Abstract
More than 80,000 tons of itaconic acid (IA) is produced worldwide each year and is sold at a price of around US$ 2/kg. The IA production yield from sugar is higher than 80 g/l. The widespread use of IA in synthetic resins, synthetic fibers, plastics, rubbers, surfactants, and oil additives has resulted in an increased demand for this product. However, at present, the IA production capacity exceeds the demand because this product has a restricted range of applications. Studies have been actively conducted in different biomedical fields—dental, ophthalmic, and drug delivery—to extend the range of applications of IA. Recently, many researchers have attempted to replace the carbon source used for microbial production of IA with cheaper alternative substrates. However, there is still a need for new biotechnology innovations that would help to reduce the production costs, such as innovative process development and strain improvement to allow the use of a low-quality carbon source. In this short review, we discuss the following aspects of IA production: strain improvement, process development, identification of the key enzyme cis-aconitic acid decarboxylase (CAD) in the IA metabolic pathway, metabolic importance of CAD, and new applications of IA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
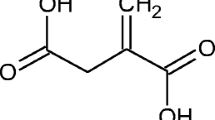
Itaconic acid (IA) is a promising organic acid. It is a white crystalline unsaturated dicarbonic acid in which one carboxyl group is conjugated to the methylene group and has a molecular weight of 130.1. IA is used worldwide in the industrial synthesis of resins such as polyesters, plastics, and artificial glass (Kin et al. 1998) and in the preparation of bioactive compounds in the agriculture, pharmacy, and medicine sectors. There is continued interest in developing biological methods to produce compounds with double bonds that are suitable for the manufacture of various polymers. IA also provides possibilities for selective enzymatic transformations to create useful polyfunctional building blocks (Ferraboschi et al. 1994).
IA was originally discovered as a product of pyrolytic distillation of citric acid. Kinoshita (1932) observed that an osmophilic strain of green Aspergillus species, which had been isolated from dried salted plums, formed IA. Aspergillus terreus was isolated as an IA-producing microorganism, and one strain (NRRL 1960 = ATCC 10020) was isolated by extensive screening. In 1955, industrial IA production by submerged fermentation was initiated by Pfizer Co. Inc. in their Brooklyn plant (Pfeifer et al. 1952). Subsequently, other plants were established in England, France, Russia, and Japan. Kobayashi (1967) and Kobayashi and Nakamura (1964) established a complete process for IA manufacture using A. terreus derived from ATCC 10020. Since the discovery of IA by Kinoshita, many attempts have been made during this century to improve the economics of this process, and as a result, optimized industrial processes have been established. The main development in IA production was batch fermentation with free suspended biomass. However, the economic and environmental circumstances around IA production have drastically changed in recent times due to increasing concerns regarding sustainability, environmental conservation, cheaper alternative substrates, and rising energy costs. Therefore, new biotechnological methodologies involving fermentation processes and technologies that use alternative cheap substrates as the carbon source are currently under investigation and development.
Strain improvement of A. terreus by mutagenesis
Several types of microorganisms have been used for IA production, as shown in Table 1. To date, there have been a few reports on strain improvement for IA production. Kobayashi and Nakamura (1964) reported that IA production was suppressed during cultivation since the growth of A. terreus was significantly inhibited by the IA produced. The IA production rate of A. terreus IFO 6365 drastically decreased in the presence of IA concentrations higher than 20 g/l (Yahiro et al. 1995). To overcome such product inhibition, it is preferable to select an IA-resistant mutant strain that would give high IA yields. A high IA-yielding strain was isolated on an IA concentration-gradient agar plate after N-methyl N′-nitro-N-nitrosoguanidine treatment. Six hundred seventy colonies that appeared in a high IA concentration region were picked, and their IA productivity was evaluated by a series of screening procedures. The mutant strain that produced more than 65 g/l of IA was selected as the most promising high IA-yielding producer, and it was designated TN-484 (Yahiro et al. 1995). This mutant strain was evaluated as shown in Table 2. For commercial IA production, the yield based on the amount of glucose consumed is a very important economic consideration because the cost of the carbon source is reported to be more than 25% of the total production cost (Kobayashi and Nakamura 1964; Rober and Kubicek 1996). The IA yield as a function of the amount of glucose consumed remained at more than 0.54 (g IA/g glucose consumed) in spite of the increase in the initial glucose concentration, which seems to be advantageous for the commercial production of IA. Moreover, the morphology of TN-484 was different from that of the parental strain; the size of the pellet mycelium was smaller than that of the parent strain, as a result of which the viscosity of the culture broth was maintained at low levels and IA productivity was improved. Industrially, more than 85 g/l of IA was produced by this strain in a 100-kl scale fermentor using a simple medium consisting of glucose, corn steep liquor, and small amounts of minerals (Role, 1997, personal communication).
