Abstract
The present study is the first report on the ability of Geobacter sulfurreducens PCA to reduce Pd(II) and produce Pd(0) nano-catalyst, using acetate as electron donor at neutral pH (7.0 ± 0.1) and 30 °C. The microbial production of Pd(0) nanoparticles (NPs) was greatly enhanced by the presence of the redox mediator, anthraquinone-2,6-disulfonate (AQDS) when compared with controls lacking AQDS and cell-free controls. A cell dry weight (CDW) concentration of 800 mg/L provided a larger surface area for Pd(0) NPs deposition than a CDW concentration of 400 mg/L. Sample analysis by transmission electron microscopy revealed the formation of extracellular Pd(0) NPs ranging from 5 to 15 nm and X-ray diffraction confirmed the Pd(0) nature of the nano-catalyst produced. The present findings open the possibility for a new alternative to synthesize Pd(0) nano-catalyst and the potential application for microbial metal recovery from metal-containing waste streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development and advance in several nanotechnology fields have favored the exploration and application of nanometric materials in many areas. The exploitation of metallic nanoparticles (NPs) has grown as their importance became recognized (Yang et al. 2010). This is the case of ultrafine transition metal NPs, which can be used in diverse fields, such as catalysis and disinfection (Hennebel et al. 2009; Mandal et al. 2006; Narayanan and El-Sayed 2005), electronics (Cui and Lieber 2001), optics (Eychmuller 2000), and even in biological and medical science (Salata 2004). Lately, the interest in palladium (Pd) NPs has grown because they can be used in both homogeneous and heterogeneous catalysis, which is due to their high surface area to volume ratio and to their high surface energy (Narayanan and El-Sayed 2005). In the field of water treatment, Pd-based catalysts offers advantages because of their ability to activate di-hydrogen (H2) and to catalyze reductive transformation of a number of priority contaminants (e.g., pollutants recognized for their adverse effects on the environment and on public health (Alvarez and Cervantes 2011; Chaplin et al. 2012)).
Moreover, there is a growing interest in synthesizing metal NPs by biological methods since the processes occur at neutral pH and close to ambient temperature and pressures. Hence, these synthesis processes are considered less labor-intensive, low-cost techniques which do not produce toxic byproducts and constitute a promising area of green chemistry (Bhattacharya and Gupta 2005; Gade et al. 2008; Mandal et al. 2006; Mukherjee et al. 2008).
The main reasons to explore bacterial cultures to produce metal nano-catalysts are their variety, abundance in different environments, relative simplicity of their manipulation, and their metabolic versatility, which allow the microbial reduction of a wide variety of metals that can serve as terminal electron acceptors to form nano-catalyst (Lloyd 2003). Previous studies have documented the capacity of different microorganisms to reduce Pd(II). Desulfovibrio desulfuricans and Shewanella oneidensis are considered the most studied organisms in the context of the bioreductive synthesis of Pd(0) NPs (De Corte et al. 2012; De Windt et al. 2006; Lloyd et al. 1998). Pd(0) NPs formed at the outer surface of the bacterial cells (De Windt et al. 2006; Lloyd et al. 1998) may be exploitable as catalysts in the transformation of contaminants, for instance. Specific examples are the application of Pd nano-catalyst in the reductive transformation of Cr(VI), ClO4 −, polychlorobiphenyls, chlorophenols, trichloroethylene, and iodinated contrast media (De Corte et al. 2012). Another environmentally beneficial application is the microbial production of Pd(0) NPs in the treatment of industrial effluents containing Pd, such as waste streams from spent automotive catalysts and printed circuit boards processes, which contain substantial amounts of Pd and other precious metals (De Corte et al. 2012).
Geobacter sulfurreducens, a delta-proteobacterium, is known for its versatile ability to couple the oxidation of acetate or hydrogen to the reduction of Fe(III), Co(III), U(VI), Tc(VII), fumarate, and humic acids (Caccavo et al. 1994; Cervantes et al. 2003; Lovley and Phillips 1988, 1987; Lovley 1991; Voordeckers et al. 2010). Furthermore, G. sulfurreducens is able to produce electricity by using anodes as terminal electron acceptor in microbial fuel cells (Bond and Lovley 2003).
