Abstract
The production of α-ketoglutaric acid by yeast Yarrowia lipolytica VKMY-2412 from ethanol and its subsequent chemical conversion to succinic acid (SA) were investigated. A highly effective and environmentally friendly process of α-ketoglutaric acid production was developed using a special pH-controlling strategy, in which the titration of the culture broth with KOH in the acid-formation phase was minimal, that allowed accumulation of only low amounts of inorganic wastes in the course of SA recovery. The culture broth filtrate containing α-ketoglutaric acid (88.7 g l−1) was directly employed for SA production; the amount of SA produced comprised 71.7 g l−1 with the yield of 70 % from ethanol consumed. SA was isolated from the culture broth filtrate in a crystalline form with the purity of 100 %. The yield of isolated SA was as high as 72 % of its amount in the culture broth filtrate. The antimicrobial and nematocidic effects of SA of microbial origin on pathogenic organisms that cause human and plant diseases were revealed for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent years demonstrate an increasing interest in microbial production of succinic acid (SA) as an alternative of a chemical SA synthesis from maleic anhydride. The microbiological synthesis of SA is considered as more perspective due to its ecological and economical advantages (Zeikus et al. 1999; Carole et al. 2004; Werpy et al. 2006; McKinlay et al. 2007; Bechthold et al. 2008; Bozell and Petersen 2010). The SA production is mostly based on the use of bacteria, such as Anaerobiospirillum succiniciproducens, Actinobacillus succinogenes, Mannheimia succiniciproducens, Escherichia coli, and Corynebacterium glutamicum (Zeikus et al. 1999; Carole et al. 2004; Werpy et al. 2006; McKinlay et al. 2007; Bechthold et al. 2008; Sauer et al. 2008; Bozell and Petersen 2010; Cheng et al. 2012). The pilot plants producing SA are known to operate in Europe, USA, and Japan for the last few years (Yuzbashev et al. 2011; De Jong et al. 2012). The SA had been also produced by fungus Penicillium simplicissimum (Gallmetzer et al. 2002) and yeasts Saccharomyces cerevisiae (Arikawa et al. 1999), Candida brumptii (Sato et al. 1972), Candida zeylanoides (Mandeva et al. 1981; Kamzolova et al. 2009a), Candida catenulata (Kamzolova et al. 2009a), and Yarrowia lipolytica (Yuzbashev et al. 2010). The SA can be also produced through the reduction of fumaric acid by using some fungi and bacteria (Moon et al. 2004).
It is known that SA is produced through several metabolic pathways: (1) the reductive part of the tricarboxylic acid cycle (TCA) due to increased activities of phosphoenolpyruvate and pyruvate carboxylase (Millard et al. 1996; Lin et al. 2005); (2) the oxidative part of the TCA cycle due to the deletion of succinate dehydrogenase (Arikawa et al. 1999; Camarasa et al. 2003; Yuzbashev et al. 2010); or (3) the glyoxylate cycle (Sato et al. 1972; Vemuri et al. 2002; Sanchez et al. 2005; Wu et al. 2007; Kamzolova et al. 2009a). SA can be also formed through the decarboxylation of α-ketoglutaric acid by hydrogen peroxide (Concise Chemical Encyclopedia 1961; Fedotcheva et al. 2006) according to the following equation:
Based on the chemical knowledge, co-authors of the present study developed the process of SA production that involved the biosynthesis of α-ketoglutaric acid by yeast Y. lipolytica and the subsequent decarboxylation of α-ketoglutaric acid to SA in the presence of H2O2 (Kamzolova et al. 2009b, 2012a, 2014). Ethanol, n-alkanes, and rapeseed oil were tested as carbon sources; it was revealed that the SA yield and hence the cost of the invented product were considerably dependent on the carbon source applied.
At present, ethanol is considered as a promising carbon substrate for the development of biotechnological processes; it can be produced from renewable resources, such as sugar cane, beet, corn, or lignocelluloses (Stephanopoulos 2007; Weusthuis et al. 2011). Chemicals produced from ethanol are permissible for usage in the food and medical industry. Ethanol was shown to be a promising substrate for production of citric acid (Kamzolova et al. 2003), α-ketoglutaric acid (Chernyavskaya et al. 2000; Kamzolova et al. 2012b), and lipid (Dedyukhina et al. 1994). As reported by Weusthuis et al. (2011), the several companies in the USA and Switzerland created the food products based on microbial biomass produced from ethanol. We performed the SA production from ethanol by Y. lipolytica (Kamzolova et al. 2009b), which resulted in the accumulation of SA up to 63.4 g l−1. However, the disadvantage of this method was low SA yield from ethanol (58 %).
The aims of the present work were (1) to develop a highly effective process of SA production from ethanol by using strain Y. lipolytica VKMY-2412, which includes a simple procedure of SA recovery and (2) to characterize of invented preparation by the evaluation of antimicrobial and nematocidic properties of SA.
Materials and methods
Microorganism
We used strain Y. lipolytica VKM Y-2412 obtained from the All-Russian Collection of Microorganisms (VKM). The strain was maintained at 4 °C on agar slants with n-alkanes as the carbon substrate.
Chemicals
All chemicals were of analytical grade (Sigma-Aldrich, St. Louis, MO, USA). Ethanol was purchased from the Kazan Ethanol Processing Company (Russia) and used as a carbon source.