Development of an economic process for IA production
Due to increasing production costs, the fermentation industry is finding it difficult to produce antibiotics, amino acids, and organic acids at internationally competitive costs. Therefore, it is necessary to reduce the costs of the fermentation process. One strategy is to use a new type of fermentor that can replace the conventional stirred tank reactor (STR). The STR is considered to be the workhorse of the fermentation industry; however, it is expensive to construct and operate and is difficult to maintain due to its complex construction. Furthermore, the STR is not suitable for filamentous microorganisms, such as fungi and Streptomycetes, because shear stress is generated by the mechanical agitation.
Therefore, various types of reactors, including the bubble column (Yoshida 1988), packed bubble column (Abraham and Sawant 1990), tubular reactor (Moser 1991), and air-lift reactor (ALR) (Siegel et al. 1986), have been examined in detail. The ALR has been widely studied because it does not require mechanical agitation and therefore does not have moving parts. Moreover, its energy demand is considerably lower than that of a STR. An ALR can be easily constructed and requires only approximately one third of the energy needed for an STR (Träger et al. 1989). Consequently, Candida utilis (Kiese et al. 1980), Pseudomonas fluorescens (Onken and Jostmann 1984), Thiobacilli (Helle and Onken 1988), Penicillium chrysogenum (König et al. 1982), and Saccharomyces cerevisiae (Wu and Wu 1991) have been tested in the ALR. Träger et al. (1992) compared gluconic acid production by Aspergillus niger in the ALR and STR and found that the ALR had good reliability and a low power requirement for pilot-scale production of gluconic acid. A. terreus (IFO-6365) was used for IA production in the ALR using a modified draft tube (Okabe et al. 1993). When this type of ALR reactor was used, the IA production rate (0.66 g l−1 h−1) increased to double the value from the STR due to morphological changes of the fungus from the filamentous form to the pellet type. Park et al. (1994) reported that repeated IA production in the ALR was possible in 21 days, and an IA production rate of 0.37 g l−1 h−1 was achieved.
As noted earlier, the IA production rate in the STR was significantly lower than that in the ALR even if the dissolved oxygen (DO) concentration was maintained at a higher level than that in the ALR. To evaluate the economic efficiency of the reactor, the power input per volume for operating both reactors was compared (Yahiro et al. 1997a). In the case of the STR, the power input was a summation of agitation and aeration (Matsushima et al. 1972). On the other hand, in the case of the ALR, the power input was used only for aeration. The power input per unit volume (P g/V) for the ALR was calculated as follows:
where ΔP and Q indicate the pressure drop between the inlet and outlet gas (kg/cm2) and the gas flow rate (l/min), respectively. The IA production rate was higher in the ALR than in the STR at each power input per unit volume (Fig. 1). In comparison to the STR, the ALR showed a higher IA production rate at less power input per unit volume.
IA producers have also been evaluated on the shake-flask scale. Even when the same strain of A. terreus was used, the IA concentration differed between the flask culture and ALR, with a slightly higher concentration of IA being produced in the flask culture than in the ALR (Okabe et al. 1993; Park et al. 1994; Yahiro et al. 1997a). This might be due to oxygen limitations in the ALR because mixing in the ALR is milder than that in the rotary shaker.
