Abstract
The present study has been focused widely on comparative account of probiotic qualities of Bacillus spp. for safer usage. Initially, 170 heat resistant flora were isolated and selected for non-pathogenic cultures devoid of cytK, hblD, and nhe1 virulence genes. Subsequently, through biochemical tests along with 16S rRNA gene sequencing and fatty acid profiling, the cultures were identified as Bacillus megaterium (AR-S4), Bacillus subtilis (HR-S1), Bacillus licheniformis (Csm1-1a and HN-S1), and Bacillus flexus (CDM4-3c and CDM3-1). The selected cultures showed 70–80 % survival under simulated gastrointestinal condition which was also confirmed through H+-ATPase production. The amount of H+-ATPase increased by more than 2-fold when grown at pH 2 which support for the acid tolerance ability of Bacillus isolates. The study also examined the influence of acidic pH on cellular fatty acid composition of Bacillus spp. A remarkable shift in the fatty acid profile was observed at acidic pH through an increased amount of even numbered fatty acid (C16 and C18) in comparison with odd numbered (C15 and C17). Additionally, the cultures exhibited various probiotic functional properties. Overall, the study increases our understanding of Bacillus spp. and will allow both industries and consumers to choose for well-defined probiotic with possible health benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of probiosis has emerged as a new science primarily as dietary supplements for animals and for the prevention of gastrointestinal disease, as well as prophylactic in human and aquaculture. Probiotics are defined as “live microorganisms when administered in adequate amounts confer a health effect on the host” (FAO/WHO 2002), which implies that demonstration of some health benefits is necessary criteria to be designated as “probiotic.” Currently, lactic acid bacteria (LAB) and bifidobacteria are well-studied probiotics that manifest beneficial properties including disease treatment and prevention, as well as nutrient digestion and absorption (Soccol et al. 2010). On the other hand, published literature information substantiating the health benefits of spore-forming Bacillus spp. that are able to survive the extremes of heat, acidity of the stomach, and bile acids is sparse (Hyronimus et al. 2000).
Bacillus spp. are heterogeneous group of Gram-positive, spore-forming, fermentative, aerobic, rod-shaped bacteria ubiquitously present in a variety of natural habitats, including soil, water, and the gastrointestinal tract (GIT) of animals. The use of Bacillus sp. in probiotic products raises safety issues because few species are known to be etiological agents in local, deep-tissue, and systemic infections (Mahler et al. 1997). In the majority of the cases, Bacillus cereus is found to be associated with food poisoning wherein enterotoxin production has been verified (From et al. 2005). Therefore, the use of Bacillus sp. requires development of strict standards with regard to safety issues. Although significant progress in legislation concerning this matter has been made in the USA, Canada, and Europe (FAO/WHO 2002; EFSA 2005), their recognition in world probiotic market is deprived. Only few strains like Bacillus subtilis and Bacillus indicus have been approved as a food supplement, and Bacillus clausii is used in pharmaceutical application. Hence, majority of Bacillus spp. claims their better niche and demand for generally regarded as safe (GRAS) status. Further, their inherent resistance to environmental stress has attracted for their application in the area of probiotics or food supplement, in vaccine technology as heat-stable oral vaccine delivery systems, and in nanobiotechnology as a tool for the efficient display of heterologous antigens on the spore surface (Sen et al. 2010).
Despite the enormous research on probiotics, the practical question arises whether a given microorganism can be considered to be a probiotic or not. In general, probiotic cultures with proper identity and origin should sustain under gastrointestinal conditions (acid and bile resistance) and supposed to be safe for consumption with additional functional beneficial properties (Havenaar et al. 1992; Sorokulova 2008). Hence, these criteria are considered in the present work to authenticate the Bacillus isolates as probiotic strains. Considering the importance of taxonomic description, various techniques including biochemical tests, as well as random amplified polymorphic DNA (RAPD)-PCR, 16S ribosomal RNA (rRNA) gene sequencing, and fatty acid profiling have been considered in the study for identification. Survival under GIT condition has been established, and their acid tolerance has been demonstrated through H+-ATPase activity and change in cellular fatty acid composition. Basic probiotic properties including safety, adhesion, and other functional properties have been validated by various in vitro studies with an ultimate goal for safer and efficient probiotic strain.
Material and methods
Media chemicals and reagents
All microbial media chemicals were purchased from HiMedia Pvt. Ltd., Mumbai, India. Ascorbic acid, 3,5-dinitrosalicylic acid (DNS), glucose, NaCl, ferric chloride, HPLC grade chloroform, and hexane were from Sisco Research Laboratory, Bangalore, India. Trypsin, pancreatin, pepsin, ox-bile, diphenylpicrylhydrazyl (DPPH), synthetic antioxidant standards, fatty acid standards, cholesterol-PEG 600, adenosine triphosphate (ATP), Taq DNA polymerase, MgCl2, dNTPs, and PCR buffer were procured from Sigma-Aldrich Inc., USA. All other chemicals used were of AR grade unless otherwise mentioned.
Bacterial cultures and growth conditions
Bacillus reference cultures Bacillus thuringienisis MCC2008, Bacillus megaterium MCC2009, B. subtilis MCC2014, Bacillus licheniformis MCC2016, Bacillus amyoliquifaceins MCC2017, Bacillus flexus MCC2011, and B. cereus MCC2015 were collected from culture collection center of Food Microbiology Department, CSIR-Central Food Technological Research Institute, Mysore, India. Standard culture B. subtilis 168 was kindly provided by Prof. KD Entien, Germany. Reference standard B. cereus F4433 was procured from Microbial Type Culture Collection (MTCC) center, Chandigarh. All the Bacillus strains were grown in Luria-Bertani (LB) medium at 37 °C under constant shaking (120 rpm). For antimicrobial activity, pathogenic and spoilage bacterial strains of Micrococcus luteus ATCC9341, Yersinia enterocolitica MTCC859, Aeromonas hydrophila B445, Staphylococcus aureus FRI722, Salmonella typhimurium MTCC1251, Escherichia coli CFR02, and Klebsiella sp. were procured from American Type Culture Collection (ATCC), USA, or MTCC, Chandigarh, India. Listeria monocytogenes ScottA was kindly provided by Dr. AK Bhunia, USA.
