Abstract
Within sustainable resource management, the recovery of nitrogen and phosphorus nutrients from waste streams is becoming increasingly important. Although the use of microalgae has been described extensively in environmental biotechnology, the potential of nitrate-accumulating microalgae for nutrient recovery has not been investigated yet. The ability of these marine microorganisms to concentrate environmental nitrate within their biomass is remarkable. The aim of this study was to investigate the application potential of nitrate-accumulating diatoms for nutrient recovery from marine wastewaters. The intracellular nitrate storage capacity was quantified for six marine diatom strains in synthetic wastewater. Amphora coffeaeformis and Phaeodactylum tricornutum stored the highest amount of nitrate with respectively 3.15 and 2.10 g N L−1 of cell volume, which accounted for 17.3 and 4.6 %, respectively, of the total nitrogen content. The growth and nitrate and phosphate uptake of both diatoms were further analyzed and based on these features P. tricornutum showed the highest potential for nutrient recovery. A mathematical model was developed which included intracellular nitrate storage and the kinetic parameters were derived for P. tricornutum. Furthermore, a simulation study was performed to compare the performance of a proposed microalgal nutrient recovery unit with a conventional denitrification system for marine wastewater treatment. Overall, this study demonstrates the potential application of P. tricornutum for saline wastewater treatment with concurrent nitrogen and phosphorus recycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Within sustainable resource management, the recovery of nutrients such as nitrogen and phosphorus is becoming more and more critical (Verstraete and Vlaeminck 2011). In developing regions of Africa and Asia the insufficient access to local nutrient sources forms a severe constraint on human nutrition and economic development (Larsen et al. 2007). Efficient nutrient management, including the recycling of nitrogen and phosphorus from human, industrial and agricultural waste streams, is therefore seen as a necessary element of sustainable development.
In other regions, wastewater phosphorus and nitrogen compounds are considered as pollutants and nutrient management primarily focuses on nutrient removal within wastewater treatment plants to prevent discharge to sensitive surface waters (Larsen et al. 2007; Verstraete and Vlaeminck 2011). The recovery of nutrients is restricted to concentrated waste streams, such as urine, manure, or digestate, which hold reactive nitrogen and phosphorus compounds in concentrations of several grams of nitrogen per liter and up to a gram of phosphorus per liter (Mulder 2003). In this concentration range, physicochemical processes, such as struvite precipitation or ammonia stripping, are available which make nutrient recovery technically and economically feasible (Maurer et al. 2006).
In low-strength waste streams, such as domestic or aquaculture wastewater, nitrogen and phosphorus are present in concentrations of milligrams per liter. Biological or physicochemical nutrient removal processes are here the preferred choice of treatment. For phosphate, conventional removal involves precipitation using iron or aluminum salts. Phosphorus recovery is facilitated using enhanced biological phosphate removal (EBPR) systems using polyphosphate accumulating organisms (PAOs; Yuan et al. 2012). For nitrogen, no recovery options are implemented and biological nitrogen removal techniques based on nitrification/denitrification, nitritation/denitritation, or partial nitritation/anammox are the preferred choice of treatment (Vlaeminck et al. 2012). Despite of their excellent pollutant removal efficiencies, these treatment strategies result in the loss of valuable reactive nitrogen compounds, while the greenhouse and ozone-depleting gas N2O is also co-emitted (Desloover et al. 2012). The nutrient recovery potential of low-strength wastewater is nonetheless substantial, as the volumes are sufficiently large to represent major nutrient fluxes (Mulder 2003; Yuan et al. 2012). The recycling of these nutrients, using sustainable biological nutrient recovery systems, could therefore reduce the dependency of inorganic fertilizers and generate revenues for wastewater treatment facilities (Verstraete et al. 2009).
The use of microalgae has been described extensively within environmental biotechnology. Microalgae efficiently utilize and remove the nitrogen and phosphorus present in wastewater and assimilate these in valuable algal biomass (Cai et al. 2013; Craggs et al. 1997). Also, the mitigation of flue gas can be included within the algal treatment process due to the ability to fix CO2 (Van den Hende et al. 2012). Depending on the supplied wastewater, the nutrient-rich algal biomass can subsequently be valorized as a natural food source in aquaculture or as a feedstock for biorefineries (Hemaiswarya et al. 2011; Wijffels and Barbosa 2010).