Several workers have tried to immobilize A. terreus in order to improve the performance of various fermentation systems (Table 3). Polyacrylamide (Horitsu et al. 1983), polyurethane foam (Kautola et al. 1990, 1991), calcium alginate (Kautola et al. 1985), celite R-626 (Kautola et al. 1985), and porous disks (Naihu and Wang 1986) have been used to immobilize the mycelia. The production rates of IA in immobilized cell bioreactors with porous disks or celite R-626 were relatively higher than those on the other materials, although the IA concentrations were still lower than 20 g/l. In the case of batch cultures, the IA production rate was similar and ranged between 0.26 and 0.32 g l−1 h−1. The production rate in continuous cultures was twofold higher than that in batch cultures. However, the IA concentration (18 or 26 g/l) was too low for industrial purposes. Although, there are many reports on repeated batch culture, the IA concentration was too low. In the ALR, the IA production rate in repeated batch culture was 0.37 g l−1 h−1, which was 40% higher than of the rate in batch cultures. Repeated batch culture without the loss of IA formation activity may be expected in the ALR.
Production of IA from cheaper alternative substrates
Microbial production of multifunctional organic acids has been of interest due to their possible applications in the food industry and potential as raw materials in the manufacture of biodegradable polymers (Tsao et al. 1999). The highest IA yield is achieved when glucose is used as the substrate, but crystalline glucose is too expensive to use as a raw material for the commercial production of IA. Therefore, other raw materials that are cheaper than crystalline glucose, such as starch, molasses, hydrolysates of corn syrup or wood, and other combinations, were also tested. The most frequently used substrates are beet or sugarcane molasses (Nubel and Ratajak 1964), which are pretreated by ion exchange or ferrocyanide (Batti and Schweiger 1963). Among the various carbohydrates available, corn starch is one of the best carbon sources, since it is very pure, inexpensive, and stable in a mass supply (Reddy and Singh 2002). However, corn starch is not a popular fermentation raw material because it is very difficult to sterilize due to gelatinization upon heating. The problem of gelatinization of corn starch upon heat sterilization was solved by hydrolyzing the starch using acid or enzymes. Hydrolysis using glucoamylase (5,000 AUN/ml) resulted in IA yields of up to 0.36 g/g starch, whereas hydrolysis with nitric acid at pH 2.0 yielded 0.35 g/g starch. When the corn starch was hydrolyzed with hydrochloric or sulfuric acid, the A. terreus cells required an additional nitrogen source for IA production even though the corn starch itself contained a small amount of the nitrogen source. However, when the starch was hydrolyzed with nitric acid, the cells grew and produced IA without any additional ingredients. These results indicate that nitric acid acts not only as an acid for the hydrolysis of corn starch but also as a nitrogen source for A. terreus. When raw corn starch was used for IA production, the production medium consisted of only corn starch that had been pretreated by partial hydrolysis with either with glucoamylase or nitric acid at pH 2 prior to autoclaving at 121°C for 20 min. More than 60 g/l of IA was produced by A. terreus TN-484 in a 2.5-l air-lift bioreactor from a medium consisting of 140 g/l of corn starch with no nitrogen source or other ingredients (Yahiro et al. 1997b). The IA yield based on the amount of corn starch consumed was more than 50% and was similar to that from crystalline glucose. In the case of sago starch, the medium containing nitric acid for both hydrolysis and IA production from sago starch was optimized, and 48.2 g/l of IA was produced with a yield of 0.34 g/g sago starch (Dwiarti et al. 2007). Market refuse, apple, and banana were also used as substrates for IA production, and IA yields of 28.5 and 31.0 g/l were obtained using acid- and α-amylase-hydrolyzed corn starch.
CAD in IA biosynthesis
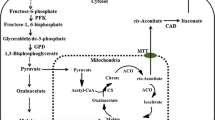
The pathway for IA biosynthesis in fungi has been studied by several groups (Kinoshita 1932; Eimhjellen and Larsen 1955; Shimi et al. 1962). Bentley and Thiessen (1957a,b,c) showed that cis-aconitic acid, which is produced in the tricarboxylic acid (TCA) cycle, could be a substrate for an A. terreus crude enzyme preparation that contained cis-aconitic acid decarboxylase (CAD; EC 4.1.1.6) and could lead to the formation of IA. Bonnarme et al. (1995) traced 14C-labeled metabolites and concluded that CAD catalyzed the decarboxylation of cis-aconitic acid to IA in the cytoplasm. These results suggest that CAD is an essential enzyme for IA biosynthesis. However, until then, the enzyme had not been purified to homogeneity due to its instability. Dwiarti et al. (2002) investigated the purification conditions for this enzyme and purified a 55-kDa protein with CAD activity to homogeneity from the high IA-producing strain A. terreus TN484-M1. The protein was stable in a buffer-containing 30% glycerol and was identified as the essential metabolic enzyme for IA production in the fungus.