Isolation and preliminary identification of Bacillus spp.
Sample collection and processing
For isolation of Bacillus spp., raw milk samples were collected from various animals including cow, buffalo, sheep, goat, and donkey from different localities of Mysore, India. Samples were collected in sterile collection tubes during milking and few samples from collection centers. Some commercially available milk-based fermented dairy products were purchased from local market as a source for Bacillus isolation. In addition, rhizobial soil, root, and leaves of various medicinal herbs were collected from different localities of Mysore.
Selective screening and characterization
Samples (1 mL or 1 g) were suspended in 9-mL physiological saline (0.85 % NaCl). For initial isolation of heat resistant flora, suspension was heat-treated at 70 °C for 20 min. Later, samples were serially diluted and appropriate dilution was plated on LB agar. For total microbial count, suspension without heat treatment was plated on nutrient agar. Plates were incubated at 37 °C for 24–48 h. Colonies were selected based on morphological differences and purified by repeated streaking. Gram-positive and catalase-positive rods were selected and then stored at −20 °C under 40 % glycerol until use. Subsequently, cultures were tested for their growth at acidic pH (3.0) and in the presence of bile (0.1 %) by growing the culture in modified LB medium as per the requirement for 6 h and checked for growth as indicated by turbidity.
Virulence or pathogenicity of the isolates
Non-hemolytic property of isolated cultures was tested by using 7 % defibrinated sheep blood agar. Lecithinase activity was tested on Baird-Parker agar enriched with egg yolk emulsion and 3 % potassium tellurite. Gelatin hydrolysis was checked in medium supplemented with 2 % gelatin. Further, absence of virulence genes like cytotoxin (cytK) gene, L1 subunit of hemolytic (hblD) gene, and NH1 subunit of non-hemolytic enterotoxin (nhe1) gene was analyzed by PCR with B. cereus F4433 as a positive control. Primers and PCR conditions are provided in Table 1.
Identification of Bacillus isolates
Morphological, phenotypical, and biochemical characterization
The vegetative cells and spores (shape and position) were observed with bright field microscope (Olympus, Japan) under submerge × 100 magnification. Biochemical and physiological characteristics of culture isolates were analyzed according to Bergey’s Manual of Systematic Bacteriology. Sugar utilization was tested using HiCarbo identification kit (HiMedia Pvt. Ltd., Mumbai, India).
Molecular characterization
Microbial grouping of the isolates was carried out by RAPD-PCR using M13 primer (Schillinger et al. 2003). RAPD banding was scored, and dendrogram was constructed using NTSYS software (Applied Biostatistics Inc., version 1.07). For Bacillus identification, 16S rRNA gene was amplified using BSF and BSR primer (Table 1) as per the conditions described by Raghavendra and Halami (2009). The amplified product was sequenced at Vimta Labs, Hyderabad, India. The taxonomical identification of Bacillus isolates was performed by BLAST search (Altschul et al. 1997). Phylogenetic tree was constructed by using neighbor-joining method with bootstrap (1,000 replicates) by Kimura two-parameter model using MEGA 4 program (Kumar et al. 2008).
Cellular fatty acid extraction and analysis
Cellular lipids of Bacillus isolates and reference cultures were extracted from cell pellet with chloroform and methanol as described by Bligh and Dyer (1959). Lipids were then methylated with 2 N KOH in methanol for 1 h. The resulting fatty acid methyl esters were analyzed by gas chromatography (GC; Shimadzu Corporation, Kyoto, Japan) using RTX-1 capillary column (100 % dimethyl polysiloxane; 30 m × 0.32 mm ID × 0.25 μm). GC condition was programmed with injector and detector temperature at 230 and 250 °C, respectively, with nitrogen flow at a rate of 1.5 mL min−1. Fatty acid methyl esters were identified by using authentic standards (Sigma Chemical Co.) and confirmed through GC-MS spectra (Model Turbomass Gold; Perkin Elmer International, Huenenberg, Switzerland).
Survival under simulated gastrointestinal condition
Survival efficiency: Survival of isolated cultures under simulated gastric condition was tested by inoculating (10 % containing 8 log cfu mL−1) washed cell pellet in buffer (glycine/HCl buffer; pH 2.0) containing pepsin (3 mg mL−1). Cultures were then incubated at 37 °C under constant shaking (100 rpm). At regular intervals (0, 30, 60, 90, and 120 min), an aliquot of the sample was drawn, serially diluted and plated on LB agar. Controls were kept under neutral pH (7.0) without pepsin. After the incubation period of 24–48 h, colonies were counted and the difference between control and test samples was expressed as a percentage of survival. Similarly, survival of isolated cultures at simulated intestinal condition was tested in buffer saline (phosphate buffer pH 8.0, 0.85 % NaCl) supplemented with 1 % trypsin or 1 % pancreatin in addition to 0.3 % ox-bile. Survival rate was estimated at 0, 3, 6, and 24 h.