The potential of nitrate-accumulating microalgae for nutrient recovery has not been investigated so far. These specific types of eukaryotic diatoms have been described in both the pelagic and benthic zones of marine ecosystems (Kamp et al. 2011). Nitrate-accumulating microalgae have the capacity to store nitrate in transitory cytoplasmic pools in concentrations up to several grams of nitrogen per liter of cell volume (Bode et al. 1997; Dortch et al. 1984; Kamp et al. 2011; Lomas and Glibert 2000; Needoba and Harrison 2004). The intracellular nitrate is then used as a source for assimilatory nitrate reduction, enabling the diatoms to overcome fluctuating nitrogen concentrations in the surrounding water and hence support growth when environmental nitrogen is depleted.
The ability of these specific microorganisms to concentrate environmental nitrate within their biomass is remarkable and holds potential for biological nitrogen recovery in saline wastewaters, such as present in intensive recirculating aquaculture systems (van Rijn 2013). The aim of this study is to explore this potential of nitrate-storing diatoms for nutrient recovery from marine wastewater. A screening was performed to determine the nitrate storage capacity of six diatom species in synthetic wastewater. For the diatoms with the highest nitrate accumulation, the growth and nutrient uptake rates were further analyzed. Based on these features, the kinetic growth and nutrient uptake parameters were derived for the best performing diatom species using a mathematical model. Finally, a simulation study was performed to compare the performance of a proposed microalgal nutrient recovery unit with a conventional denitrification system for the treatment of aquaculture wastewater.
Material and methods
Strains and cultivation
The benthic diatom strains Amphora coffeaeformis (CCMP 127) and Nitzschia punctata (CCMP 561) and the pelagic strains Skeletonema dohrnii (CCMP 782), Thalassiosira nordenskioeldii (CCMP 995) and Thalassiosira weissflogii (CCMP 1336) were obtained from the Provasoli–Guillard National Center for Marine Algae and Microbiota (NCMA). The pelagic strain Phaeodactylum tricornutum (CCAP 1055/1) was obtained from the Flanders Institute for Biotechnology (VIB). In all experiments the diatoms were cultured axenically in sterile synthetic wastewater, modified from artificial seawater (ESAW) medium (Berges et al. 2001). Nitrate and phosphate concentrations were 100 mg NO3 −–N L−1 and 44 mg PO4 3−–P L−1, respectively, i.e., a mass and molar N:P ratio of 2.3 and 5, respectively, to prevent phosphorus limitation in the medium. Cultures were aerated at 0.7 Lair Lmedium −1 with 0.22 μm filter sterilized 2 % CO2. The cultivation temperature was 20 °C, the pH was maintained at 8.0–8.2 and continuous illumination was provided from the top by means of cool white lamps (Osram, Dulux L) at a light intensity of 110 μmol photons m−2 s−1. Bacterial contamination of the cultures was checked throughout the experiments using phase contrast microscopy.
Quantification of intracellular nitrate
Diatoms were cultured in 0.8 L batch tests in synthetic wastewater. Tests were performed in quadruplicate and growth was monitored by optical density (OD) measurement at 450 and 670 nm. Intracellular nitrate storage was quantified under exponential and stationary growth phase. The cell disruption method was modified from Dortch (1982). 50 mL of algal suspension was filtered on a 0.45 μm Whatman glass fiber filter. Cells were washed three times with 50 mL of 3 % NaCl to remove extracellular nitrate. Filters were frozen at −80 °C for 15 min, after which cells were broken by adding three times 10 mL of hot (80 °C) distilled water to extract intracellular nitrate. Nitrate, nitrite, ammonium, and phosphate were determined after filtration as detailed below. Determination of the cell density was carried out using light microscopy with a cell counting chamber (KOVA® Glasstic, USA) at 100× magnification (Zeiss Axisoskop 2, Germany). The width and length of cells (n = 100) was determined at 1,000× magnification using the image processing software tool ImageJ (Schneider et al. 2012). The cell volume was estimated by assuming a biconical cell shape for P. tricornutum and a cylindrical shape for the other five species (Kamp et al. 2011).
Kinetic experiments
Growth experiments were performed for A. coffeaeformis and P. tricornutum in 0.8 L batch tests. Cells in exponential growth were inoculated in synthetic wastewater at a cell density of 3 × 103 cells mL−1. Tests were performed during 10 days and nitrate, nitrite, ammonium, and phosphate concentrations in the medium were measured daily. Growth was monitored daily by cell count (KOVA® Glasstic, USA), and cell concentrations were correlated to biomass concentrations by determining the dry weight (DW) content at different stages of the growth curve in parallel tests. Additional batch tests were performed for P. tricornutum, collected at the late stationary phase (t = 14 days) after spiking with nitrate to reach the maximal cell density. Nutrient depleted cells at an initial cell density of 4 × 107 cells mL−1 were spiked with nitrate and phosphate to a final concentration of 100 mg N L−1 and 44 mg P L−1. The cell density and nitrate, nitrite, ammonium, and phosphate in the medium were monitored during 3.5 days. All batch tests were performed in quadruplicate.