Batch cultivation of Y. lipolytica in a fermenter
Strain Y. lipolytica VKM Y-2412 was cultivated in a 10-l ANKUM-2 M fermenter (SKB, Pushchino, Russia) with an initial volume of 5 l in the medium containing (g l−1) the following: (NH4)2SO4, 6.0; МgSO4·7H2O, 1.4; NaCl, 0.5; Ca(NO3)2, 0.8; KH2PO4, 2.0; K2HPO4, 0.2; and Burkholder’s trace element solution (Burkholder et al. 1944). In the course of cultivation, solution of (NH4)2SO4 (1 g l−1) was added into a fermenter at 48, 72, 96, and 168 h. In preliminary experiments, it was indicated that (NH4)2SO4 added to the medium in concentration of 10 g l−1 before inoculation inhibited growth and acid-formation of producer. However, (NH4)2SO4 added in concentration of 6 g l−1 at the onset of incubation and subsequently by 1 g l−1 at 48, 72, 96, and 168 h did not affect physiology of the producer. The medium contained increased amounts of ZnSO4·7H2O (1.32 mg l−1) and FeSO4·7H2O (5.96 mg l−1). The thiamine concentration was 1.2 μg l−1. The fermentation conditions were maintained automatically at the constant level: temperature (29 ± 0.5 °C); the medium pH was adjusted with 20 % KOH as described in the text; dissolved oxygen concentration (pO2) was 20 and 60 % (of air saturation) for the growth- and the acid-production phase, respectively; and agitation was 800 rpm. Pulsed addition of ethanol (by 1.6 g l−1) was performed as the pO2 value increased by 10 % indicating a decrease in respiratory activity of cells due to the total consumption of the carbon source. Cultivation was carried out for 8 days.
Analytical methods
Biomass was determined gravimetrically.
Thiamine concentration in the medium was determined by microbiological method based on a thiamine-auxotrophic strain Y. lipolytica 695.
To analyze organic acids, the culture broth was centrifuged (8,000 g, 3 min); 1 ml of the supernatant was diluted with an equal volume of 6 % HClO4, and the concentration of organic acids was determined using high-performance liquid chromatography (HPLC) on an HPLC chromatograph (Pharmacia, LKB, Uppsala, Sweden) equipped with an Inertsil ODS-3 reversed-phase column (250 × 4 mm, Elsiko, Russia) at 210 nm; 20 mM phosphoric acid was used as a mobile phase with the flow rate of 1.0 ml min−1, and the column temperature was maintained at 35 °C. Quantitative determination of organic acids was carried out using the calibration curves for SA, α-ketoglutaric acid (Sigma-Aldrich, St. Louis, MO, USA), and citric, isocitric, acetic, and fumaric acids (Boehringer Manheim, Germany) as standards. Additionally, SA was analyzed enzymatically using biochemical kit (Boehringer Mannheim/R-Biopharm).
The inhibitory activity of SA
The antimicrobial activity of SA was estimated against Gram-positive bacteria (Staphylococcus aureus 209B (IMV) and Bacillus subtilis 912B (RCM)), Gram-negative bacteria (E. coli 567B (RCM) and Erwinia carotovora 1247B (RCM)), and fungi (Trichothecium roseum 750 F (RCM), Fusarium oxysporum f.sp.lycopersici 840 F (RCM), Fusarium napiforme 152 F (IMV), Cylindrocarpon gracile 918 F (RCM), Aspergillus flavus 25 F (RCM), and Penicillium casei 542 F (IMV)).
Nematocidic activity of SA was studied against phytoparasitic stem nematodes (potato rot nematode) Ditylenchus destructor, which were isolated directly from potato tubers commercially sold in the Almaty region of Kazakhstan; nematodes were collected in fresh state for each experiment. Potato pieces were placed in sterile water in Petri dishes; the nematodes were removed with forceps and transferred into watch glasses containing either SA solution (50 mg ml−1) or control solutions (5 % ethanol or sterile water) or comparators (5 % acetic acid and 5 % lactic acid). Each glass contained 30 nematodes in 2 ml of SA or control solutions. Glasses were incubated 72 h at 26 °C. All nematodes in each glass were then examined for their motility and recorded as active or inactive ones. Each nematode was then transferred into sterile water for additional 24-h incubation, and its motility was re-examined. Nematodes that remained inactive after transferring to sterile water were considered as dead. The treatment procedures were repeated three times per experiment. The SA effect was considered as toxic if motility of nematodes stopped and was not restored for 24 h after their transferring into water or as nematostatic (temporary) if motility was restored in water.
Calculation of fermentation parameters
The biomass yield was calculated as follows: Yx/s = X/S, where X is the total amount of biomass in the culture liquid at the end of fermentation (g) and S is the amount of ethanol consumed (g).
To take into account the medium dilution due to the addition of KOH solution for maintaining the constant pH value, the total amount of α-ketoglutaric acid in the culture broth was used for calculations of the mass yield of α-ketoglutaric acid (Y KGA), volumetric α-ketoglutaric acid productivity (Q), and specific acid production rate (q KGA).
The mass yield of α-ketoglutaric acid production (Y KGA), expressed in grams of α-ketoglutaric acid per gram of ethanol, was calculated from
The volumetric α-ketoglutaric acid productivity (Q KGA), expressed in g/(l h), was calculated from
The specific α-ketoglutaric acid production rate (q KGA), expressed in g/g (cells)·h, was calculated from
where P is the total amount of α-ketoglutaric acid in the culture liquid at the end of cultivation (g), S is the total amount of ethanol consumed (g), V is the initial volume of culture liquid (l), t is the fermentation duration (h), and X is the average working biomass in the fermentor (g).
Statistical analysis
All the data presented are the mean values of three experiments and two measurements for each experiment; standard deviations were calculated (SD < 10 %).