H+-ATPase assay
Acid tolerance of Bacillus isolates was assessed by measuring the ATPase activity in permeabilized cell as described by Belli and Marquis (1991) with slight modification. Overnight grown culture (25 mL) was centrifuged (8,000 rpm for 15 min at 4 °C), and the washed cells were suspended in the buffer of different pH (2, 3, 4, and 7). After 2 h of incubation, cells were centrifuged and resuspended in 2.5 mL of 75 mM Tris-HCl buffer (pH 7.0) with 10 mM MgSO4. Toluene (250 μL) was added to the cell suspension and mixed vigorously, followed by incubation for 5 min at 37 °C. Cell suspension was then subjected to three cycles of freezing at −80 °C and thawing at 37 °C. Permeabilized cells were then harvested by centrifugation at 10,000 rpm for 15 min. They were then resuspended in 1 mL of 75 mM Tris-HCl buffer (pH 7.0) with 10 mM MgSO4. The suspension was rapidly frozen and stored at −80 °C. Ten microliters of a permeabilized cell suspension was added to 1.0 mL of 50 mM Tris-maleate buffer (pH 6.0) with 10 mM MgSO4 at 37 °C. The ATPase reaction was initiated by the addition of 125 μL of 0.02 M ATP (pH 6.5) and was allowed to proceed at 37 °C for 5 min. The liberated phosphate was measured at 700 nm after adding 1.5 mL of color reagent, which is prepared freshly before using by mixing four volumes of 2.5 % ammonium molybdate solution in 5.5 % sulfuric acid and one volume of 2.5 % ferrous sulfate solution. ATPase activities were expressed as micromoles of phosphate released from ATP per minute per milligram of protein.
Effect of acidic pH on cellular fatty acids
Change in the lipid composition of Bacillus isolates under acidic pH was analyzed by GC. Overnight grown cultures were inoculated (10 %) into fresh media adjusted to varying pH (2, 3, 4, and 7) and incubated at 37 °C for 12 h. Later, cell pellet was collected by centrifugation (8,000 rpm for 15 min at 4 °C), washed with saline, and used for extraction of lipid as described earlier. Fatty acid methyl esters (FAME), as analyzed by GC, were compared with standards.
Cell hydrophobicity, autoaggregation, and mucin binding ability of Bacillus isolates
Cell hydrophobicity assay
Adhesion ability of Bacillus isolates was analyzed by hydrophobicity assay using different hydrocarbons, i.e., xylene, toluene, and hexadecane as described by Rosenberg et al. (1980). Cell surface hydrophobicity or percent adhesion was calculated using the following formula:
where A 1 is final OD600 after 30-min incubation, and A 0 is the initial absorbance.
Autoaggregation assay
Autoaggregation of Bacillus isolates were studied according to Del Re et al. (2000). Overnight grown culture was centrifuged (8,000 rpm, 15 min), and the washed cell pellet was suspended in PBS buffer (pH 7.0) until the OD595 reaches 0.5–0.6. Later, the cell suspension (4 mL) was vortexed and incubated at 37 °C. After 30 min and 1 h, absorbance of the upper layer was measured at 595 nm using a spectrophotometer. Percentage of autoaggregation was measured by using the formula, 1 − (A t / A 0) × 100, where A t represents absorbance at time 30 min or 1 h, and A 0 represents absorbance at time = 0.
Mucin binding assay
Ability of Bacillus spp. to adhere to mucin was assessed using microtiter plate according to Jonsson et al. (2001). Percent adhesion of culture to mucin was calculated by the difference in the initial and final cell count of the culture after 1-h incubation.
Additional functional properties of Bacillus isolates
Antimicrobial activity
Antimicrobial activity of Bacillus isolates against M. luteus ATCC9341, Y. enterocolitica MTCC859, A. hydrophila B445, S. aureus FRI722, S. typhimurium MTCC1251, E. coli CFR02, Klebsiella sp., and L. monocytogenes ScottA was tested by agar well diffusion assay as described by Xie et al. (2009).
Enzyme production
Caseinase, amylase, cellulase, and lipase productions were determined by using casein/skim milk powder (2 %), starch (2 %), carboxymethyl cellulose (2 %), and tributirin (1 %) agar plates, respectively. Zone of clearance on incubation with Bacillus isolates indicated the enzyme production. Caseinolytic activity was quantified in positive isolates using azocasein as substrate. One unit (U) of hydrolytic activity of the protease was defined as the amount of enzyme required to cause an increase of 0.001 A 440 unit in 1 min per milligram of protein. Phytase activity was measured by using sodium phytate (Sigma, USA) as substrate. One unit of phytase activity was defined as the amount required to liberate 1 mmol of phosphate per minute per milligram of protein under the assay condition. The activity of α-amylase was assayed by incubating 0.1 mL enzyme with 1.0 mL of soluble starch (1.0 w/v) prepared in 0.1 M phosphate buffer (pH 7.5). After incubation at 50 °C for 5 min, the reaction was stopped and the reducing sugars released were determined by the addition of 1.0 mL of DNS reagent with maltose as a standard. One unit of enzyme activity was defined as the amount of enzyme required for releasing 1 μg of maltose from the substrate per minute per milligram of protein. Cellulase activity was assayed by incubating 0.1 mL enzyme in 1.0 mL CMC. After 30 min of incubation at 37 °C, reducing sugar released was determined by using DNS reagent with glucose as a standard. One unit of enzyme was defined as the amount of enzyme releasing 1 μg of glucose from the substrate per minute per milligram of protein.
Antioxidant activity
Antioxidant activity of Bacillus isolates was tested by diphenyl picryl hydrazyl (DPPH) scavenging assay, reducing potential, total phenolic content, H2O2 scavenging activity, and metal chelation ability according to the protocol described by Sowmya and Sachindra (2012).
Antibiotic susceptibility assay
Antibiotic susceptibility of Bacillus isolates to various antibiotics was analyzed by using HiMedia Octadics.
Cholesterol assimilation assay
Bacillus isolates were grown in LB media supplemented with 100 mg L−1 of cholesterol-PEG 600, and their cholesterol assimilation ability was determined by the method described by Gilliland et al. (1985). Results were expressed as percent assimilation to that of initial concentration.
Results
Isolation and selective screening of Bacillus sp.