Analytical techniques
Nitrate, nitrite and phosphate were analyzed after sample filtration using anion chromatography (Metrohm 761 Compact IC, Switzerland). Ammonium (Nessler method), total Kjeldahl nitrogen, and total phosphorus (molybdene–vanadate method) were determined according to standard methods (Greenberg et al. 1992). The total carbon and nitrogen contents of the biomass were determined using an elemental analyzer (ANCA-GSL PDZ Europe, UK).
Model development and parameter estimation
To describe the microalgal growth and nutrient uptake, a kinetic model was developed using the modeling and simulation platform WEST® (Vanhooren et al. 2003). The model was constructed based on the experimental observations and the algal kinetics model developed by Decostere et al. (2013). The latter describes inorganic carbon uptake, oxygen production, and algal growth and decay and was extended in this study with nitrate and phosphate uptake and intracellular nitrate storage. Growth on nitrate and phosphate were expressed by a Monod equation (Aslan and Kapdan 2006; Baldia et al. 1991). Growth on intracellular stored nitrate when the ambient nitrate becomes limiting was included and defined by the same kinetics as direct nitrate assimilation. This growth on intracellular stored nitrate will only occur when the concentration of intracellular stored nitrate becomes small compared to the ambient nitrate concentration, as indicated by the term \( \left(\frac{X_{S TN}}{S_{N{ O}_3^{-}}+{X}_{S TN}}\right) \). As other nitrogen species, such as ammonium and nitrite, were not detected, only kinetics for nitrate were considered.
Growth limitation due to the self-shading effect was described by an empirical function, based on the observed growth rate which was determined by cell count (Eq. 1). The microalgal biomass concentration is represented by X ALG (g DWL−1). The biomass inhibition constant K i_X (g DWL−1) and the inhibition exponent n X were set to 1 and 10, respectively, in order to describe the observed reduction in growth rate at higher biomass concentrations.
The nitrate storage kinetics were expressed as a function of the algal biomass concentration, the ambient nitrate concentration \( {S}_{N{ O}_3^{-}} \) (mg NO3 −–N L−1) and the nitrate storage constant kSTO (L g−1 DW d−1; Eq. 2). A nitrate accumulation limitation factor was also included and was based on the P-uptake model by Henze et al. (2000). This limitation factor is a function of the maximum nitrate uptake capacity K cap (mg NO3 −–N g−1 DW), the microalgal biomass concentration, and the effective concentration of nitrate stored in the algal pools X STN (mg NO3 −–N L−1; Table 1):
The half-saturation coefficient for nitrate and the nitrate storage rate were fitted to the experimental data by using the Simplex algorithm (Nelder and Mead 1965), while the maximum specific growth rate μmax (1.05 d−1) and the half-saturation coefficient for phosphate K PO4 (8.3 × 10−3 mg P L−1) were obtained from literature (Fawley 1984; Tyrrell 1999). An overview of the modeled processes and the reaction rates developed in this research is given in the Gujer matrix of the model presented in Table 1. Microalgae decay, gas–liquid transfer of O2 and CO2 and inorganic carbon equilibrium were also incorporated in the model, but are not given in Table 1 as these processes were taken over from Decostere et al. (2013).
The goodness-of-fit between experimental and simulated values was quantified by calculating the Theil’s inequality coefficient (TIC), in which y i represents simulated data and y m,i represents measured data points (Eq. 3). A TIC value lower than 0.3 hereby indicates a good agreement between model predictions and measured data (Audenaert et al. 2010; Zhou 1993):
Furthermore, a local sensitivity analysis was performed. The relative sensitivity function (RSF) was adopted to evaluate the sensitivity of the model output (concentration of nitrate, phosphate and algal biomass) to a change of the model parameters. The RSF was calculated out of the sensitivity function (SF) by the finite forward difference method with a relative perturbation factor of 0.1 % (De Pauw and Vanrolleghem 2006). Model parameters are defined as not (RSF < 0.25), moderately (0.25 < RSF < 1), very (1 < RSF < 2) or extremely (RSF > 2) influential (Jiang et al. 2005).