Results
SA production in the culture broth
The process of SA production included the following stages: (1) microbial synthesis of α-ketoglutaric acid by yeast strain Y. lipolytiсa, (2) separation of cells with the subsequent treatment of the culture broth filtrate containing α-ketoglutaric acid with hydrogen peroxide for the production of SA, and (3) the recovery of SA from the culture broth filtrate.
We used earlier selected strain Y. lipolytica VKM Y-2412 that was characterized by high production of α-ketoglutaric acid from ethanol (Kamzolova et al. 2012b) and rapeseed oil (Kamzolova and Morgunov 2013). In order to achieve high concentration of α-ketoglutaric acid, we modified the fermentation conditions described earlier (Kamzolova et al. 2012b) by using a special pH-controlling strategy. We applied a three-stage pH-controlling method in which pH was 4.5 in the growth and the growth retardation phase up to 72 h, then pH was maintained at 3.5 from 72 to 144 h in the period of the α-ketoglutaric acid synthesis, and in the later phase of acid production, the titration by KOH was switched off.
The time courses of Y. lipolytica VKM Y-2412 growth, thiamine consumption, α-ketoglutaric acid production, and the calculated parameters of the process are shown in Fig. 1. The growth curve had three phases: the exponential growth phase (up to 18 h), the growth retardation phase (18–72 h), and the stationary phase (after 72 h). The maximum specific growth rate (μmax) calculated from the linear segment of the growth curve amounted to 0.220 h−1; a value comparable with literature data obtained for Y. lipolytica cultivated on glucose (Workman et al. 2013), glycerol (Workman et al. 2013), and fatty acids (Papanikolaou et al. 2002). No accumulation of α-ketoglutaric acid was observed during the exponential phase. The transition of Y. lipolytica VKM Y-2412 cells from the exponential growth to the growth retardation phase caused by thiamine exhaustion in the medium was accompanied by a decrease in specific growth rate (μ) to 0.051 h−1 and by stimulation of α-ketoglutaric acid production. Intense synthesis of α-ketoglutaric acid was observed in the stationary phase. The dynamics of KOH addition indicated that KOH was consumed at a rate of 0.083 g KOH (l h)−1 during the first hours of cultivation; in the period from 24 to 144 h, KOH consumption increased 1.5–2.3-fold, and later, the synthesis of α-ketoglutaric acid proceeded without KOH addition. By the end of cultivation (240 h), yeast produced α-ketoglutaric acid (102 g l−1) as a major product, and the production of SA and the other organic acids (citric, isocitric, acetic, and fumaric) was less than 0.5 g l−1. The mass yield of α-ketoglutaric acid from ethanol consumed (Y KGA) in Y. lipolytica VKM Y-2412 was 0.70 g g−1. As can be seen from the data presented in Fig. 1, the specific culture productivity (q p) of Y. lipolytica VKM Y-2412 remained constant (0.030–0.040 g (g cells)−1 h−1) during the growth retardation and the stationary phases and decreased by two to three times after 192 h; therefore, prolongation of the process over 8 days was ineffective.
In a series of experiments, the attempt to increase the concentration of productive cells of Y. lipolytica VKM Y-2412 was made. For that, in the culture medium, concentration of thiamine was increased up to 2.0 and 3.0 μg l−1, and the amount of ammonium sulfate was increased to 14 g l−1 (nitrogen was added at the beginning of the process (6 g l−1) and by 2 g l−1 at 48, 72, 96, and 168 h). In the course of fermentation, the pO2 value was maintained at the constant level (as mentioned in the “Materials and methods” section) by changing the stirring rate and by increasing the aeration rate (up to two to three volumes of air per volume of medium). It should be noted that at thiamine concentration of 3.0 μg l−1, aeration was so high that the water was carrying-out from a fermentor with air.
The growth of Y. lipolytica VKM Y-2412, α-ketoglutaric acid production, and the calculated parameters of the process at different thiamine concentrations are shown in Table 1. The best results were obtained at thiamine concentration of 1.2 μg l−1 and the biomass amount of 14.7 g l−1. When thiamine concentration was increased to 2.0 and 3.0 μg l−1, biomass was increased to 20.3 and 26.0 g l−1, respectively, but production of α-ketoglutaric acid was enhanced only by 5 and 7 %, respectively.
We calculated the productivity of α-ketoglutaric acid biosynthesis (P) for each variant, expressing it as the amount of α-ketoglutaric acid (g) produced by 1 g of cells. The productivity of cells was maximal at biomass of 14.7 g l−1 and gradually decreased at a higher level of biomass (20.3 and 26.0 g l−1). The volumetric α-ketoglutaric acid productivity (Q KGA) and the mass yield (Y KGA) by Y. lipolytica VKM Y-2412 reached 0.727 g l−1 h−1 and 0.70 g g−1, respectively, at biomass of 14.7 g l−1 and gradually decreased at higher level of biomass. The accumulation of by-products (SA and citric, isocitric, acetic, and fumaric acids) was drastically increased at a higher level of biomass.
The results demonstrated that it is reasonable to work with biomass of 14–15 g l−1 in order to obtain the maximum concentration of α-ketoglutaric acid and high productivity. At this level of biomass, it is possible to maintain aeration at the level of 60 % (from saturation) during the α-ketoglutaric acid biosynthesis using a fermentor of the given construction.
In further experiments on transferring α-ketoglutaric acid into SA, the strain was grown under optimal conditions during 192 h at thiamine concentration in the medium of 1.2 μg l−1. By the end of the Y. lipolytica VKM Y-2412 cultivation, biomass was separated from the culture broth by centrifugation; filtrate contained potassium salts of α-ketoglutaric acid, traces of other organic acids (SA, citric, isocitric, acetic, and fumaric), and the residues of unconsumed mineral salts.