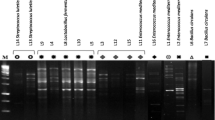
Initially, heat-resistant flora with Gram-positive and catalase-positive property were isolated from different raw milk samples (n = 78), from commercially available milk-based dairy products (n = 58), and rhizobial soil, root, and leaf samples of various medicinal herbs (n = 48) (Table S1). Among 170 cultures isolated, 79 isolates were showing antibacterial activity against Micrococcus sp. with inhibition zone ranging from 15 to 22-mm diameter/40 μL of the culture supernatant. Further, among the tested cultures, only 43 isolates were able to grow in acidic pH (3.0) and in the presence of bile (0.1 % bile). Forty three cultures showing the above property along with reference strains were selected and subjected to RAPD profiling. RAPD-PCR gave distinct banding pattern from selected Bacillus isolates. Computer-assisted numerical processing of RAPD banding profiles using cluster analysis with unweighted pair group method (UPGMA) along with and arithmetic average algorithm yielded a dendrogram which consists of two major groups (I and II), which were further divided into several groups at similarity level ranging from 25 to 100 % (Fig. 1). All the cultures were clubbed into 17 groups with >70 % similarity. Selected cultures were further tested for non-hemolytic property as well as lack of lecithinase and gelatinase activity. All the details of the representative cultures are depicted in Fig. 1. Further data from PCR amplification of virulence genes (hblD, cytK, and nhe1) demonstrated that only six strains (AR-S4, CDM3-1, CDM4-c, CSM1-1a, HN-S1, and HR-S1) were devoid of all the tested virulent genes. Hence, these cultures were considered for further studies.
Dendrogram drawn using RAPD profile of Bacillus isolates. UPGMA bootstrapping values of this clade was 95–99. Culture isolates were grouped by considering the significances in bootstrap values, interior branch lengths, and diversification rate. Asterisks indicate reference strains. In case of growth of culture in pH 3 and bile (0.1 %), +++ = OD600 > 1.0; ++ = OD600 0.5–0.6; + = OD600 < 0.2. H hamolysis, G gelatinase, L lecithinase activity, “+” positive, “−”negative
Identification of Bacillus isolates
Biochemical and physiological characteristics of selected Bacillus isolates were studied according to Bergey’s Manual of Systematic Bacteriology. Further, they were identified by partial 16S rRNA gene sequencing. The sequences containing at least 700–750 bp (related to V2–V4 variable region of 16S rRNA gene of E. coli) were used for database query. Using megablast tool of GenBank (http://www.ncbi.nlm.nih.gov/), sequences were subjected for BLAST analysis and the cultures were identified as AR-S4-B. megaterium, CDM3-1/CDM4-3c-B. flexus, Csm1-1a/HN-S1-B. licheniformis, and HR-S1-B. subtilis. Representatives of maximum homologous (95–99 %) sequences of each isolate were obtained from NCBI GenBank and were used for the construction of the phylogenetic tree (Fig. 2a). Nucleotide sequences of the isolates were deposited at NCBI DNA database with accession number KF668669 to KF668674. The cultures were deposited at Microbial Culture Collection (MCC), National Centre for Cell Science, Pune, with the following accession numbers: B. megaterium strain AR-S4 (MCC2336), B. flexus strain CDM3-1 (MCC 2458), B. flexus strain CDM4-3c (MCC2427), B. licheniformis strain Csm1-1a (MCC 2514), B. licheniformis strain HN-S1 (MCC2512), and B. subtilis strain HR-S1 (MCC 2507).
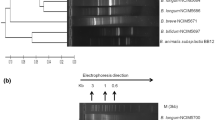
a Phylogenetic relationship of native isolates with strains from GenBank with highest similarities (accession numbers in parentheses). The neighbor-joining tree and subtree are based on approximately 700–750 bp of the 16S rRNA gene. Numbers show the level of bootstrap support from 1,000 repetitions. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons. b Dendrogram based on cluster analysis of cellular fatty acid composition of native Bacillus isolates with reference cultures by using Jaccard coefficient similarity
Although, 16S rRNA gene sequence analysis is the most commonly used method for identifying bacteria and for studying phylogenetic relationships, its usefulness is limited because of the high percentage of sequence similarity between closely related species. Thus, comparison of cellular fatty acid profile of Bacillus isolates with the reference cultures has been carried out for authentication of culture identity. The major fatty acids observed in all the cases were C16, C18, C18:1, and C18:3. Dendrogram was constructed using PAST software, version 2.14 (htt://folk.uio.no/ohammer/past). Jaccard similarity coefficient (r = 0.85) clustered with the associated cultures in bootstrap test (1,000 replicates) depicted >85 % similarities between the native isolates and corresponding reference strains (Fig. 2b). The data was comparable with 16S rRNA gene sequence homology. The two strains, Csm1-1a and HN-S1, clustered with Me-1 (B. licheniformis MCC2016) but were distantly linked with other group as comparable with 16S rRNA gene data (Fig. 2a, b).
Survival under simulated gastrointestinal condition
All the six cultures selected showed good survival even after 1 h under simulated gastric condition (pH 2.0) with a survival rate ranging from 78.7 to 99.6 %, and in the presence of pepsin (3 mg mL−1), cell count reduced to 60.7–88.4 %. After 2-h incubation, significant reduction (p < 0.05) in cell count was observed (15.3–40.6 % survival). Maximum survival (40.9 %) was observed in HR-S1 (B. subtilis MCC2507) isolated from rhizobial soil, followed by CDM3-1 (B. flexus MCC2458; 40.6 %) from cow milk. Similarly, under simulated intestinal condition (pH 8.0), 80.6–92.7 % survival was observed after 24 h of incubation. In the presence of trypsin (0.1 %) as a digestive enzyme, 63.6–84.1 % survival was observed and with pancreatin (0.1 %) 68.9–90.9 % cell survived.