Recently, several studies have documented the important contribution of humic substances and quinone analogues to the reduction of metals, such as Fe(III), Mn(IV), Tc(VII), and Cr(VI) (Van der Zee and Cervantes 2009; Van Trump et al. 2006). The addition of catalytic concentrations of humic substances or quinones to microbial cultures has significantly increased the rate of reduction of these metals. In some cases, the presence of humic substances or quinones as redox mediators is even a prerequisite to achieve the microbial reduction process. Moreover, it has recently been reported that different quinone redox mediators enhanced the production of Se(0) and Te(0) NPs by Escherichia coli (Wang et al. 2011).
The aim of this work was to evaluate the capacity of G. sulfurreducens to use Pd(II) as terminal electron acceptor with the overall goal to produce Pd(0) NPs during cultivation of this strain. The study further explored the catalytic effects of the quinone model compound, anthraquinone-2,6-disulfonate (AQDS), on the microbial production of Pd(0) NPs, which had not been previously reported.
Materials and methods
Microorganism and culture conditions
G. sulfurreducens strain PCA (DSM 12127; ATCC51573) used in this study was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ). The strain was routinely cultured anaerobically in acetate/fumarate medium as previously described (Coppi et al. 2001; Lovley et al. 1993) at 30 °C.
Microbial reduction of Pd(II)
Late logarithmic phase cultures of G. sulfurreducens were harvested by centrifugation and washed twice with a sterilized, osmotically balanced buffer (g/L): NaHCO3 (2.5), NH4Cl (0.25), NaH2PO4·H2O (0.006), KCl (0.1), NaCl (1.75) (Shelobolina et al. 2007). Washed cells were re-suspended in glass serum bottles using 100 mL of a designed reaction buffer (Shelobolina et al. 2007) containing (g/L): NaHCO3 (2.5), NH4Cl (0.25), NaH2PO4·H2O (0.006), and KCl (0.1). Serum bottles were capped with inert viton stoppers and all anaerobic media were flushed with N2/CO2 (80/20 %). Headspace volume (22 mL) was also flushed with the same gas mixture.
Medium was inoculated with washed cells to yield one of two different final cell concentrations, 400 or 800 mg/L in terms of cell dry weight (CDW). The basal medium contained 5 mM acetate to serve as electron donor and Na2PdCl4 (Sigma-Aldrich) as sole terminal electron acceptor at a concentration of 25 mg Pd(II) per liter. Depending on the experimental set-up, basal medium was supplemented with 100 μM of AQDS as redox mediator. Cell suspensions were dispensed into sterile glass serum bottles (100 mL of medium into 122-mL glass serum bottles) and incubated for 24 h at 30 °C. All experimental treatments were set-up in triplicate. The setup was sampled at specified time intervals in order to follow the reduction kinetics by analyzing Pd concentration and acetate consumption.
When hydrogen was supplied as electron donor, the headspace of the bottles was saturated with a mixture of H2/CO2 (80/20 %). Abiotic controls were incubated in cell-free media.
A diagram of Pd speciation in the medium at 0.5 pH intervals between pH 6 and 8 at 30 °C (total Pd concentration of 25 mg/L in the medium) was constructed using Visual MINTEQ® software ver. 3.0.
Analytical techniques
To document microbial reduction of Pd(II), 5 mL of appropriate samples of incubated inoculated or uninoculated Pd medium were filtered using 0.22 μm membrane filters (Millipore, Bedford, USA). Filtrate and filter deposit (Pd-associated with biomass or agglomerated) were analyzed separately by inductively coupled plasma-optic emission spectroscopy (ICP-OES, Varian 730-ES). The fraction retained on the filters was boiled with aqua regia, diluted, and filtered with the same type of membrane described above.
Acetate consumption was analyzed by capillary electrophoresis (Agilent 1600A, Waldbronn, Germany). Acetate was quantified by comparison with high purity standard. A fused silica capillary column (Agilent, id 50 μm, 80.5 cm long, effective length 72 cm) and a basic anion buffer (Agilent, pH 12.1) were used. The temperature and voltage applied were 20 °C and −30 kV, respectively. Samples were injected with a pressure of 300 mbar for 6 s. Detection was carried out with indirect UV using a diode-array detector. The signal wavelength was set at 350 nm with a reference at 230 nm. A buffer flush for 4 min at 1 bar was performed prior to each run.