Results
Intracellular nitrate storage capacity of the diatom species
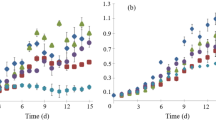
A stepwise screening was performed to analyze which diatom species has the highest potential for nutrient recovery. First, the intracellular nitrate storage capacity was determined for six marine diatom species during both exponential and stationary growth and was normalized to their cell volume. A large variation in nitrate storage was observed between the different species (Fig. 1). A. coffeaeformis and P. tricornutum stored the highest amount of nitrate with respectively 3.15 g NO3 −–N L−1 of cell volume and 2.10 g NO3 −–N L−1 of cell volume. The capacity to accumulate nitrate was not correlated to the cell size or the habitat type (benthic or pelagic) of the studied diatoms. Furthermore, no significant difference (p < 0.05) in the intracellular nitrate concentration between the exponential growth phase and the stationary growth phase was observed. Although phosphate was never limiting in the medium, intracellular phosphate storage was not detected for any of the diatoms. Moreover, nitrite and ammonium were not observed to be stored intracellularly.
Based on their nitrate storage capacity A. coffeaeformis and P. tricornutum were selected and their potential for nutrient recovery was further examined by analyzing growth and nutrient uptake of the two species. Inocula from both diatoms in their exponential growth phase were incubated and the evolution of the cell density and nitrate and phosphate concentrations was analyzed. Although both microalgae showed a similar growth curve, the nitrate uptake rate was higher for P. tricornutum (Fig. 2). Moreover, the total nitrogen content in exponential growth of P. tricornutum (8.1 %) was found to be higher compared to A. coffeaeformis (3.0 %) (Table 2). The intracellular nitrate pool of P. tricornutum only accounted for 4.6 % of the total nitrogen content, while it accounts for 17.3 % of total nitrogen for A. coffeaeformis. The initial decrease in phosphate concentration in both experiments is the result of precipitation rather than assimilation, which was visually noticeable and also shown by a small discrepancy in the phosphorus mass balance of 2.6 mg P L−1 (P. tricornutum) and 6.6 mg P L−1 (A. coffeaeformis; Fig. 2 and Table 2).
To enable validation of the kinetic model, additional batch experiments were performed for P. tricornutum in which nitrogen-depleted cells in the late stationary phase at maximal cell density (1.55 g DW L−1) were spiked with nitrate and phosphate to a final concentration of 100 mg N L−1 and 44 mg P L−1 (Fig. 3, right). Nitrate was removed completely from the medium after 3 days, resulting in an increase of the total nitrogen content of the biomass by 34.2 % (Table 2). Also 36 % of the phosphate was removed from the medium, which increased the phosphorus content in the biomass by 6.4 %. However, a difference of 12 mg P L−1 between the phosphate removed from the medium and the increase in phosphorus in the biomass indicated that also physicochemical precipitation occurred.
Kinetic parameter estimation for P. tricornutum
A mathematical model was developed for P. tricornutum to determine the kinetic parameters using the results of the exponential growth experiment. Model validation was conducted using the stationary growth test. The half-saturation coefficient for nitrate KNO3 was estimated at 0.02 mg N L−1, and the nitrate storage rate k STO at 29 L g−1 DW d−1 with a negligible error (Table 3). Due to identifiability issues, the values for μmax (1.05 d−1) and the half-saturation coefficient for phosphate (8.3 × 10−3 mg P L−1) were chosen from literature and not estimated. Using these assigned values, the kinetic model was able to describe the algal metabolism accurately for the exponential growth experiment (Fig. 3), as confirmed by the low TIC values for nitrate (TIC = 0.02), phosphate (TIC = 0.06), and algal biomass (TIC = 0.04).
The model validation using the stationary growth experiment showed a larger deviation, as the simulated environmental nitrate concentration at the end of the experiment reached 13 mg N L−1, instead of the experimental 0 mg N L−1. This can be explained by an increase of the dry weight concentration in the system and the increase of the organic nitrogen content, which was unaccounted for in the model.
In addition, a local sensitivity analysis of the kinetic model was executed in order to get an insight in the influence of the parameters on the model simulations (Fig. 4). The exponential growth experiment was used for this local sensitivity analysis and the same experimental conditions and (calibrated) parameter values were applied. From this local sensitivity analysis, it became clear that mainly the relative sensitivity functions related to μmax were influential (RSF > 1, Fig. 4a). This stresses the need for a meticulous selection of this chosen parameter to be able to obtain an accurate prediction of the algal metabolism. Results showed that the nitrate concentration was only influenced by the nitrate storage rate at the end of the experiments when nitrate was mostly depleted (Fig. 4b). Furthermore, the sensitivity function for the half-saturation coefficients for nitrate and phosphate almost equaled to zero during the experiment, which is explained by the high initial nitrate and phosphate concentrations in the synthetic wastewater.
Model-based comparison of the nutrient recovery system with a conventional denitrification system
The potential for nutrient recovery of P. tricornutum was further assessed by simulating a microalgal nutrient recovery system using the calibrated kinetic model. The performance of the proposed nutrient recovery unit, which requires marine conditions, was compared to a conventional submerged moving bed biofilm reactor for seawater denitrification (Labelle et al. 2005). The algal biomass concentration in the system was controlled at 1 g L−1, while both influent characteristics and reactor volume were derived from Labelle et al. (2005).