H+-ATPase assay
ATPase activity of permeabilized cells was in the range of 0.56–2.82 μM pi mg−1 min−1. As per the data, H+-ATPase activity was found to increase when the culture was exposed to acidic pH (Fig. 3). At pH 2.0, B. subtilis strain HR-S1 that had maximum survival efficiency under gastric condition showed almost 8.2-fold increase in the ATPase activity at pH 2.0 in comparison to neutral pH (7.0), indicating the acid tolerant ability of the culture. Cell growth and H+-ATPase activity were found to have a negative correlation (r = −0.85 to −0.96), i.e., acid-tolerant cell had higher H+-ATPase than non-tolerant cultures.
a Survival of Bacillus isolates at various pH. b ATPase activity under different pH. ATPase activities were expressed as micromoles of phosphate released from ATP per minute per milligram of protein. AR-S4 B. megaterium, CDM3-1 and CDM4-3c B. flexus, Csm1-1a and HN-S1 B. licheniformis, HR-S1 B. subtilis. Values are mean ± SD of triplicate trials
Cellular fatty acid response to change in pH
Acidic pH induced a characteristic change in the relative proportion of fatty acids (Fig. 4). Although species-specific variation was observed in the composition of fatty acids, overall increase in the total fatty acids was observed in all the isolates with the maximum being at pH 3. At pH 2.0, slight reductions were observed in all the cases. C18 was the major fatty acid in HR-S1, Csm1-1a, CDM4-3c, and CDM3-1 accounting for 33.26, 30.59, 33.5, and 37.9 % of total fatty acid, respectively. In case of HN-S1 and AR-S4, C18.1 (27.5 %) and C16 (22.9 %) were the major fatty acids, respectively. Monounsaturated fatty acid (C18.1) enhanced in all the isolates under acidic condition.
Cellular fatty acid profile of Bacillus sp. under various pH condition. a B. megaterium AR-S4, b B. flexus CDM3-1, c B. flexus CDM4-3c, d B. licheniformis Csm1-1a, e B. licheniformis HN-S1, f B. subtilis HR-S1. pH condition (x-axis); concentration of fatty acid (μg mL−1) (y-axis). Values are mean ± SD of duplicate trials
Cell hydrophobicity, autoaggregation, and mucin binding ability of Bacillus isolates
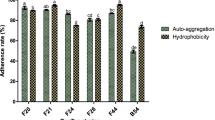
The percent cell hydrophobicity of the Bacillus isolates to xylene, toluene, and hexadecane are presented in Table 2. The hydrophobic values of isolates to xylene were in the range of 25.7–50.5 %; for toluene, it was 30.1–64.8 %; and for hexadecane, it was 11.1–58.3 %. In all the cases, Csm1-1a, identified as B. licheniformis MCC2514, exhibited maximum hydrophobicity. Cell aggregation potential of Bacillus isolates was determined by sedimentation ability. After 30 min of incubation, the cultures showed 0.7–25.4 % aggregation which increased to 7.8–39.1 % after 1 h (Table 2). B. licheniformis strain Csm1-1a exhibited maximum aggregation (39.1 %), followed by B. subtilis strain HR-S1 (33.8 %). Further, adhesion capability of Bacillus isolates was analyzed by mucin binding ability (Table 2). The cultures exhibited 35–65 % adhesion to mucin. Taking into account the various in vitro adhesion methods tested Csm1-1a (B. licheniformis MCC2514) exhibited maximum adhesion.
Functional properties of Bacillus isolates
Antimicrobial activity
The Bacillus cultures tested exhibited antimicrobial activity against M. luteus with inhibition zone ranging from 12 to 24-mm diameter. Maximum activity was observed in B. licheniformis HN-S1 (24 mm). In addition to M. luteus, HN-S1 was able to inhibit S. aureus (20 mm), L. monocytogenes (16 mm), Salmonella typhi (16 mm), Klebsiella sp. (16 mm), E. coli (12 mm), and Aeromonas sp. (12 mm). Csm1-1a was able to inhibit S. aureus (16 mm), Klebsiella sp. (16 mm), and Aeromonas sp. (12 mm).
Enzyme production
Enzyme activity of Bacillus isolates are depicted in Table 3. Protease activity of isolates was in the range of 18.6–31.0 U mg−1 protein. On starch agar medium, only two cultures, CDM4-3c (B. flexus) and HN-S1 (B. licheniformis), showed inhibition zone with an activity of 499.0 and 507.9 U mg−1, respectively. In case of cellulase production, CDM3-1 (B. flexus) exhibited maximum production (22.5 μM mg−1), followed by B. licheniformis HN-S1 (18.5 μM mg−1) and B. megaterium AR-S1 (18.4 μM mg−1). Lipase production as analyzed on tributyrin agar plates indicated activity in Csm1-1a (B. licheniformis), HN-S1 (B. licheniformis), and CDM4-3c (B. flexus). Phytase degrading ability was observed only in two cultures, B. megaterium AR-S4 (0.41 U mg−1) and B. licheniformis HN-S1 (0.42 U mg−1).
Antioxidant activity
Antioxidant substances scavenge the free radicals by donating hydrogen ion which in turn helps in protecting the body from degenerative diseases. The data of various methods used for evaluating antioxidant activity of Bacillus isolates are presented in Table 3. The highest polyphenol content was observed in B. licheniformis Csm1-1a (6.4 ± 0.22 μg mL−1 gallic acid equivalent). Reducing potential, DPPH, and H2O2 scavenging, as well as metal chelation ability of current isolates, indicated their antioxidant phenomenon.
Cholesterol assimilation ability
As per the data obtained (Table 3), B. subtilis HR-S1 demonstrated maximum cholesterol assimilation (76.02 %), followed by B. flexus CDM4-3c (66.44 %).
Antibiotic susceptibility assay
Bacillus isolates selected were found to be susceptible to all the antibiotics tested (Table 4). None of the cultures were resistant to any of the antibiotic tested.
Discussion
The advantages of probiotic culture have been attributed due to their ability to survive under GIT stress, establish in the colon, and exert beneficial effect. In such connection, the present work has been carried out to isolate and exemplify a safer and effective Bacillus sp. for probiotic application.