Aliquots of the culture broth filtrate containing 88.7 g l−1 of α-ketoglutaric acid (608 mM) were incubated for 1 h with 608 mM H2O2. After a 1-h incubation with H2O2, the culture broth filtrate contained SA but not α-ketoglutaric acid; finally, 88.7 g l−1 of α-ketoglutaric acid (608 mM) was completely oxidized up to 71.7 g l−1 of SA (608 mM).
Recovery of SA from the culture broth
The culture broth containing SA as a monopotassium salt and trace amount of H2O2 was boiled in the presence of manganese dioxide as a catalyst for the decomposition of residual H2O2. The content of H2O2 traces and peroxyacids (organic acids in which one or several hydroxyl groups are substituted for the hydrogen peroxide residue) can be easily determined by using very sensitive reaction with phenolphthalein in the presence of one drop of 0.01 M copper sulfate. After that, the filtrate was clarified by filtration through charcoal cardboard AKS-4 (Germany) to remove colored impurities of manganese dioxide. After that, the filtrate was acidified with sulfuric acid up to pH 2.85–2.95 to convert potassium salts of SA to free SA. It should be noted that the acidification of solution to lower pH values decreased purity of the product, whereas at higher pH values, the yield of the product was reduced. The filtrate containing free SA and residual K2SO4 was evaporated to paste concentrate. After that, the concentrate was treated with 96 % ethanol for SA extraction. The use of ethanol at the stage of SA recovery made it possible to extract SA which is poorly soluble in other organic solvents and to get rid of the major portion of inorganic salts (in particular, potassium sulfate is insoluble in ethanol).
The solution was decanted and filtered through a glass filter. The ethanol fraction containing SA was evaporated, and dry residue was recrystallized with ethanol. The purity of the obtained crystalline SA reached 100 %. The yield of SA was as high as 72 % of its amount in the culture broth filtrate.
Inhibitory activity of SA
The antimicrobial activity of SA was evaluated against pathogenic bacteria and fungi causing human and plant diseases.
Ethanol was used as a control in our experiments, because it is a well-known antimicrobial agent which is active against Gram-positive and Gram-negative bacteria and viruses that cause human and plant diseases. Acetic and lactic acids were investigated as comparators of inhibitory effects on pathogenic organisms. Numerous literature data indicate that acetic, citric, lactic, formic, and sorbic acids have been used as safe antimicrobial agents (Bjornsdottir et al. 2006; Baimark et al. 2008; In et al. 2013; Lastauskienė et al. 2014).
The results of antimicrobial activity of SA are shown in Table 2. It was found that SA selectively inhibited growth of three out of ten tested cultures: S. aureus, E. carotovora, and P. casei (zones of inhibition of the test culture growth reached 22, 16, and 17 mm, respectively). Lactic acid selectively inhibited growth of E. coli, S. aureus, E. carotovora, and B. subtilis, while acetic acid inhibited the growth of most of the studied bacteria and fungi.
The nematocidic activity of SA was evaluated against the potato rot nematode D. destructor. As seen from Table 3, after a 72-h treatment with SA, the number of inactive nematodes, which lost motility, reached 72 % of the initial amount; after the transition into water, 13.7 % of inactive nematodes restored their motility. Therefore, lethality of nematodes under the action of SA was 58.3 %. The lethality of nematodes under the action of acetic and lactic acids amounted to 100 and 73 %, respectively.
Discussion
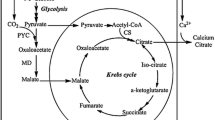
The principal scheme of the process of SA production is presented in Fig. 2. The method of SA production involves the biosynthesis of α-ketoglutaric acid from ethanol by the yeast Y. lipolytica VKM Y-2412 and the subsequent decarboxylation of α-ketoglutaric acid to SA in the presence of H2O2. The oxidation of ethanol to acetaldehyde is catalyzed by NAD-dependent alcohol dehydrogenase (EC 1.1.1.1), after which NAD-dependent aldehyde dehydrogenase (EC 1.2.1.2) catalyzes oxidation of acetaldehyde to acetate and then acetate is transformed into acetyl-CoA, the main substrate of TCA (Barth and Gaillardin 1996; Kamzolova et al. 2009a). High activities of the TCA enzymes, including citrate synthase (EC 4.1.3.7), aconitate hydratase (ЕС 4.2.1.3), and NAD-isocitrate dehydrogenase (ЕС 1.1.1.41), and low activity of α-ketoglutarate dehydrogenase (ЕС 1.2.4.2) are necessary conditions for intensive α-ketoglutaric acid production in view of the fact that α-ketoglutaric acid formed in the TCA cycle is excreted from the yeast cell rather than metabolized through the TCA cycle. Moreover, the assimilation of ethanol involves the functioning of glyoxylate cycle (Finogenova et al. 2002); in this case, unlike the TCA cycle, isocitrate is converted into glyoxylate and succinate by isocitrate lyase (EC 4.1.3.1.). The shortcut cycle is completed by the formation of malate from glyoxylate and acetyl-CoA.