The N-terminal sequence and four internal amino acid sequences of purified CAD were determined, and the gene was cloned by referring to the A. terreus genome database provided by the Broad Institute (http://www.broad.mit.edu; Kanamasa et al. 2008). The gene was classified as ATEG_09971 in the database and is represented as CAD1. A fragment containing CAD1 was amplified from the A. terreus IFO6365 genome by PCR and then sequenced (accession number AB326105). The predicted CAD1 gene encoded a polypeptide of 490 amino acid residues with a calculated molecular mass of 52,721 Da. This was consistent with the experimentally determined molecular weight of purified CAD (55 kDa on sodium dodecyl sulfate polyacrylamide gel electrophoresis). The CAD1 gene was functionally expressed in yeast, and the results proved that the obtained CAD1 gene encoded the A. terreus CAD protein (Kanamasa et al. 2008).
The CAD protein contains a conserved domain of the MmgE/PrpD family of proteins of bacteria and fungi, which includes several 2-methylcitrate dehydratases of bacteria that are involved in propionate catabolism. The protein that showed the highest identity (53%) with CAD in the DNA Data Bank of Japan was an unnamed protein from Aspergillus oryzae that possessed a conserved region of the PrpD family (accession no. AP007175).
Regarding the localization of CAD, there has been some debate as to whether it is present in the mitochondria or in the cytoplasm because cis-aconitic acid is produced in the TCA cycle while IA is finally secreted into the culture broth. The WoLF PSORT (Horton et al. 2006) algorithms predicted that this protein would be localized in the cytoplasm, suggesting that cis-aconitic acid was transported from the mitochondria to the cytoplasm in A. terreus.
No typical sequence for the TATA box exists in the 5′-untranslated region of CAD1, while consensus binding motifs for the HAP complex (CCAAT), a global transcription activator identified in eukaryotes including filamentous fungi (Goda et al. 2005; Kato et al. 1998; Xing et al. 1993), are present upstream of CAD1, suggesting that it is a highly transcribed gene. The inhibitory effect of IA on IA production by A. terreus was reported by Lockwood and Reeves (1945). This phenomenon could be caused by feedback inhibition by IA at the transcriptional level of CAD1. However, it was found that the transcription of CAD1 was not inhibited in the presence of IA (Kanamasa et al. 2008).
To clarify the role of CAD1 in the high-producing strain TN484-M1, the CAD1 gene was sequenced. There were no differences in the nucleotide sequences of CAD1 from the wild type and TN484-M1 strains, but the CAD1 transcription level of the TN484-M1 strain was fivefold higher than that of the wild-type strain (Kanamasa et al. 2008). This suggests that high IA productivity was not caused by the substitution of the amino acid sequence of CAD but was caused by the higher expression levels of CAD1 in the high-producing strain in comparison to the wild-type strain. The CAD1 will provide a way for enhancement of the IA productivity by biotechnological methods.
Process for the industrial production of IA
The process for the industrial production of IA from the culture broth consists of five steps, as shown in Fig. 2. The culture broth is filtered to remove mycelia and other suspended solids. The filtrate of the IA culture is concentrated to a value higher than 350 g/l and crystallized at 15°C. This crystallization process is carried out twice in series. The IA crystals from the two crystallization processes are decolorized by active carbon treatment at 80°C. However, this step can be omitted in the case of the industrial-grade product. The decolorized broth is evaporated and recrystallized. The recrystallized IA is dried and packaged. If IA of high purity is required, further purification steps such as solvent extraction, ion exchange, and re-decolorization are required. The IA recovery yield is 95% in the filtration process, 98% in the concentration process, and 95% in the crystallization and drying processes. The total IA recovery yield from cultivation to final packaging is approximately 80%.
Schematic diagram of IA production and recovery process from A. terreus culture. a Medium preparation, b pre-culture, c fermentor, d filter, e evaporator, f First crystallization, g separator, h second crystallization, i decolorization, j heat exchangher, k recrytallization, l drying shelves, m packaging
To reduce the manufacturing costs, waste starch may be used in IA production. When sago starch was used as the carbon source, the IA recovery yield was almost the same as that obtained when glucose was used; however, the purity was slightly lower than that obtained when glucose was used as the carbon source (Dwiarti 2006; Dwiarti et al. 2007). IA purified from glucose and sago starch had a purity of 99.0% and 97.2%, respectively. The melting points of these two samples were 166–169°C and 166–167°C, respectively. Although the form and whiteness of both crystal products from sago starch were the same as those of the authentic IA standard, an extra purification step might be required to obtain higher purity.