The conventional denitrification system achieved a nitrate removal efficiency of 88 % at a hydraulic retention time (HRT) of 1 h (Table 4). The simulated microalgal nutrient recovery system resulted in a nitrate removal efficiency of 92 % at a HRT of 36 h. Also a 55 % phosphate removal efficiency was obtained. The increase of the HRT lowered the possible volumetric loading rate of the reactor from 1.3 to 0.04 g N L−1 d−1 compared to Labelle et al. (2005) (Fig. 5). A maximal algal biomass production of 109 g DW d−1 was obtained at an HRT of 24 h, which results in a nitrate and phosphate removal efficiency of 67 and 37 %, respectively (Fig. 5).
Discussion
A screening was performed to determine the nitrate storage capacity of six marine diatoms in synthetic wastewater. The applied nutrient concentrations are representative for the wastewater in marine recirculating aquaculture systems (RAS) (Hamlin et al. 2008; Menasveta et al. 2001; Suzuki et al. 2003). The capacity to accumulate nitrate varied strongly between the diatom species, but was in line with values for microalgal nitrate accumulation in literature (De La Rocha et al. 2010; Dortch et al. 1984; Kamp et al. 2011; Lomas and Glibert 2000; Needoba and Harrison 2004). However, previous research was performed under ecologically relevant conditions, with nitrate concentrations varying from 1 mg N L−1 (Lomas and Glibert 2000) to 13 mg N L−1 (Kamp et al. 2011), while the applied nitrate concentration of 100 mg N L−1 in this study is representative for aquaculture wastewaters (van Rijn 2013). This did not result in a larger intracellular nitrate pool (Fig. 1). No nitrate accumulation was observed for T. nordenskioeldii. As also a low growth rate was observed for this diatom, the lack of nitrate storage resulted from the suboptimal cultivation temperature for this diatom, which blooms in cold water habitats (2–15 °C; Hoppenrath et al. 2007). A. coffeaeformis and P. tricornutum stored the highest amount of nitrate. The evolution of the biomass composition (Table 2) also indicates that nitrate is stored as long as environmental nitrate is not depleted. However, the difference in biomass composition between the two species showed not only that the capacity to store nitrate can vary between species but also that its contribution towards the total nitrogen content is flexible. A range of 0.1 to 4 % of inorganic N of the total N is typically described in literature (Dortch et al. 1984), yet inorganic nitrogen can account for up to 20 % of the total cellular nitrogen (Bode et al. 1997). This is confirmed in this study by the high ratio of intracellular nitrate to total nitrogen of 17.3 % for A. coffeaeformis (Table 2).
Based on the growth and biomass composition, P. tricornutum proved to be the most suitable for nutrient recycling. This diatom is studied extensively in biotechnology, as it functions as a model organism in genomic and transcriptomic research (Fabris et al. 2012). However, most research concerning the cultivation of P. tricornutum focuses on nitrate- and/or phosphate-depleted conditions. In such conditions, P. tricornutum is known to accumulate lipids to concentrations of up to 19 % of the dry weight, making it a valuable resource for biofuel production (Chen et al. 2011; Valenzuela et al. 2013). In contrast, this study demonstrated the application potential of P. tricornutum in conditions with elevated nitrate and phosphate concentrations. In these conditions, P. tricornutum reached a total nitrogen content of 8.1 to 11.0 % of the dry weight (Table 2). According to the specific total nitrogen-to-protein conversion factors for P. tricornutum, this corresponds to a protein content of 39.7 to 59.3 % of the dry weight, which is higher than the measured 35 % protein content in exponential growth described by Lourenco et al. (2004). The protein content is similar to that of Chlorella (60 %) and Spirulina (70 %), demonstrating the potential of P. tricornutum for applications as single cell protein (Cai et al. 2013; Habib et al. 2008; Lourenco et al. 2004)
To determine the kinetic parameters of P. tricornutum, a mathematical model was built based on Decostere et al. (2013). Most microalgal kinetic models describe the photosynthetic activity as a function of a single factor such as light intensity, inorganic carbon or temperature (Ogbonna et al. 1995). Only a few models take into account the combination of different environmental conditions (Bernard and Remond 2012; Bougaran et al. 2010; Filali et al. 2011). The model applied in this work includes the inorganic carbon equilibrium and uptake of inorganic carbon, nitrate, and phosphate. For the first time, intracellular nitrate accumulation was included to describe the algal metabolism. The parameters related to growth on nitrate and nitrate storage were calibrated using the experimental data, and the low TIC values showed that a good model agreement was obtained. The derived half-saturation coefficient for nitrate (0.02 mg N L−1) is in the range of the values for diatoms obtained by Collos et al. (2005) (0.028–0.14 mg N L−1) and is lower than the values of denitrification systems (0.2–0.5 mg N L−1; Henze et al. 1986). This shows that the algal system is superior for mitigation at low nitrogen concentrations.