The SA production by yeast Y. lipolytica through two steps: α-ketoglutaric acid synthesis via the TCA under deficiency of the α-ketoglutarate dehydrogenase complex and chemical conversion of α-ketoglutarate to SA in the presence of H2O2. ADH NAD-dependent alcohol dehydrogenase, AlDH NAD-dependent aldehyde dehydrogenase, ACS acetyl-CoA synthase, CS citrate synthase, AH aconitate hydratase, NAD-IDH NAD-dependent isocitrate dehydrogenase, KGDH α-ketoglutarate dehydrogenase, ICL isocitrate lyase, MS malate synthase, SDH succinate dehydrogenase, FU fumarase, MDH malate dehydrogenase
The yeasts Y. lipolytica were selected as producers of α-ketoglutaric acid because of their inability to synthesize the pyrimidine moiety of the thiamine molecule (Barth and Gaillardin 1996; Stottmeister et al. 2005; Finogenova et al. 2005; Zhou et al. 2010; Otto et al. 2011; Groenewald et al. 2014). During the cultivation of Y. lipolytica under the thiamine deficiency conditions when thiamine proves to be a growth-limiting factor, the cells convert ethanol into the incompletely oxidized product, α-ketoglutaric acid. The acid is excreted into the medium and can be accumulated in large amounts. α-Ketoglutaric acid is successively decarboxylated to SA in the presence of deliberately added H2O2.
It is well known that the most expensive stage of microbial SA production is the isolation of SA from the culture broth, since the conversion of produced SA salts into free SA is required. This stage made up 60–70 % of the product cost (Zeikus et al. 1999). It is considered that the promising process should imply the fermentation without any titration agent or with its limited use where the free SA is the main product (Yuzbashev et al. 2011). However, the traditional producers of SA, bacteria A. succiniciproducens, A. succinogenes, M. succiniciproducens, recombinant strains of E. coli, and C. glutamicum, should be cultivated at neutral pH, since at lower pH (5–6), significant amounts of by-products (acetate, formate, ethanol, and others) are accumulated (Zeikus et al. 1999; Carole et al. 2004; Werpy et al. 2006; McKinlay et al. 2007; Bechthold et al. 2008; Bozell and Petersen 2010; Cheng et al. 2012). To maintain optimal pH value, it is necessary to add KOH, NaOH, and other bases which react with SA; as a result, SA salts are produced rather than the desired free SA; therefore, additional stage of medium acidifying was required before the SA extraction.
In the present study, we used a three-stage pH-controlling regime, in which pH was 4.5 in the growth phase, then it was maintained at 3.5 from 72 to 144 h, and finally, in the later phase of acid production, the titration with KOH was switched off (Fig. 1). The use of a three-stage pH-shift technology during the growth and the biosynthesis phases is a principal approach for the development of highly effective and environmentally friendly processes of the α-ketoglutaric acid production. In this case, the calculated value of KOH expenditure was only 250 g per 1 kg of SA, while Yuzbashev et al. (2011) reported that for the production of 1 ton of succinate with the use of bacteria, about half a ton of alkali (KOH) needs to be added to maintain neutral pH. According to literature data, the yeast Y. lipolytica can efficiently grow in a wide range of pH and produce practically valuable metabolites at low pH values (Zinjarde 2014). There is information on the enhanced production of SA (Yuzbashev et al. 2010) and erythritol by Y. lipolytica from glycerol at pH 3.0 (Mirończuk et al. 2014). Under optimal fermentation conditions, the engineered strain S. cerevisiae with deleted GPD1 was able to produce SA at pH 3.8 (Yan et al. 2014). The pH shift from 5.0 during the growth phase to pH 3–4 in the phase of acid synthesis was used in order to develop highly effective process of α-ketoglutaric acid production from on n-paraffins (195 g l−1) (Weissbrodt et al. 1988). High KGA production from rapeseed oil (115 g l−1) was revealed in fed-batch culture of Y. lipolytica H222-27-11 at pH 5.0 in growth phase, and pH 3.5 after 48 h, during acid formation (Aurich et al. 2012). Using a two-stage pH control strategy, in which pH was buffered by CaCO3 in the growth phase and then maintained at 3.0 in the acid production phase, the concentration of α-ketoglutaric acid reached 66.2 g l−1 with Y. lipolytica WSH-Z06, grown on glycerol (Yu et al. 2012).
The strain Y. lipolytica VKM Y-2412 excreted up to 88.7 g l−1 of α-ketoglutaric acid with yield of 0.70 g g−1 (Fig. 1) under the three-stage pH-controlling regime. Earlier, the production of 49 g l−1 of α-ketoglutaric acid by ethanol-grown mutant Y. lipolytica N 1 was reported at pH 4.5 (Chernyavskaya et al. 2000). The high α-ketoglutaric acid production from ethanol (172 g l−1) was achieved using a two-stage controlling strategy (pH 5.0 in the growth phase and pH 3.5 in the acid-producing stage), but volumetric productivity was low due to a long-term cultivation for 324 h (Kamzolova et al. 2012a). Using a three-stage pH-controlling method in the case of yeast cultivation on rapeseed oil as a carbon source, we achieved 106.5 g l−1 of α-ketoglutaric acid with mass yield of 0.95 g g−1 (Morgunov et al. 2013).
As seen from Fig. 1, the prolongation of the process over 192 h is inefficient because the specific culture productivity (q p) of Y. lipolytica VKM Y-2412 decreased by two to three times as compared with that in the earlier stationary phase. Possible reasons for a decrease in the metabolic activity of producer are aging of the cells due to the absence of growth in the thiamine-limited medium. The disadvantages of a long-time cultivation mentioned above dictate the necessity to use the other approaches for improving biosynthesis of α-ketoglutaric acid.