Application trends
IA has been used in a wide range of industries (Table 4). During the 1950s, IA was used in industrial adhesives. Overall, during this period, IA was used at an industrial scale, and large amounts of it were required. The alkali salt or sulfonated form of poly IA is used as a detergent and in shampoos. The polymerized methyl, ethyl, or vinyl esters of IA are used as plastics, adhesives, elastomers, and coatings. In the textile industry, IA was employed in nonwoven fabric binders.
Since the 1990s, the applications of IA have been extended to biomedical fields, such as the dental, ophthalmic, and drug delivery fields. A major problem in ophthalmic drug delivery is retention of an adequate concentration of the therapeutic agent in the pre-corneal area. Polycarboxylic carriers such as polyacrylic acid and polyIA in a subcolloidal nanoparticulated hydrogel form (De et al. 2004; Stanojević et al. 2006) have high potential uses in sustained drug release during ocular delivery. Therefore, poly(N-isopropylacrylamide/IA) (Tasdelen et al. 2004) and poly (N-vinyl 2-pyrrolidone/IA) (Sen and Yakar 2001) were tested for the delivery of lidocaine and terbinafine hydrochloride, respectively. Further, poly(acrylamide(A)-co-monomethyl itaconate) hydrogel was used for the dermal delivery of a bupivacaine-loaded formulation that could be used as a dressing against wound pain (Blanco et al. 2003).
Another potential application of IA is in the preparation of glass ionomer cement (GIC). GICs were introduced 30 years ago and have been shown to be very useful adjuncts in restorative dentistry. GIC is composed of a calcium-aluminosilicate glass powder and an adequate solution of an acrylic acid homo- or copolymer. These cements possess certain unique properties that make them useful as restorative and adhesive materials: They adhere to the tooth structure and base metals, exhibit anticariogenic properties due to release of fluoride, are thermally compatible with tooth enamel, and are biocompatible (Nagaraja and Kishore 2005). Crisp and Wilson (1980) synthesized a copolymer of acrylic and IA that proved to be indefinitely stable in aqueous solution. This copolymer was the first commercial marketable cement. Recently, an N-vinylcaprolactam-containing copolymer of acrylic-IA (Moshaverinia et al. 2009) and poly(acrylic acid-co-IA) (Culbertson 2006) was developed for use in functional and mechanical GICs. These materials are finding increasing applications in clinical dentistry.
Supply and demand
IA is an important intermediate in polymer production. It is extremely useful in the industrial production of synthetic resins, synthetic fibers, pesticides, plastic, rubbers, surfactants, ion-exchange agents, and lubricating oil additives. The applications of IA have been extended to the production of special glass fiber reinforced plastics, special optical lens, artificial dental cements, and drug delivery.
China is one of four IA-producing countries in the world and has become increasingly important in terms of the global IA supply (Table 5). China plays a key role in maintaining the supply and demand of IA from the viewpoint of production, manufacture, and worldwide competitiveness. In the early 1990s, the IA output was very low, and China mainly relied on imports to meet the domestic demand. After 1993, China began to set up IA-producing units in order to satisfy the domestic demand for IA. In 2000, China had ten normally functioning enterprises that had a combined total output of 20,000 tons, and it became the second largest IA producer after the USA. Currently, unofficial statistics estimate that the annual IA production capacity of China has reached 30,000 tons. IA consumption can be roughly accounted for as follows: 40% is used in the production of nitrilon, which is an acrylonitrile-based synthetic fiber that contains 93% acrylonitrile, 5.7% methyl acrylate, and 1.3% IA (Gong and Wang 2002); 30% is consumed in the ion-exchange resin sector; 10% in papermaking; 10% in the water treatment sector; and 10% in other sectors.