To further assess the potential of P. tricornutum for nutrient recovery and wastewater treatment, a microalgal nutrient recovery unit was simulated for the treatment of aquaculture wastewater and its performance was compared to a conventional denitrification system (Labelle et al. 2005). The simulation study showed that P. tricornutum can obtain the same nitrate removal efficiency as the denitrification reactor by increasing the HRT of the system from 1 to 36 h, thereby reducing the loading rate of the system. The microalgal system on the other hand also results in simultaneous phosphate recovery. This valuable nutrient stays untreated in the denitrification reactor, which necessitates further treatment with iron or aluminum salts to remove it from the effluent before discharge. Also the dosage of an additional carbon source under the form of methanol is not required in the microalgal system. From a wastewater treatment perspective the algal unit is however unable to compete with the denitrification system due to the large reactor volume required. This restricts the system in the proposed configuration to treatment sites where the surface area is not constrained. Here, the algal system is competitive with conventional periphyton beds, applied in intensive aquaculture, which achieve 23 % protein recovery with a hydraulic retention time of several days (Crab et al. 2007). The algal biomass can subsequently be valorized as feed in aquaculture or can be commercially applied as a slow-release fertilizer (Mulbry et al. 2005).
From a sustainable resource management perspective, the transition from a nutrient removal to a nutrient recycling approach is inevitable. This case study demonstrates the potential use P. tricornutum for marine wastewater treatment and the production of renewable resources under the form of microalgal biomass. Future research is, nonetheless, required to further assess the feasibility of P. tricornutum for nutrient recovery. A continuous reactor setup using real wastewater and a natural light regime is indispensable to illuminate the potential of these nitrate-accumulating microalgae for nutrient recovery.
References
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28(1):64–70. doi:10.1016/j.ecoleng.2006.04.003
Audenaert WTM, Callewaert M, Nopens I, Cromphout J, Vanhoucke R, Dumoulin A, Dejans P, Van Hulle SWH (2010) Full-scale modelling of an ozone reactor for drinking water treatment. Chem Eng J 157(2–3):551–557. doi:10.1016/j.cej.2009.12.051
Baldia SF, Nishijima T, Hata Y, Fukami K (1991) Growth characteristics of a blue-green alga Spirulina platensis for nitrogen utilization. Nippon Suisan Gakkaishi 57(4):645–654. doi:10.2331/suisan.57.645
Berges JA, Franklin DJ, Harrison PJ (2001) Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J Phycol 37(6):1138–1145 doi:10.1046/j.1529-8817.2001.01052.x
Bernard O, Remond B (2012) Validation of a simple model accounting for light and temperature effect on microalgal growth. Bioresour Technol 123:520–527. doi:10.1016/j.biortech.2012.07.022
Bode A, Botas JA, Fernandez E (1997) Nitrate storage by phytoplankton in a coastal upwelling environment. Mar Biol 129(3):399–406. doi:10.1007/s002270050180
Bougaran G, Bernard O, Sciandra A (2010) Modeling continuous cultures of microalgae colimited by nitrogen and phosphorus. J Theor Biol 265(3):443–454. doi:10.1016/j.jtbi.2010.04.018
Cai T, Park SY, Li YB (2013) Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew Sust Energ Rev 19:360–369. doi:10.1016/j.rser.2012.11.030
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81. doi:10.1016/j.biortech.2010.06.159
Collos Y, Vaquer A, Souchu P (2005) Acclimation of nitrate uptake by phytoplankton to high substrate levels. J Phycol 41(3):466–478 doi:10.1111/j.1529-8817.2005.00067.x
Crab R, Avnimelech Y, Defoirdt T, Bossier P, Verstraete W (2007) Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270(1–4):1–14. doi:10.1016/j.aquaculture.2007.05.006
Craggs RJ, McAuley PJ, Smith VJ (1997) Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Res 31(7):1701–1707. doi:10.1016/s0043-1354(96)00093-0