In a series of experiments, the attempt to increase the concentration of productive cells of the SA producer Y. lipolytica VKM Y-2412 was made. An increase in thiamine concentration in the medium to 2.0 and 3.0 μg l−1 resulted in increased biomass level to 20.3 and 26.0 g l−1, respectively; however, production of α-ketoglutaric acid was enhanced only by 5 and 7 %, respectively. In further experiments on transferring α-ketoglutaric acid into SA, the strain was grown under optimal conditions during 192 h at thiamine concentration in the medium of 1.2 mg l-1.
The culture broth filtrate containing 88.7 g l−1 of α-ketoglutaric acid was directly employed for SA conversion by H2O2 treatment; the amount of SA produced reached 71.7 g l−1 with the yield of 70 % from ethanol consumed. These results were higher by 13.2 and 20.6 %, respectively, than the values previously published for the SA production from ethanol (Kamzolova et al. 2009b) and comparable with the data reported recently for SA production from rapeseed oil (Kamzolova et al. 2014). For comparison, bacteria M. succiniciproducens produced 52.4 g l−1 of SA with yield of 0.76 g g−1 in the medium containing glucose as a carbon source (Lee et al. 2006). Yuzbashev et al. (2010) constructed Y. lipolytica strains with deleted SDH2 gene coding one of subunits of succinate dehydrogenase, which was able to produce 45 g l−1 of SA. A high-efficiency process of SA production from glycerol was developed by using a combination of the cell-recycled cultivation of A. succiniciproducens with subsequent electrodialysis in order to concentrate SA; as a result, SA concentration of 83 g l−1 with the yield of 0.89 g g−1 was achieved (Meynial-Salles et al. 2008). High SA production (80–110 g l−1) was obtained in the monofluoroacetate-resistant mutant of rumen bacteria Bacterium sp. 130Z grown anaerobically in carbohydrate-containing medium in the presence of CO2 (Guettler et al. 1996). The highest SA production (146 g l−1) was reached with a genetically-modified strain C. glutamicum, which was cultivated at high cell density under oxygen limitation with pulsed addition of sodium bicarbonate and glucose (Okino et al. 2008).
We succeeded in the isolation of SA from the culture broth filtrate in a crystalline form; the purity of crystalline SA reached 100 %, and the yield of SA was as high as 72 % of its amount in the culture broth filtrate. The recovery of SA from the culture broth involved the decomposition of H2O2 in the filtrate, the filtrate bleaching and acidification with a mineral acid, the evaporation of the filtrate, the extraction of SA by ethanol, the concentration of SA, and its crystallization.
Two important points should be taken into account in the course of SA isolation from the culture broth. (1) Before the SA extraction with ethanol, the culture broth should be at first acidified and then concentrated; otherwise, acidification of the concentrate would result in its dilution and the evaporation procedure must be repeated. (2) The use of ethanol at the stage of SA recovery made it possible to extract SA, which is poorly soluble in other organic solvents, and to get rid of the major portion of inorganic salts (in particular, potassium sulfate is insoluble in ethanol).
It should be noted that the invented process is characterized by low accumulation of by-product, K2SO4 (~400 g per 1 kg of SA), since the KOH expenditure was only 250 g per 1 kg of SA (Fig. 1). K2SO4 could be utilized as a fertilizer; it does not contain chloride, which can be harmful for some crops. For comparison, Yuzbashev et al. (2011) reported that for the production of 1 ton of succinate, about half a ton of alkali (KOH) needs to be added to maintain neutral pH; subsequently, to obtain free acid in a downstream purification process, about 1 ton of sulfuric acid will be used. As a result, more than 1 ton of inorganic salts will be formed.
We studied antimicrobial and nematocidic activities of SA produced by Y. lipolytica. As seen from Table 2, SA inhibited growth of S. aureus, E. carotovora, and P. casei. It should be noted that antimicrobial activity of SA was almost similar to that of the standard drugs. It was reported that penicillin G tested against Gram-positive bacteria formed the inhibition zone of 24.6 mm; streptomycin tested against Gram-negative bacteria formed the inhibition zone of 26.4 mm, whereas amphotericin tested against fungi formed the inhibition zone of 26.7 mm (Mohamed et al. 2014). We found that lactic acid selectively inhibited growth of E. coli, S. aureus, E. carotovora, and B. subtilis, while acetic acid inhibited the growth of most of the studied bacteria and fungi.
We have also tested the inhibitory effect of SA on potato rot nematode D. destructor, which is a pest of many agriculturally important crops. As seen from Table 3, the treatment of nematodes with SA during 72 h resulted in the inactivation of 72 % of nematodes. The nematostatic activity expressed as the amount of nematodes, which restored their motility after the transfer into water, comprised 13.7 %; nematocidic activity reached 58.3 %. The lethality of nematodes under the action of acetic and lactic acids amounted to 100 and 73 %, respectively. To our knowledge, it is the first report on the inhibitory effects of SA and acetic and lactic acids on the survival of plant-parasitic nematode D. destructor.
It should be noted that it is the first report on antibacterial and nematocidic activities of SA. The mechanism of SA action on bacteria and nematodes is unclear; it is possible that SA penetrated inside the cells and decreased intracellular pH that resulted in the inhibition of metabolism of bacteria and nematodes; however, it cannot be excluded that the effect of SA on intracellular metabolism of test cultures was more complicated. The effect of exogenous SA on metabolism of bacteria and nematodes requires special investigations.
The obtained results make it possible to suggest that SA of microbial origin may be used for the control of potentially pathogenic microorganisms and thereby offers natural alternative to the antibiotic treatment.