Isolation and identification of Bacillus sp
Spore-forming Bacillus spp. are ubiquitously present in a variety of natural habitats, including soil, water, and the gastrointestinal tract of animals, as well as extreme temperature and climatic condition due to their inherent resistance. Typically, prevalence of Bacillus has been studied as a source of contamination or infective agent. Studies carried out specifically for the isolation of Bacillus sp. for probiotic application is sparse (Fakhry et al. 2008; Chaiyawan et al. 2010). In the present study, raw milk samples, fermented dairy products, and rhizobial soil were used as a source for isolation of probiotic Bacillus sp. Usually, in these samples, LAB are predominantly present; hence, selective screening of Bacillus was carried out by heat treatment at 70 °C. Amongst, 170 heat-resistant microfloras were isolated; majority (68.2 %) was from rhizobial soil followed by raw milk (24.7 %). All the selected cultures were aerobic, Gram-positive, catalase-positive, motile, rod-shaped, and spore formers. Antimicrobial assay depicted 46.5 % strains with activity against M. luteus, among which only 43 isolates were able to tolerate low pH (3.0) and bile concentration (0.1 %). A total of 17 strains from rhizobial soil of medicinal herbs and 3 from raw milk showed hemolytic activity. The production of lecithinase, proteases, gelatinase, and enterotoxins are recognized as a putative virulence factor that is required by invasive bacterial pathogens to elicit successful systemic infections (Rowan et al. 2001). In this connection, lecithinase test revealed positive reaction among 14 Bacillus and 10 were gelatinase-positive.
As the safety of probiotic cultures is of vital importance, virulence genes such as non-hemolytic enterotoxin (nhe) and cytotoxin K (cytK), along with hemolysin BL (hbl), which are known to cause diarrhoeal syndrome (Granum et al. 1999; Guinebretière et al. 2002), were analyzed in the present isolates. Accordingly only six strains (AR-S4, CDM3-1, CDM4-c, CSM1-1a, HN-S1, and HR-S1) were found devoid of all the tested virulent genes. These cultures were identified as AR-S4-B. megaterium, CDM3-1/CDM4-3c-B. flexus, Csm1-1a/HN-S1-B. licheniformis, and HR-S1-B. subtilis through biochemical assays and 16S rRNA gene sequencing. Further, the identity of cultures was confirmed by comparison of fatty acid profile of native isolates with that of reference cultures. Although slight variation in the concentration of fatty acids was observed, salient differential fatty acids were reproducible which support the authenticity of the strains. Similarly, Peak et al. (2011) observed slight variation in the fatty acid profile of Bacillus spp. and have suggested that variation in consistency may be due to physiological age, temperature, incubation time, and growth condition.
Cellular fatty acid profiles are known to vary widely but are consistent within the Bacillus spp. Siegel et al. (1997) have compared cellular fatty acid composition of Bacillus sphaericus, a human isolate, with that of Bacillus sphaericus of mosquito larvicide. Roberts et al. (1994) distinguished Bacillus mojavensis sp. nov., from Bacillus subtilis by analyzing divergence in DNA sequence along with fatty acid composition. They reported 14:O iso, 15:O iso, 150 anteiso, 16:O iso, 16:O, 17:O iso, 17:O anteiso, 16:l cis5, and 17:l cis7 iso as major fatty acids in the tested Bacillus strains. In the present study, dendrogram scaled with Jaccard similarity distance was comparable with 16S rRNA gene data. Native isolates as identified by 16S rRNA gene clustered with the respective reference strains with >90 similarity.
Survival under simulated gastrointestinal condition
Survival under simulated GIT condition is an important criterion for any culture to be considered probiotic. They have to survive the acid and bile stress of GIT and establish in the colon to exhibit beneficial effect. Under simulated gastric condition (pH 2.0; pepsin 3 mg mL−1), all the selected isolates showed 60.7–88.4 % survival and under simulated intestinal condition (pH 8.0), 80.6–92.7 % survival was observed. Variability among survival rate of Bacillus spp. under simulated GIT has been demonstrated by a large number of researchers (Lee et al. 2012). Sahadeva et al. (2011) examined GIT tolerance of probiotic culture isolated from commercially cultured milk drinks available in the Malaysian market. They reported that the strains showed good tolerance to pH 3.0 with >106 CFU mL−1 after 3-h incubation and could tolerate 0.3 % bile. Lee et al. (2012) reported 55 % survival at pH 2 after 2 h of incubation, and a reduction of 9–20 % was observed in the presence of bile (0.3 %).
H+-ATPase assay
In order to evaluate the acid tolerance of Bacillus isolates, membrane ATPase activity was studied. H+-ATPase activity was found to increase by more than 2-fold under acidic pH (2.0). Similarly, Miwa et al. (1997) reported that acid-intolerant bacteria contain less H+-ATPase and have less capacity to enhance its activity in response to low pH. In general, it is known that when internal pH reaches a threshold value, cellular functions are inhibited and the cells die. In such a situation, FoF1-ATPase is the mechanism used by Gram-positive organisms to protect themselves against acidic stress. H+-ATPase pumps H+ out of cells at the expense of ATP hydrolysis. Miwa et al. (1997) confirmed that acid-tolerant cellulolytic bacteria contain 4- to 5-fold more H+-ATPase than those of acid-intolerant bacteria. FoF1-ATPase generates a proton motive force, via proton expulsion, resulting in an increase of intracellular pH (Fortier et al. 2003). The FoF1-ATPase was upregulated as a result of acid stress in lactobacilli (Kullen and Klaenhammer 1999). Further, O’Sullivan and Condon (1999) indicated that when the internal pH was changed by modifying the pH of medium, the level of H+-ATPase changed and the ability of cells to export protons was balanced with the need to support the growth rate. They suggested that H+-ATPase is acid-tolerant response protein that probably plays a role in the ability of lactococcal cells to survive in acid environment.