Transmission electron microscopy
Samples from different assays were prepared for transmission electron microscopy (TEM) by fixation in 3 % glutaraldehyde for 2 h followed by three washing cycles (6,000×g for 10 min) with the buffer described above. Samples were collected on copper grids (mesh size, 200 μm) covered with a carbon-coated Formvar film, and stained with 2 % uranyl acetate. Preliminary observations were carried out with a JEOL electron microscope model 200 CX, operated under standard conditions at 80 kV in order to assess the best requirements for fine analysis of samples.
By using high-resolution transmission electron microscopy (HRTEM), electron-dense zones were located, where palladium could be deposited. In order to corroborate these data, energy-dispersive X-ray microanalysis (EDX) was used. Samples were examined by using a FEI field emission transmission electron microscope, model TECNAI F30.
X-ray diffraction analysis
Analysis of the palladium deposited on G. sulfurreducens cells was conducted in an X-Ray diffractometer Bruker D8 Advance. Samples were washed with chloroform-methanol (1:1, vol/vol) and then with acetone, and subsequently dried at room temperature as described previously by Lloyd et al. (1998). X-ray diffraction (XRD) patterns were recorded from 20° to 90° 2θ with a step time of 2 s and step size of 0.01° 2θ.
Results
Reduction of Pd(II) by G. sulfurreducens
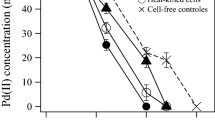
The speciation diagram (Fig. 1) showed that Pd(NH3)4 2+ is the predominant species, representing 99.08 % of the total palladium concentration (which correspond to 24.77 mg/L Pd(NH3)4 2+) followed by Pd(NH3)3 2+ representing 0.89 % (corresponding to 0.22 mg/L Pd(NH3)3 2+). Other palladium species, such as Pd(NH3)2 2+, PdCl4 2−, PdCl3 −, PdCl2(aq), Pd(NH3)2+, PdOH+, PdCl+, and Pd2+ represented less than 0.001 % (Table 1). From these results, it was assumed that under the experimental conditions applied, no significant formation of the precipitate Pd(OH)2(s) was produced, since this solid represented just 0.02 % of the total concentration of Pd (0.0047 mg/L of Pdtotal). Therefore, considering these negligible interferences, results derived from microbial incubations demonstrated that G. sulfurreducens readily reduced Pd(II) to Pd(0) linked to the anaerobic oxidation of acetate. The microbial reduction of Pd(II) mainly occurred during the first 2 h of incubation reaching a plateau after this period of time when the inoculum was 800 mg/L (CDW; Fig. 2a). According to the results summarized in Table 2, for the experiments conducted with 800 mg/L of CDW, palladium reduction was 2.6 times faster than with an inoculum of 400 mg/L (CDW; Fig. 2b). Increase in biomass, in 24 h of incubation, also resulted in more extensive palladium deposition on the biomass (Table 2). Addition of AQDS as redox mediator further enhanced the microbial reduction of Pd(II) by G. sulfurreducens (Table 2). Indeed, the specific reduction rate of Pd(II) increased 1.35- and 2-fold by the supply of this redox mediator in microbial incubations performed with 800 and 400 mg/L of CDW, respectively, as compared to controls lacking added AQDS. Neither acetate oxidation nor reduction of Pd(II) was detected in uninoculated controls with or without added AQDS (data not shown). Moreover, the pH remained constant (7.1 ± 0.1.) during the microbial reduction of Pd(II) by G. sulfurreducens in all experimental treatments. The reduction of Pd(II) was concomitant with an evident color change of the biomass which turned black.