References
Arikawa Y, Kuroyanagi T, Shimosaka M, Muratsubaki H, Enomoto K, Kodaira R, Okazaki M (1999) Effect of gene disruptions of the TCA cycle on production of succinic acid in Saccharomyces cerevisiae. J Biosci Bioeng 87:28–36
Aurich A, Specht R, Müller RA, Stottmeister U, Yovkova V, Otto C, Holz M, Barth G, Heretsch P, Thomas FA, Sicker D, Giannis A (2012) Microbiologically produced carboxylic acids used as building blocks in organic synthesis. In: Wang X, Chen J, Quinn P (eds) Reprogramming microbial metabolic pathways. Springer-Dortrecht-Heidelberg, New York, pp 391–424
Baimark Y, Threeprom J, Dumrongchai N, Srisuwan Y, Kotsaeng N (2008) Utilization of wood vinegars as sustainable coagulating and antifungal agents in the production of natural rubber sheets. J Environ Sci Technol 1:157–163
Barth G, Gaillardin C (1996) Yarrowia lipolytica. In: Wolf K (ed) Non conventional yeasts in biotechnology: a handbook. Springer, Berlin, pp 313–388
Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A (2008) Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol 31:647–654
Bjornsdottir K, BreidtJr F, McFeeters RF (2006) Protective effects of organic acids on survival of Escherichia coli O157: H7 in acidic environments. Appl Environ Microbiol 72:660–664
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10”. Green Chem 12:539–554
Burkholder P, McVeigh J, Moyer D (1944) Studies on some growth factors of yeasts. J Bacteriol 48:385–391
Camarasa C, Grivet JP, Dequin S (2003) Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology 149:2669–2678
Carole TM, Pellegrino J, Paster MD (2004) Opportunities in the industrial biobased products industry. Appl Biochem Biotechnol 115:871–885
Cheng KK, Zhao XB, Zeng J, Zhang JA (2012) Biotechnological production of succinic acid: current state and perspectives. Biofuels Bioprod Biorefin 6:302–318
Chernyavskaya OG, Shishkanova NV, Il’chenko AP, Finogenova TV (2000) Synthesis of α-ketoglutaric acid by Yarrowia lipolytica yeast grown on ethanol. Appl Microbiol Biotechnol 51:152–158
Concise Chemical Encyclopedia (1961) Knunyanz LI. et al. (Eds), Vol. 2. Sovetskaya Entsiklopediya, Moscow, p. 552
De Jong E, Hidson A, Walsh P, Welisch M (2012) Product development in the biobased chemical arena. Biofuels Bioprod Biorefin 6:606–624
Dedyukhina EG, Kamzolova SV, Eroshin VK (1994) Investigation of lipid synthesis and biomass composition in the constitutive lipid producer Debaryomyces globosus under conditions of continuous cultivation. Mikrobiologiya 63(3):1007–1014 (in Russian)
Fedotcheva NI, Sokolov AP, Kondrashova MN (2006) Nonenzymatic formation of succinate under oxidative stress. Free Radic Biol Med 41:56–64
Finogenova TV, Kamzolova SV, Dedyukhina EG, Shishkanova NV, Il’chenko AP, Morgunov IG, Chernyavskaya OG, Sokolov AP (2002) Biosynthesis of citric and isocitric acids from ethanol by mutant Yarrowia lipolytica N 1 under continuous cultivation. Appl Microbiol Biotechnol 59:493–500
Finogenova TV, Morgunov IG, Kamzolova SV, Chernyavskaya OG (2005) Organic acid production by the yeast Yarrowia lipolytica: a review of prospects. Appl Biochem Microbiol 41:418–425
Gallmetzer M, Meraner J, Burgstaller W (2002) Succinate synthesis and excretion by Penicillium simplicissimum under aerobic and anaerobic condition. FEMS Microbiol Lett 210:221–225
Groenewald M, Boekhout T, Neuvéglise C, Gaillardin C, van Dijck PW, Wyss M (2014) Yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Crit Rev Microbiol 40(3):187–206
Guettler MV, Jain MK, Rumler D (1996) Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. US Patent 5573931
In YW, Kim JJ, Kim HJ, Oh SW (2013) Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J Food Safety 33:79–85
Kamzolova SV, Morgunov IG (2013) α-Ketoglutaric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl Microbiol Biotechnol 97:5517–5525
Kamzolova SV, Shishkanova NV, Morgunov IG, Finogenova TV (2003) Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res 3:217–222
Kamzolova SV, Yusupova AI, Dedyukhina EG, Chistyakova TI, Kozyreva TM, Morgunov IG (2009a) Succinic acid synthesis by ethanol-grown yeast. Food Technol Biotechnol 47(2):144–152
Kamzolova SV, Yusupova AI, Vinokurova NG, Fedotcheva NI, Kondrashova MN, Finogenova TV, Morgunov IG (2009b) Chemically assisted microbial production of succinic acid by the yeast Yarrowia lipolytica grown on ethanol. Appl Microbiol Biotechnol 83:1027–1034
Kamzolova SV, Vinokurova NG, Yusupova AI, Morgunov IG (2012a) Succinic acid production from n-alkanes. Eng Life Sci 12:560–566
Kamzolova SV, Chiglintseva MN, Lunina JN, Morgunov IG (2012b) α-Ketoglutaric acid production by Yarrowia lipolytica and its regulation. Appl Microbiol Biotechnol 96:783–791
Kamzolova SV, Vinokurova NG, Dedyukhina EG, Samoilenko VA, Lunina JN, Mironov AA, Allayarov RK, Morgunov IG (2014) The peculiarities of succinic acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl Microbiol Biotechnol 98:4149–4157
Lastauskienė E, Zinkevičienė A, Girkontaitė I, Kaunietis A, Kvedarienė V (2014) Formic acid and acetic acid induce a programmed cell death in pathogenic Candida species. Curr Microbiol. doi:10.1007/S00284-014-0585-q