Cellular fatty acid response to change in pH
Considering the importance of cellular fatty acids in regulation of membrane fluidity in response to environmental stress, the fatty acid profile of present isolates were analyzed on exposure to varying pH (7, 4, 3, and 2). As compared to pH 7, concentration of even-numbered fatty acids (C16 and C18) was found to be higher (29–56 %) at pH 3, whereas odd-numbered fatty acids (C15 and C17) accounted only 0.7–5.6 %. Monounsaturated fatty acid (C18.1) enhanced in all the isolates under acidic condition. Similar observation has been made in Streptococcus mutants and Lactobacillus casei (Fozo et al. 2004).Correspondingly, Petrackova et al. (2010) described increased C14 and C16 at an expense of reduced C15 and C17 in B. subtilis when exposed to pH 5. In L. monocytogenes, it has been reported that straight-chain fatty acid was higher (C14:0 and C16:0) at pH 5.5 and the level of C18:0 reduced (van Schaik et al. 1999). Similarly, increase in C16 has been observed in E. coli (Yuk and Marshall 2004). As suggested by Giotis et al. (2007), this consistent change or balance in the membrane fatty acids could be critical in pH adaptation of present isolates. These results substantiate the survival ability of isolates under gastric pH.
Cell hydrophobicity, autoaggregation, and mucin binding ability of Bacillus isolates
Bacterial adhesion is an important criterion of probiotics that determine the colonization capability of a microorganism in the GIT preventing their immediate elimination by peristalsis as well as provide competitive advantage by preventing pathogens by specific blockage on cell receptors (Otero et al. 2004). In vitro models like cell hydrophobicity, autoaggregation, and mucin adhesion are considered vital in selecting probiotic candidates and are primary tool for the study of surface adhesion (Patel et al. 2009). Accordingly, the present isolates exhibited good cell hydrophobicity toward xylene, toluene, and hexadecane. Patel et al. (2009) reports 6–62 % hydrophobocity to xylene and 32.58 % autoaggregation. Thirabunyanona and Thongwittaya (2012) analyzed adhesion of Bacillus sp. isolated from chicken intestine and accounted 17–57 % (n-hexadecane), 31–62 % (xylene), and 29–59 % (toluene) hydrophobicity. Nithya and Halami (2013) examined Bacillus isolates having 10–80 % adhesion to hexadecane. Further, adhesion capability of Bacillus isolates was analyzed by mucin binding ability. Mucins are a family of high molecular weight, heavily glycosylated proteins produced by epithelial cells that form viscoelastic gel-like layer over epithelial surfaces in the mammalian GIT. Overall study indicates Csm1-1a (B. licheniformis MCC2514) had maximum adhesion ability. Cell hydrophobicity plays an important role in specific and non-specific factors that enable a microorganism to bind and persist in the host gut (Prakash et al. 1997). Cellular aggregation helps in transient colonization as well as provides a protective shield to the host system due to formation of a bacterial biofilm over the host tissue. Del Re et al. (2000) have also reported that strains were able to adhere to cell monolayers if they autoaggregate and manifest a good degree of hydrophobicity to hydrocarbons. Hence, the potential hydrophobicity, autoaggregation, and mucin binding ability observed in the current study explain the possible adhesion of the present isolates to GIT and their probiotic nature.
Functional properties of Bacillus isolates
All the selected isolates showed antimicrobial activity against various pathogens tested, indicating their potential application as biopreservative agents. Enzyme study revealed a variation in the production of enzymes among different isolates. This indicates that the combination among Bacillus strains producing different extracellular enzymes may generate a synergistic mediated improvement of the production performance and nutrient digestibility in host animals. Further, the antioxidant activity suggest their possible application in scavenge the free radicals by donating hydrogen ion which in turn helps in protecting the body from degenerative diseases.
In conclusion, the present research data highlights the safer probiotic isolates with supporting pieces of evidence for authentic identity, survival efficiency, and functional attributes. All the selected Bacillus sp. demonstrated tolerance to acidic pH as analyzed by cell growth, H+-ATPase activity, and modification in cellular fatty acids. On the basis of the present investigation, the in vitro models tested suggested Bacillus sp. as a possible candidate with probiotic phenotype and will lead to their application in various functional foods for potential health claim.
References
Altschul SF, Maddan TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Belli WA, Marquis RE (1991) Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol 57:1134–1138
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Chaiyawan N, Taveeteptaikul P, Wannissorn B, Ruengsomwong S, Klungsupya P, Buaban W, Itsaranuwat P (2010) Characterization and probiotic properties of Bacillus strains isolated from broiler. Thai J Vet Med 40(2):207–214
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442
EFSA (2005) European Food Safety Authority, Opinion of the scientific committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J 226:1–12
Fakhry S, Sorrentini I, Ricca E, De Felice M, Baccigalupi L (2008) Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J Appl Microbiol 105:2178–2186
FAO/WHO (2002) Joint FAO/WHO (Food and Agriculture Organization/World Health Organization) working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada
Fortier LC, Tourdot-Marechal R, Divie’s C, Lee BH, Guzzo J (2003) Induction of Oenococcus oeni H+-ATPase activity and mRNA transcription under acidic conditions. FEMS Microbiol Lett 222:165–169
Fozo EM, Kajfasz JK, Quivey RG Jr (2004) Low pH-induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol Lett 238:291–295
From C, Pukall R, Schumann P, Hormazabal V, Granum PE (2005) Toxin producing ability among Bacillus spp. outside the Bacillus cereus group. Appl Environ Microbiol 71:1178–1183
Gilliland SE, Nelson CR, Maxwell C (1985) Assimilation of cholesterol by Lactobacillus acidophilus. Appl Environ Microbiol 49(2):377–381