Kinetics of Pd(II) reduction and acetate consumption for a CDW 800 mg/L and b CDW 400 mg/L. Filled triangles Pd(0) associated with biomass G. sulfurreducens. Unfilled triangles acetate consumed G. sulfurreducens. Filled circle Pd(0) associated with biomass G. sulfurreducens + AQDS. Unfilled circle acetate consumed G. sulfurreducens + AQDS
Further experiments were performed to evaluate the capacity of G. sulfurreducens to reduce Pd(II) with H2 as electron donor (data not shown). Attempts to distinguish between the microbial and chemical reduction of Pd(II) did not clearly establish a reduction attributable to G. sulfurreducens activity under these conditions. In both microbial and abiotic incubations complete reduction of Pd(II) occurred within 1 h (data not shown).
Characterization of microbially produced Pd nano-catalyst
Palladium-covered cells were observed and analyzed by means of TEM and HRTEM. From Fig. 3a it can be seen that palladium reduction by G. sulfurreducens resulted in the deposition of Pd NPs ranging from 5 to 15 nm on the cell surface. Moreover, TEM images obtained from AQDS-amended cultures revealed that produced Pd NPs were not only associated to G. sulfurreducens, but also formed extracellularly at an average distance of up to 300 nm from the cell surface (Fig. 3b).
Pd(0) NPs recovered from incubations of G. sulfurreducens provided with acetate as electron donor viewed by TEM in the absence (a) and in the presence of AQDS (b). HRTEM images and high angle annular dark field characterization of samples derived from incubation in the absence (c) and in the presence of AQDS (d). e and f HRTEM images showing planes of Pd(0) NPs
Samples were also analyzed by field emission transmission electron microscopy using high angle annular dark field mode. By this method, it was possible to observe the nanoparticle distribution on the cell surface both in the presence and in the absence of AQDS (Fig. 3c and d). Also elemental EDX microanalysis at areas of black precipitate confirmed that these were rich in palladium content (Fig. 4a).
By using HRTEM it was possible to confirm the crystalline nature of Pd NPs obtained. All Pd nanoparticles observed in HRTEM images showed lattice images indicating that they were single crystallites. The layers observed correspond to planes with spacing of d111 = 0.228 nm (analyzed by DigitalMicrograph® 3.7.0; Fig. 3e and f). The analysis of Pd NPs confirmed their heterogeneous distribution on the cell surface (see Fig. 3e and f).
XRD analysis confirmed the formation of Pd(0) NPs with an average diameter of 8.54 nm (analyzed by PowderCell® 2.4) in microbial incubations with both acetate and hydrogen as electron donors (Fig. 4b). The XRD pattern showed five strong Bragg reflections at 2 < theta > values around 40.11, 46.66, 68.13, 82.11 which correspond to planes (111), (200), (220), and (311) of a face-centered cubic lattice (fcc) (XRD pattern was indexed to ICDD card 89–4897 (fcc palladium syn)). HRTEM images, EDX and XRD pattern thus clearly showed that the Pd NPs were crystalline in nature.
Discussion
The present study demonstrates that G. sulfurreducens is capable of using Pd(II) as terminal electron acceptor and is the first report on the reduction of this transition metal by this organism. The Pd(II) reduction by G. sulfurreducens resulted in the formation of nanoparticles of Pd(0), which became associated with the cell surface. The produced nanoparticles may have a potential use as nano-catalysts.
Regarding the metabolic capabilities of G. sulfurreducens, it has previously been reported that this strain can use a diversity of electron acceptors coupled to the oxidation of acetate or hydrogen under anaerobic conditions. Some examples of metals that G. sulfurreducens is able to reduce are Fe(III), Tc(VII), Co(III), and U(VI) (Afkar et al. 2005; Caccavo et al. 1994; Lloyd et al. 2000; Lovley and Phillips 1988, 1987; Lovley 1991). Furthermore, this organism can reduce humic acids and AQDS (Cervantes et al. 2003), which are reduced at the outer surface of this organism, where c-type cytochromes are intimately involved in the process (Voordeckers et al. 2010). Thus, the present study expands our understanding of the versatility of G. sulfurreducens in reducing several distinct terminal electron acceptors.