De La Rocha CL, Terbruggen A, Volker C, Hohn S (2010) Response to and recovery from nitrogen and silicon starvation in Thalassiosira weissflogii: growth rates, nutrient uptake and C, Si and N content per cell. Mar Ecol Prog Ser 412:57–68. doi:10.3354/meps08701
De Pauw DJW, Vanrolleghem PA (2006) Practical aspects of sensitivity function approximation for dynamic models. Math Comput Model Dyn Syst 12(5):395–414. doi:10.1080/13873950600723301
Decostere B, Janssens N, Alvarado A, Maere T, Goethals P, Van Hulle SWH, Nopens I (2013) A combined respirometer-titrimeter for the determination of microalgae kinetics: experimental data collection and modelling. Chem Eng J 222:85–93. doi:10.1016/j.cej.2013.01.103
Desloover J, Vlaeminck SE, Clauwaert P, Verstraete W, Boon N (2012) Strategies to mitigate N2O emissions from biological nitrogen removal systems. Curr Opin Biotechnol 23(3):474–482. doi:10.1016/j.copbio.2011.12.030
Dortch Q (1982) Effect of growth-conditions on accumulation of internal nitrate, ammonium, amino-acids and proteins in marine diatoms. J Exp Mar Biol Ecol 61(3):243–264 doi:10.1016/0022-0981(82)90072-7
Dortch Q, Clayton JR, Thoresen SS, Ahmed SI (1984) Species differences in accumulation of nitrogen pools in phytoplankton. Mar Biol 81(3):237–250. doi:10.1007/bf00393218
Fabris M, Matthijs M, Rombauts S, Vyverman W, Goossens A, Baart GJE (2012) The metabolic blueprint of Phaeodactylum tricornutum reveals an eukaryotic Entner–Doudoroff glycolytic pathway. Plant J 70(6):1004–1014. doi:10.1111/j.1365-313X.2012.04941.x
Fawley MW (1984) Effects of light intensity and temperature interactions on growth characteristics of Phaeodactylum tricornutum. J Phycol 20(1):67–72. doi:10.1111/j.0022-3646.1984.00067.x
Filali R, Badea AC, Tebbani S, Dumur D, Diop S, Pareau D, Lopes F, Ieee (2011) Optimization of the interval approach for Chlorella vulgaris biomass estimation. IEEE, New York
Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Habib MAB, Parvin M, Huntington TC, Hasan MR (2008) A review on culture, production and use of Spirulina as food for humans and feed for domestic animals and fish. Food and Agriculture Organization of the United Nations (FAO), Rome, p 41
Hamlin HJ, MichaelS JT, Beaulaton CM, Graham WF, Dutt W, Steinbach P, Losordo TM, Schrader KK, Main KL (2008) Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquac Eng 38(2):79–92. doi:10.1016/j.aquaeng.2007.11.003
Hemaiswarya S, Raja R, Kumar RR, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27(8):1737–1746. doi:10.1007/s11274-010-0632-z
Henze M, Gujer W, Mino T, van Loosdrecht M (2000) Activated sludge models ASM1, ASM2, ASM2D and ASM3 IWA Scientific and Technical Report. vol 9. IWA, London, UK, p 130
Henze M, Grady CPLJ, Gujer W, Marais GvR, Matsuo T (1986) Activated sludge model no. 1. IAWPRC Scientific and Technical Report No 1. IAWPRC London, UK
Hoppenrath M, Beszteri B, Drebes G, Halliger H, Van Beusekom JEE, Janisch S, Wiltshire KH (2007) Thalassiosira species (Bacillariophyceae, Thalassiosirales) in the North Sea at Helgoland (German bight) and sylt (North Frisian Wadden Sea) — a first approach to assessing diversity. Eur J Phycol 42(3):271–288. doi:10.1080/09670260701352288
Jiang T, Liu X, Kennedy MD, Schippers JC, Vanrolleghem PA (2005) Calibrating a side-stream membrane bioreactor using activated sludge model no. 1. Water Sci Technol 52(10–11):359–367
Kamp A, de Beer D, Nitsch JL, Lavik G, Stief P (2011) Diatoms respire nitrate to survive dark and anoxic conditions. Proc Natl Acad Sci 108(14):5649–5654. doi:10.1073/pnas.1015744108
Labelle MA, Juteau P, Jolicoeur M, Villemur R, Parent S, Comeau Y (2005) Seawater denitrification in a closed mesocosm by a submerged moving bed biofilm reactor. Water Res 39(14):3409–3417. doi:10.1016/j.watres.2005.06.001
Larsen TA, Maurer M, Udert KM, Lienert J (2007) Nutrient cycles and resource management: implications for the choice of wastewater treatment technology. Water Sci Technol 56(5):229–237. doi:10.2166/wst.2007.576
Lomas MW, Glibert PM (2000) Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. J Phycol 36(5):903–913. doi:10.1046/j.1529-8817.2000.99029.x
Lourenco SO, Barbarino E, Lavin PL, Marque UML, Aidar E (2004) Distribution of intracellular nitrogen in marine microalgae: calculation of new nitrogen-to-protein conversion factors. Eur J Phycol 39(1):17–32. doi:10.1080/0967026032000157156