In 2005, China’s IA production capacity exceeded the demand. Ten years ago, the price of this product ranged between US$ 4/kg (Willke and Vorlop 2001) and US$ 4.3/kg (Bresser and Braun 2000). However, at present, it is US$ 2/kg. The domestic price in China has fallen to below US$ 1.5/kg. The main reason for the low utilization of the capacity is the restricted range of applications of IA, with the main consumption being in the nitrilon and ion-exchange resin sectors.
Concluding remarks
IA is a promising organic acid that has been categorized as one of the “top 12” building block molecules from sugars in advance biorefineries (Kurian 2005). The market for IA and its derivatives is still growing. Moreover, investigations into new properties of this compound have opened up possibilities for novel applications in the fields of polymer chemistry, pharmacy, and agriculture. To ensure efficient supply of IA, further studies on reducing the production costs are essential. Sugar, used as the carbon source, should be replaced by cheaper alternative substrates such as cellulolytic biomass because most starch is used in food. Moreover, innovations by which the process becomes more energy-saving are necessary. Strain improvement by genetic and metabolic engineering is also an important aspect, since it would allow cheaper alternative substrates to be utilized. In this regard, the development of an IA producer that is capable of utilizing lignocellulosic biomass as the carbon source is highly recommended.
References
Abraham M, Sawant SB (1990) Hydrodynamics and mass transfer characteristics of packed bubble columns. Chem Eng J 43:95–105
Batti M, Schweiger LB (1963) Process for the production of itaconic acid. US Patent 3,078,217 (to Miles Laboratories)
Bentley R, Thiessen CP (1957a) Biosynthesis of itaconic acid in Aspergillus terreus. I. Tracer studies with 14C-labeled substrates. J Biol Chem 226:673–687
Bentley R, Thiessen CP (1957b) Biosynthesis of itaconic acid in Aspergillus terreus. II. Early stages in glucose dissimilation and the role citrate. J Biol Chem 226:689–701
Bentley R, Thiessen CP (1957c) Biosynthesis of itaconic acid in Aspergillus terreus. III. The properties and reaction mechanism of cis-aconitic acid decarboxylase. J Biol Chem 226:703–720
Blanco MD, Bernardo MV, Teijón C, Sastre RL, Teijón JM (2003) Transdermal application of bupivacaine-loaded poly(acrylamide(A)-co-monomethyl itaconate) hydrogels. Int J Pharm 255:99–107
Bonnarme P, Gillet B, Sepulchre AM, Role C, Beloeil JC, Ducrocq C (1995) Itaconate biosynthesis in Aspergillus terreus. J Bacteriol 177:3573–3578
Bresser E, Braun S (2000) Conversion of citric acid to itaconic acid in a novel liquid membrane bioreactor. J Chem Technol Biotechnol 75:66–72
Christiansen A (1980) Surface active amide and amideazolines. GB Patent 1,574,916 (to Miranol Chemical)
Crisp S, Wilson AD (1980) Cements. US Patent 4,222,920 (to Mat’l Res Dev Co. England)
Culbertson BM (2006) New polymeric materials for use in glass-ionomer cements. J Dent 34:556–565
De TK, Bergey EJ, Chung SJ, Rodman DJ, Bharali DJ, Prasad PN (2004) Polycarboxylic acid nanoparticles for ophthalmic drug delivery: an ex vivo evaluation with human cornea. J Microencapsul 21:841–855
Dwiarti L (2006) Study of biorefinery of sago starch for itaconic acid production. PhD thesis, United Graduate School of Agricultural Science, Gifu University (Shizuoka University)
Dwiarti L, Yamane K, Yamatani H, Kahar P, Okabe M (2002) Purification and characterization of cis-aconitic acid decarboxylase from Aspergillus terreus TN484-M1. J Biosci Bioeng 94:29–33
Dwiarti L, Otsuka M, Miura S, Yaguchi M, Okabe M (2007) Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour Technol 98(17):3329–37
Eimhjellen KE, Larsen H (1955) The mechanism of itaconic acid formation by Aspergillus terreus. 2. The effect of substrates and inhibitors. Biochem 60:139–147