Giotis ES, McDowell DA, Blair IS, Wilkinson BJ (2007) Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl Environ Microbiol 73:997–1001
Granum PE, O'Sullivan K, Lund T (1999) The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol Lett 177:225–229
Guinebretière MH, Broussolle V, Nguyen-The C (2002) Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J Clin Microbiol 40:3053–3056
Havenaar R, Ten Brink B, Huis in’t Veld JHJ (1992) Selection of strains for probiotic use. In: Fuller R (ed) Probiotics. The Scientific Basis Chapman and Hall, London, pp 209–221
Hyronimus B, Le Marrec C, Sassi AH, Deschamps A (2000) Acid and bile tolerance of spore-forming lactic acid bacteria. Int J Food Microbiol 61:193–197
Jonsson H, Strom F, Roos S (2001) Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol Lett 204:19–22
Kullen MJ, Klaenhammer TR (1999) Identification of the pH inducible, proton-translocating F1F0-ATPase (atpBEFHAGDC) operon of Lactobacillus acidophilus by differential display: gene structure, cloning and characterization. Mol Microbiol 33:1152–1161
Kumar S, Nei M, Dudley J, Tamura K (2008) Mega: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinforma 9:299–306
Lee J, Park I, Choi Y, Cho J (2012) Bacillus strains as feed additives: in vitro evaluation of its potential probiotic properties. Rev Colomb Cienc Pecu 25:577–585
Mahler H, Pasi A, Kramer JM, Schulte P, Scoging AC, Baer W, Kraehenbuehl S (1997) Fulminant liver failure associated with the emetic toxin of Bacillus cereus. The New Engl J Med 336:1143–1148
Miwa T, Esaki H, Umemori J, Hino T (1997) Activity of H+-ATPase in ruminal bacteria with special reference to acid tolerance. Appl Environ Microbiol 63(6):2155–2158
Nithya V, Halami PM (2013) Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann Microbiol 6(1):129–137
O’sullivan E, Condon S (1999) Relationship between acid tolerance, cytoplasmic pH, and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl Environ Microbiol 65(6):2287–2293
Otero MC, Ocana VS, Macias ENM (2004) Bacterial surface characteristics applied to selection of probiotic microorganisms. Method Mol Biol 268:435–440
Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Chincholkar SB (2009) Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res Int 42:505–510
Peak KK, Duncan KE, Luna VA, King DS, McCarthy PJ, Cannons AC (2011) Bacillus strain most closely related to Bacillus nealsonii are not effectively circumseribed within the taxonomic species definition. Int J Microbiol. doi:10.1155/2011/673136.
Petrackova D, Vecer J, Svobodova J, Herman P (2010) Long term adaptation of Bacillus subtilis 168 to extreme pH affects chemical and physical properties of the cellular membrane. J Membr Biol 233(1–3):73–83
Prakash R, Sinha PR, Sinha RN, Singh B (1997) Adherence of lactobacilli to epithelial cells and hexadecane for use of probiotics. Ind J Dairy Sci 10:43–47
Raghavendra P, Halami PM (2009) Screening, Selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine and their probiotic properties. Int J Food Microbiol 133:129–134
Roberts MS, Nakamura LK, Cohan FM (1994) Bacillus mojavensis sp. nov., distinguishable from Bacillus subtilis by sexual isolation, divergence in DNA sequence, and differences in fatty acid composition. Int J Sys Bacteriol 44:256–264
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Rowan NJ, Deans K, Anderson JG, Gemmell CG, Hunter IS, Chaithong T (2001) Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl Environ Microbiol 67(9):3873–3881
Ryan PA, MacMillan JD, Zilinskas BA (1997) Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J Bacteriol 179:2551–2556
Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan HY, Tong EV, Wong SYW, Chan HK (2011) Survival of commercial probiotic strains to pH and bile. Int Food Res J 18(4):1515–1522
Schillinger U, Yousif NMK, Sesar L, Franz CMAP (2003) Use of group-specific and RAPD-PCR analyses for rapid differentiation of Lactobacillus strains from probiotic yogurts. Curr Microbiol 47:453–456
Sen R, Pal D, Kodali VP, Das S, Ghosh SK (2010) Molecular characterization and in vitro analyses of a sporogenous bacterium with potential probiotic properties. Probiotics Antimicrob Proteins 2:152–161
Siegel JP, Smith AR, Novak RJ (1997) Comparison of the cellular fatty acid composition of a bacterium isolated from a human and alleged to be Bacillus sphaericus with that of Bacillus sphaericus isolated from a mosquito larvicide. Appl Environ Microbiol 63(3):1006–1010
Soccol CR, de Souza Vandenberghe LP, Spier MR, Medeiros ABP, Yamaguishi CT, De Dea Lindner J, Pandey A, Thomaz-Soccol V (2010) The Potential of probiotics: a review. Food Technol Biotechnol 48(4):413–434
Sorokulova I (2008) Preclinical testing in the development of probiotics: a regulatory perspective with Bacillus strains as an example. CID 46(Suppl 2):S92–S95
Sowmya R, Sachindra NM (2012) Evaluation of antioxidant activity of carotenoid extract from shrimp processing by products by in-vitro assays and in membrane model system. Food Chem 134:308–314
Xie J, Zhang R, Shang C, Guo Y (2009) Isolation and characterization of a bacteriocin produced by an isolated Bacillus subtilis LFB112 that exhibits antimicrobial activity against domestic animal pathogens. Afr J Biotechnol 8(20):5611–5619
Thirabunyanona M, Thongwittaya N (2012) Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella enteritidis infection. Res Vet Sci 93:74–81
van Schaik W, Gahan CGM, Hill C (1999) Acid-adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotics nisin and lacticin 3147. J Food Prot 62(5):536–539
Yuk HG, Marshall DL (2004) Adaptation of Escherichia coli O157:H7 to pH alters membrane lipid composition, verotoxin secretion, and resistance to simulated gastric fluid acid. Appl Environ Microbiol 70(6):3500–3505
Acknowledgments
The authors wish to thank the Director, CFTRI, for all the facilities. This study is a part of start-up project funded by SERB-Department of Science and Technology, Government of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 59 kb)
Rights and permissions
About this article
Cite this article
Shobharani, P., Halami, P.M. Cellular fatty acid profile and H+-ATPase activity to assess acid tolerance of Bacillus sp. for potential probiotic functional attributes. Appl Microbiol Biotechnol 98, 9045–9058 (2014). https://doi.org/10.1007/s00253-014-5981-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5981-3