A recent report provides a comprehensive overview of bacterial species that are able to reduce Pd(II) to Pd(0) (De Corte et al. 2012). Most noteworthy are the Pd(II) reducing capacities of the sulfate-reducing bacterium, D. desulfuricans (Lloyd et al. 1998) and the iron-reducing bacterium S. oneidensis (De Windt et al. 2005). In both cases, NPs with remarkably narrow size distribution are formed within minutes at the outer surface of the bacterial cells (Bennett et al. 2010; De Windt et al. 2006). Even though the two studies reported that high concentrations of Pd(II) were effectively reduced to Pd(0) (200 mg Pd(II)/L by D. desulfuricans and up to 1,000 mg Pd(II)/L by S. oneidensis), there remains the challenge to decreased the size of the NPs in order to increase their catalytic activity (De Corte et al. 2012).
In the present study, the Pd(II)-reducing activity of G. sulfurreducens, in terms of mg Pd(II) reduced per liter per hour, was comparable to that of S. oneidensis. The ability of G. sulfurreducens to reduce metals and other terminal electron acceptors is due to its effective extracellular electron transfer processes accomplished through mechanisms, such as electrically conductive pili, cytochromes, and multicopper proteins (Ding et al. 2006; Malvankar et al. 2011; Reguera et al. 2005; Qian et al. 2007). Additionally, the ability of Geobacter species to use quinone moieties as potential electron acceptors is noteworthy (Scott et al. 1998). Therefore, palladium reduction rate (and parallel Pd(0) NPs production) by G. sulfurreducens can be enhanced by supplying catalytic quantities of redox mediators, such as AQDS, which promotes extracellular deposition of Pd(0) NPs with sizes ranging from 5 up to 15 nm in the present study. The effect of quinone redox mediators has previously been reported by Wang et al. (2011); who observed enhancement on the reduction of selenite (Se(IV)) and tellurite (Te(IV)) by E. coli with the consequent formation and accumulation of extracellular precipitates of Se(0) nanospheres or Te(0) nanorods, respectively.
Addition of AQDS influenced the mechanism of Pd(0) NPs deposition, which occurred extracellularly and on the cell surface of G. sulfurreducens, by allowing a long-distance electron transfer to Pd(II) promoted by the electron shuttling capacity of AQDS. The last observation implies that Pd(II) was reduced by AH2QDS (reduced form of AQDS), which was microbially produced. Under these conditions, Pd(II) does not need to be in direct contact to the cell surface to be reduced to Pd(0), thus the prevalence of extracellular deposition of Pd NPs (Fig. 3b). Unlike other microorganisms such as Shewanella species, which can secrete extracellular electron shuttles (Von Castein et al. 2008) G. sulfurreducens depends (when there has not been an electron shuttle added) on direct contact to Pd(II) (and other metals) to reduce it due to the location of cytochromes (extracellular space and outer membrane) necessary for electron transfer (Ding et al. 2006).
Thus, the mechanism of Pd(II) reduction (when an extracellular electron shuttle is not present) can be described in two steps (Lloyd et al. 1998; Rotaru et al. 2012): (1) adsorption or deposition of the metal on the cell and (2) enzymatic reduction and nucleation. This mechanism explains the results found for different CDW concentrations considering that cells represent the surface area available for Pd(0) NPs deposition. Namely, by providing a higher cellular density in microbial incubations, a faster reduction of Pd(II) and deposition of Pd(0) NPs is expected, a scenario which was shown to occur in the present study (Table 2). Although the exact mechanisms is not yet understood, it can be inferred from the standard reduction potentials values that the order in which the Pd species are reduced is PdOH+ > Pd2+ > PdCl+ > PdCl2(aq) > PdCl4 2− > PdCl3 > Pd(NH3)2+ > Pd(NH3)2 2+ > Pd(OH)2(s) > Pd(NH3)3 2+ > Pd(NH3)4 2+ (see Table 1). However, the reduction mechanism and contribution of each of these species on this process require further studies.
The promotion of extracellular nucleation of Pd(0) NPs by the role of AQDS as redox mediator represents an attractive alternative to facilitate the recovery of produced nano-catalyst from microbial incubations because a significant fraction could easily be separated from microbial cells.
