Xie HM (2011) Optimization of response surface and the mechanism study of microbial flocculants on capturing Cu(II). Chin J Environ Eng 5:2111–2416
Ghaed S, Shirazi E, Marandi R (2013) Biosorption of copper ions by Bacillus and Aspergillus species. Adsorpt Sci Technol 31:869–890
Ghorai S, Sinhamahpatra A, Sarkar A, Panda AB, Pal S (2012) Novel biodegradable nanocomposite based on XG-g-PAM/SiO2: application of an efficient adsorbent for Pb2+ ions from aqueous solution. Bioresour Technol 119:181–190
Guo JY, Yu J (2014) Sorption characteristics and mechanisms of Pb(II) from aqueous solution by using bioflocculant MBFR10543. Appl Microbiol Biotechnol 98:6431–6441
Guo JY, Yang CP, Peng LY (2014) Preparation and characteristics of bacterial polymer using pre-treated sludge from swine wastewater treatment plant. Bioresour Technol 152:490–498
Gupta VK, Rastogi A (2008) Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414
Han RP, Li HK, Li YH, Zhang JH, Xiao HJ, Shi J (2006) Biosorption of copper and lead ions by waste beer yeast. J Hazard Mater 137:1569–1576
Hao YM, Man C, Hu ZB (2010) Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J Hazard Mater 184:392–399
Immamuglu M, Tekir O (2008) Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 228:108–113
Karthikeyan S, Balasubramanian R, Iyer CSP (2007) Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol 98:452–455
Lee BJ, Schlautman MA, Toorman E, Fettweis M (2012) Competition between kaolinite flocculation and stabilization in divalent cation solutions dosed with anionic polyacrylamides. Water Res 46:5696–5706
Liu WJ, Wang K, Li BZ, Yuan HL, Yang JS (2010) Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour Technol 101:1044–1048
Ofomaja AE, Naidoo EB (2011) Biosorption of copper from aqueous solution by chemically activated pine cone: a kinetic study. Chem Eng J 175:260–270
Pehlivan E, Altun T, Parlay S (2009) Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J Hazard Mater 164:982–986
Riahi K, Thayer BB, Mammou AB, Ammar AB (2009) Biosorption characteristics of phosphates from aqueous solution onto Phoenix dactylifera L. date palm fibers. J Hazard Mater 170:511–519
Sarı A, Tuzen M (2008) Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J Hazard Mater 160:349–355
Sarı A, Tuzen M, Uluozlu OD, Soylak M (2007) Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem Eng J 37:151–158
Sarı A, Mendil D, Tuzen M, Soylak M (2008) Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (Hylocomium splendens) biomass: Equilibrium, kinetic and thermodynamic studies. Chem Eng J 144:1–9
Saygideger S, Gulnaz O, Istifli ES, Yucel N (2005) Adsorption of Cd(II), Cu(II) and Ni(II) ions by Lemna minor L.: effect of physicochemical environment. J Hazard Mater 126:96–104
Sun J, Zhang XH, Miao XJ, Zhou JT (2012) Preparation and characteristics of bioflocculants from excess biological sludge. Bioresour Technol 126:362–366
Tsekova K, Ianis M, Dencheva V, Ganeva S (2007) Biosorption of binary mixtures of copper and cobalt by Penicillium brevicompactum. Z Naturforsch C 62:261–264
Tsekova K, Todorova D, Dencheva V, Ganeva S (2010) Biosorption of copper(II) and cadmium(II) from aqueous solutions by free and immobilized biomass of Aspergillus niger. Bioresour Technol 101:1727–1731
Velmurugan N, Hwang G, Sathishkumar M, Choi TK, Lee KJ, Oh BT, Lee YS (2010) Isolation, identification, Pb(II) biosorption isotherms and kinetics of a lead adsorbing Penicillium sp. MRF-1 from South Korean mine soil. J Environ Sci 22:1049–1056
Vilar VJ, Loureiro JM, Botelho CM, Boaventura RA (2008) Continuous biosorption of Pb/Cu and Pb/Cd in fixed-bed column using algae Gelidium and granulated agar extraction algal waste. J Hazard Mater 154:1173–1182
Yahaya YA, Don MM, Bhatia S (2009) Biosorption of copper (II) onto immobilized cells of Pycnoporus sanguineus from aqueous solution: equilibrium and kinetic studies. J Hazard Mater 161:189–195
Yahya SK, Zakaria ZA, Samin J, Raj ASS, Ahmad WA (2012) Isotherm kinetics of Cr(III) removal by non-viable cells of Acinetobacter haemolyticus. Colloids Surf B: Biointerfaces 94:362–368
You Y, Ren N, Wang A, Ma F, Gao L, Peng Y, Lee D (2008) Use waste fermenting liquor to produce bioflocculants with isolated strains. Int J Hydrogen Energy 33:3295–3301
Yuvaraja G, Krishnaiah N, Subbaiah MV, Krishnaiah A (2014) Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf B: Biointerfaces 114:75–81
Zhu B, Fan T, Zhang D (2008) Adsorption of copper ions from solution by citric acid modified soybean straw. J Hazard Mater 153:300–308
Acknowledgments
The authors are thankful to the project (KYTZ201405) supported by the Scientific Research Foundation of Chengdu University of Information Technology (CUIT), and the National Natural Science Foundation of China (Grant No. 51408076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, J. Characteristics and mechanisms of Cu(II) sorption from aqueous solution by using bioflocculant MBFR10543. Appl Microbiol Biotechnol 99, 229–240 (2015). https://doi.org/10.1007/s00253-014-6103-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6103-y