Lee SJ, Song H, Lee SY (2006) Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl Environ Microbiol 72:1939–1948
Lin H, San KY, Bennet GN (2005) Effect of Sorghum vulgare phosphoenolpyruvate carboxylase and Lactococcus lactis pyruvate carboxylase coexpression on succinate production in mutant strains of Escherichia coli. Appl Microbiol Biotechnol 67:515–523
Mandeva RD, Ermakova IT, Lozinov AB (1981) Metabolite excretion by yeasts of the genus Candida in media lacking sources N, P, S, or Mg and having different carbon sources. Mikrobiologiia 50:62–68
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740
Meynial-Salles I, Dorotyn S, Soucaille P (2008) A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol Bioeng 99:129–135
Millard CS, Chao Y-P, Liao JC, Donnely MI (1996) Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl Environ Microbiol 62:1808–1810
Mirończuk AM, Furgała J, Rakicka M, Rymowicz W (2014) Enhanced production of erythritol by Yarrowia lipolytica on glycerol in repeated batch cultures. J Ind Microbiol Biotechnol 41:57–64
Mohamed NA, Mohamed RR, Seoudi RS (2014) Synthesis and characterization of some novel antimicrobial thiosemicarbazone O-carboxymethyl chitosan derivatives. Int J Biol Macromol 63:163–169
Moon SK, Wee YJ, Yun JS, Ryu HW (2004) Production of fumaric acid using rice bran and subsequent conversion to succinic acid through a two-step process. Appl Biochem Biotechnol 113–116:843–855
Morgunov IG, Kamzolova SV, Samoilenko VA (2013) Enhanced α-ketoglutaric acid production and recovery in Yarrowia lipolytica yeast by effective pH controlling. Appl Microbiol Biotechnol 97:8711–8718
Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H (2008) An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol 81:459–464
Otto C, Yovkova V, Barth G (2011) Overproduction and secretion of α-ketoglutaric acid by microorganisms. Appl Microbiol Biotechnol 92:689–695
Papanikolaou S, Chevalot I, Komaitis M, Marc I, Aggelis G (2002) Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl Microbiol Biotechnol 58(3):308–312
Sanchez AM, Bennet GN, San KY (2005) Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity. Metab Eng 7:229–239
Sato M, Nakahara T, Yamada K (1972) Fermentative production of succinic acid from n-paraffin. Agric Biol Chem 36:1969–1974
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol 26:100–108
Stephanopoulos G (2007) Challenges in engineering microbes for biofuels production. Science 315:801–804
Stottmeister U, Aurich A, Wilde H, Andersch J, Schmidt S, Sicker D (2005) White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. J Ind Microbiol Biotechnol 32:651–664
Vemuri GN, Eiteman MA, Altman E (2002) Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 68:1715–1727
Weissbrodt E, Barth G, Weber H, Stottmeister U, Duresch R, Richter P (1988) Production of 2-oxoglutaric acid by yeasts. Patent DD 267999
Werpy T, Frye J, Holladay J (2006) Succinic acid—a model building block for chemical production from renewable resources. In: Kamm B, Gruber PR, Kamm M (eds) Biorefineries—industrial processes and products. Status quo and future directions, vol 2. Wiley-VCH Verlag, Weinheim, pp 367–379
Weusthuis R, Aarts J, Sanders J (2011) From biofuel to bioproduct: is bioethanol a suitable fermentation feedstock for synthesis of bulk chemicals? Biofuels Bioprod Biorefin 5:486–494
Workman M, Holt P, Thykaer J (2013) Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. AMB Express 3(1):58. doi:10.1186/2191-0855-3-58
Wu H, Li ZM, Zhou L, Ye Q (2007) Improved succinic acid production in the anaerobic culture of an Escherichia coli pflB ldhA double mutant as a result of enhanced anaplerotic activities in the preceding aerobic culture. Appl Environ Microbiol 73:7837–7843
Yan D, Wang C, Zhou J, Liu Y, Yang M, Xing J (2014) Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour Technol 156:232–239
Yu Z, Du G, Zhou J, Chen J (2012) Enhanced α-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by an improved integrated fed-batch strategy. Bioresour Technol 114:597–602
Yuzbashev TV, Yuzbasheva EY, Sobolevskaya TI, Laptev IA, Vybornaya TV, Larina AS, Matsui K, Fukui K, Sineoky SP (2010) Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol Bioeng 7:673–682
Yuzbashev TV, Yuzbasheva EY, Laptev IA, Sobolevskaya TI, Vybornaya TV, Larina AS, Sineoky SP (2011) Is it possible to produce succinic acid at a low pH? Bioengineered 2:115–119
Zeikus JG, Jain MK, Elankova PE (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552
Zhou J, Zhou H, Du G, Liu L, Chen J (2010) Screening of a thiamine-auxotrophic yeast for α-ketoglutaric acid overproduction. Lett Appl Microbiol 51:264–271
Zinjarde CC (2014) Food-related applications of Yarrowia lipolytica. Food Chem 152:1–10
Acknowledgments
This work was partially supported by the Russian Foundation for Basic Research (project no. 12-08-01157-a).
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamzolova, S.V., Vinokurova, N.G., Shemshura, O.N. et al. The production of succinic acid by yeast Yarrowia lipolytica through a two-step process. Appl Microbiol Biotechnol 98, 7959–7969 (2014). https://doi.org/10.1007/s00253-014-5887-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5887-0