Another advantage of the application of G. sulfurreducens with redox mediators to produce Pd(0) nano-catalyst is the use of acetate as electron donor, which is economic and safer than hydrogen. Hydrogen has been used as electron donor with hydrogenotrophic mircoorganisms (De Corte et al. 2012). In fact, G. sulfurreducens is the first acetoclastic Pd(II)-reducing microorganism reported in the literature to the best of our knowledge.
Bio-palladium produced by different microbial species has been proposed as a nano-catalyst to promote the biodegradation of priority contaminants, such as: chlorinated, iodinated, and brominated organic compounds (e.g., chlorinated ethane and diatrizoate), through hydrodehalogenation; oxyanions (e.g., perchlorate, chlorate, and nitrate) by hydrodeoxygenation and azo-dyes through N–N hydrogenolysis (Chaplin et al. 2012). The application of bio-palladium includes micropollutant removal, such as pharmaceuticals and pesticides, which constitute a major concern in wastewater treatment (De Corte et al. 2012; Forrez et al. 2011; Hennebel et al. 2009).
References
Afkar E, Reguera G, Schiffer M, Lovley DR (2005) A novel Geo-bacteraceae-specific outer membrane protein J (OmpJ) is essential for electron transport to Fe(III) and Mn(IV) oxides in Geobacter sulfurreducens. BMC Microbiol 5:41
Alvarez LH, Cervantes FJ (2011) (Bio)Nanotechnologies to enhance environmental quality and energy production. J Chem Technol Biotechnol 86:1354–1363
Bard AJ, Parsons R, Jordan J (1985) Standard potentials in aqueous solutions. Marcel Dekker, New York
Bennett JA, Creamer NJ, Deplanche K, Macaskie LE, Shannon IJ, Wood J (2010) Palladium supported on bacterial biomass as a novel heterogeneous catalyst: a comparison of Pd/Al2O3 and bio-Pd in the hydrogenation of 2-pentyne. Chem Eng Sci 65:282–290
Bhattacharya D, Gupta RK (2005) Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 25:199–204
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555
Bratsch SG (1989) Standard electrode potentials and temperature coefficients in water at 298.15 K. J Phys Chem Ref Data 18:1–21
Caccavo F, Lonergan DJ, Lovley DR, Davis M, Stoltz JF, Mclnerney MJ (1994) Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol 60:3752–3759
Cervantes FJ, Duong-Dac T, Ivanova A, Roest K, Akkermans ADL, Lettinga G, Field JA (2003) Selective enrichment of Geobacter sulfurreducens from anaerobic granular sludge with quinones as terminal electron acceptors. Biotechnol Lett 25:39–45
Chaplin BP, Reinhard M, Schneider WF, Schüth C, Shapley JR, Strathmann TJ, Werth CJ (2012) Critical review of Pd-based catalytic treatment of priority contaminants in water. Environ Sci Technol 46:3655–3670
Coppi MV, Leang C, Sandler SJ, Lovley DR (2001) Development of a genetic system for Geobacter sulfurreducens. Appl Environ Microbiol 67:3180–3187
Cui Y, Lieber CM (2001) Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 291:851–853
De Corte S, Hennebel T, De Gusseme B, Verstraete W, Boon N (2012) Bio-palladium: from metal recovery to catalytic applications. Microb Biotechnol 5:5–17
De Windt W, Aelterman P, Verstraete W (2005) Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol 7(3):314–325
De Windt W, Boon N, Van den Bulcke J, Rubberecht L, Prata F, Mast J, Hennebel T, Verstraete W (2006) Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Antonie van Leeuwenhoek 90:377–389
Ding YH, Hixson KK, Giometti CS, Stanley A, Esteve-Nunez A, Khare T, Tollaksen SL, Zhu W, Adkins JN, Lipton MS, Smith RD, Mester T, Lovley DR (2006) The proteome of dissimilatory metal-reducing microorganism Geobacter sulfurreducens under various growth conditions. Biochim Biophys Acta 1764:1198–1206
Eychmuller A (2000) Structure and photophysics of semiconductor nanocrystals. J Phys Chem B 104:6514–6528
Forrez I, Carballa M, Finck G, Wick A, Hennebel T, Vanhaecke L, Ternes T, Boon N, Verstraete W (2011) Biogenic metals for the oxidative and reductive removal of pharmaceuticals, biocides and iodinated contrast media in a polishing membrane bioreactor. Water Res 45:1763–1773
Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK (2008) Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy 2:1–5
Hennebel T, De Gusseme B, Boon N, Verstraete W (2009) Biogenic metals in advanced water treatment. Trends Biotechnol 27:90–97
Lloyd JR (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425
Lloyd JR, Yong P, Macaskie LE (1998) Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl Environ Microbiol 64:4607–4609
Lloyd JR, Sole VA, Van Praagh CV, Lovley DR (2000) Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl Environ Microbiol 66:3743–3749
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism–organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Giovannoni SJ, White DC, Champine JE, Phillips EJ, Gorby YA, Goodwin S (1993) Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol 159:336–344
Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen M, Lovley DR (2011) Tunable metallic-like conductivity in microbial nanowires networks. Nat Nanotechnol 6:573–579
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P (2006) The use of microorganisms for formation of metal nanoparticles and their application. Appl Environ Microbiol 69:485–492
Meng X, Han KN (1996) The principles and applications of ammonia leaching of metals. A Review. Min Process Extr Metall Rev 16:23–61
Milazzo G, Caroli S, Sharma VK (1978) Tables of standard electrode potentials. Wiley, Chichester
Mukherjee P, Roy M, Mandal BP, Dey GK, Mukherjee PK, Ghatak J, Tyagi AK, Kale SP (2008) Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 19:075103.1–075103.7
Narayanan R, El-Sayed MA (2005) Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability. J Phys Chem B 109:12663–12676
Qian X, Reguera G, Mester T, Lovley DR (2007) Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins. FEMS Microbiol Lett 277:21–27
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Rotaru AE, Jiang W, Finster K, Skrydstrup T, Meyer RL (2012) Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnol Bioeng 109:1889–1897
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnol 2:3
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol 32:2984–2989
Shelobolina ES, Coppi MV, Korenevsky AA, DiDonato LN, Sullivan SA, Konishi H, Xu H, Leang C, Butler JE, Kim BC, Lovley DR (2007) Importance of c-Type cytochromes for U(VI) reduction by Geobacter sulfurreducens. BMC Microbiol 7:1–15
Van der Zee FP, Cervantes FJ (2009) Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnol Adv 27:256–277
Van Trump JI, Sun Y, Coates JD (2006) Microbial interactions with humic substances. Adv Appl Microbiol 60:55–96
Von Castein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Voordeckers JW, Kim BC, Izallalen M, Lovley DR (2010) Role of Geobacter sulfurreducens outer surface c-Type cytochromes in reduction of soil humic acid and anthraquinone-2,6-disulfonate. Appl Environ Microbiol 76:2371–2375
Wang X, Liu G, Zhou J, Wang J, Jin R, Lv H (2011) Quinone-mediated reduction of selenite and tellurite by Escherichia coli. Bioresour Technol 102:3268–3271
Yang X, Li Q, Wang H, Huang J, Lin L, Wang W, Sun D, Su Y, Opiyo JB, Hong L, Wang Y, He N, Jia L (2010) Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J Nanopart Res 12:1589–1598
Acknowledgments
This study was financially supported by the Council of Science and Technology of Mexico (Grant SEP-CONACYT 155656). Pat-Espadas thanks to CONACYT for the PhD fellowship number 221388. Authors also thank Araceli Patron Soberano, Beatriz Rivera, Nicolas Cayetano, Maria del Carmen Rocha, Dulce Partida, and Guillermo Vidriales for their technical support. We thank Dr. Katy Juárez for providing the strain of G. sulfurreducens. Finally, we greatly acknowledge the support from the national laboratories LANBAMA and LINAN for their contribution in sample analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pat-Espadas, A.M., Razo-Flores, E., Rangel-Mendez, J.R. et al. Reduction of palladium and production of nano-catalyst by Geobacter sulfurreducens . Appl Microbiol Biotechnol 97, 9553–9560 (2013). https://doi.org/10.1007/s00253-012-4640-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4640-9