Maurer M, Pronk W, Larsen TA (2006) Treatment processes for source-separated urine. Water Res 40(17):3151–3166. doi:10.1016/j.watres.2006.07.012
Menasveta P, Panritdam T, Sihanonth P, Powtongsook S, Chuntapa B, Lee P (2001) Design and function of a closed, recirculating seawater system with denitrification for the culture of black tiger shrimp broodstock. Aquac Eng 25(1):35–49. doi:10.1016/s0144-8609(01)00069-3
Mulbry W, Westhead EK, Pizarro C, Sikora L (2005) Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour Technol 96(4):451–458. doi:10.1016/j.biortech.2004.05.026
Mulder A (2003) The quest for sustainable nitrogen removal technologies. Water Sci Technol 48(1):67–75
Needoba JA, Harrison PJ (2004) Influence of low light and a light: dark cycle on NO3 − uptake, intracellular NO3 −, and nitrogen isotope fractionation by marine phytoplankton. J Phycol 40(3):505–516. doi:10.1111/j.1529-8817.2004.03171.x
Nelder JA, Mead R (1965) A simplex-method for function minimization. Comput J 7(4):308–313
Ogbonna JC, Yada H, Tanaka H (1995) Light-supply coefficient - a new engineering parameter for photobioreactor design. J Ferment Bioeng 80(4):369–376 doi:10.1016/0922-338x(95)94206-7
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. doi:10.1038/nmeth.2089
Suzuki Y, Maruyama T, Numata H, Sato H, Asakawa M (2003) Performance of a closed recirculating system with foam separation, nitrification and denitrification units for intensive culture of eel: towards zero emission. Aquac Eng 29(3–4):165–182. doi:10.1016/j.aquaeng.2003.08.001
Tyrrell T (1999) The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400(6744):525–531. doi:10.1038/22941
Valenzuela J, Carlson RP, Gerlach R, Cooksey K, Peyton BM, Bothner B, Fields MW (2013) Nutrient resupplementation arrests bio-oil accumulation in Phaeodactylum tricornutum. Appl Microbiol Biotechnol 97(15):7049–7059. doi:10.1007/s00253-013-5010-y
Van den Hende S, Vervaeren H, Boon N (2012) Flue gas compounds and microalgae: (bio-)chemical interactions leading to biotechnological opportunities. Biotechnol Adv 30(6):1405–1424. doi:10.1016/j.biotechadv.2012.02.015
van Rijn J (2013) Waste treatment in recirculating aquaculture systems. Aquac Eng 53:49–56. doi:10.1016/j.aquaeng.2012.11.010
Vanhooren H, Meirlaen J, Amerlinck Y, Claeys F, Vangheluwe H, Vanrolleghem PA (2003) WEST: modelling biological wastewater treatment. J Hydroinformatics 5:27–50
Verstraete W, Vlaeminck SE (2011) ZeroWasteWater: short-cycling of wastewater resources for sustainable cities of the future. Int J Sustain Dev World Ecol 18(3):253–264. doi:10.1080/13504509.2011.570804
Verstraete W, de Caveye PV, Diamantis V (2009) Maximum use of resources present in domestic “used water”. Bioresour Technol 100(23):5537–5545. doi:10.1016/j.biortech.2009.05.047
Vlaeminck SE, De Clippeleir H, Verstraete W (2012) Microbial resource management of one-stage partial nitritation/anammox. Microb Biotechnol 5(3):433–448. doi:10.1111/j.1751-7915.2012.00341.x
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(5993):796–799. doi:10.1126/science.1189003
Yuan ZG, Pratt S, Batstone DJ (2012) Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol 23(6):878–883. doi:10.1016/j.copbio.2012.08.001
Zhou X (1993) A new method with high confidence for validation of computer simulation models for flight systems. Chin J Syst Eng Electron 4(4):43–52
Acknowledgments
J.C. was supported by a PhD grant from the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT-Vlaanderen, SB-101187). S.E.V. was supported as a postdoctoral fellow from the Research Foundation Flanders (FWO-Vlaanderen). The authors thank Christophe Van de Weygert for assistance with the algae cultivation and Alberto Scoma, Marta Coma Bech, and Stephen J. Andersen for inspiring scientific discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coppens, J., Decostere, B., Van Hulle, S. et al. Kinetic exploration of nitrate-accumulating microalgae for nutrient recovery. Appl Microbiol Biotechnol 98, 8377–8387 (2014). https://doi.org/10.1007/s00253-014-5854-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5854-9