Ellis EJ, Olson AP, Bonafini JR (1994) Improved itaconic acid copolymeric compositions for contact lenses. WO Patent 9,423,314 (to Polymer Technology Corp., MA)
Ferraboschi P, Casati S, Grisenti P, Santaniello E (1994) Selective enzymatic transformations of itaconic acid derivates: An access to potentially useful building blocks. Tetrahedron 50:3251–3258
Goda H, Nagase T, Tanoue S, Sugiyama J, Steidl S, Tuncher A, Kobayashi T, Tsukagoshi N, Brakhage AA, Kato M (2005) Nuclear translocation of the heterotrimeric CCAAT binding factor of Aspergillus oryzae is dependent on two redundant localising signals in a single subunit. Arch Microbiol 184:93–100
Gong B, Wang Y (2002) ICP-AES determination of traces of noble metal ions pre-concentrated and separated on a new polyacrylacrylaminothiourea chelating fiber. Anal Bioanal Chem 372:597–600
Gordon AA, Coupland K (1980) Mehrzweckschmiermittel. DE Patent 3,001,000 (to Exxon Research and Engineering)
Hashimoto K, Shray Y, Tanigaki M (1989) Culture method for microorganism and plant cell. JP Patent 01,296,977 (to Kao Co., Japan)
Helle U, Onken U (1988) Continuous microbial leaching of a pyritic concentrate by Leptospirillum-like bacteria. Appl Microbiol Biotechnol 28:553–558
Horitsu H, Takahashi Y, Tsuda J, Kawai K, Kawano Y (1983) Production of itaconic acid by Aspergillus terreus immobilized in polyacrylamide gels. Eur J Appl Microbiol Biotechnol 18:358–360
Horton P, Park K, Obayashi T, Nakai K (2006) Protein Subcellular Localization Prediction with WoLF PSORT. Proceedings of the 4th Annual Asia Pacific Bioinformatics Conference APBC06, Taipei, Taiwan. pp. 39–48
Kanamasa S, Dwiarti L, Okabe M, Park EY (2008) Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl Microbiol Biotechnol 80:223–229
Kato M, Aoyama A, Naruse F, Tateyama Y, Hayashi K, Miyazaki M, Papagiannopoulos P, Davis MA, Hynes MJ, Kobayashi T, Tsukagoshi N (1998) The Aspergillus nidulans CCAAT-binding factor AnCP/AnCF is a heteromeric protein analogous to the HAP complex of Saccharomyces cerevisiae. Mol Gen Genet 257:404–411
Kautola H, Vahvaselka M, Linko YY, Linko P (1985) Itaconic acid production by immobilized Aspergillus terreus from xylose and glucose. Biotechnol Lett 7:167–172
Kautola H, Vassilev N, Linko YY (1990) Continuous itaconic acid production by immobilized biocatalysts. J Biotechnol 13:315–323
Kautola H, Rymowicz W, Linko YY, Linko P (1991) Itaconic acid production by immobilized Aspergillus terreus with varied metal additions. Appl Microbiol Biotechnol 35:154–158
Kawamura D, Furuhashi M, Saito O, Matsui H (1981) Production of itaconic acid by fermentation. JP Patent 56,137,893 (to Iwata)
Kiese S, Ebner HG, Onken U (1980) A simple laboratory air-lift fermentor. Biotechnol Lett 2:345–350
Kin R, Sai T, So S (1998) Itaconate copolymer with quadratic nonlinear optical characteristic. JP Patent 10,293,331
Kinoshita K (1932) Über die Produktion von Itaconsäure und Mannit durch einen neuen Schimmelpilz Aspergillus itaconicus. Acta Phytochim 5:271–287
Kobayashi T (1967) Itaconic acid fermentation. Process Biochem 2:61–65
Kobayashi T, Nakamura I (1964) Dynamics in mycelia concentration of A. terreus K26 in steady state of continuous culture. J Ferment Technol 44:264–274
König B, Schügerl K, Seewald C (1982) Strategies for penicillin fermentation in tower-loop reactors. Biotechnol Bioeng 24:259–280
Kurian JV (2005) A new polymer platform for the future—Sorona from corn derived 1, 3-propanediol. J Pol Env 13:159–167
Lancashire E (1969) Soap compositions having improved curd-dispersing properties. US Patent 3,454,500 (to Procter and Gamble)
Lockwood LB, Reeves MD (1945) Some factors affecting the production of itaconic acid by Aspergillus terreus. Arch Biochem 6:455–469
Matsushima H, Maeda K, Fukaya H, Kasahara K, Mase Y (1972) Scale-up of fermentors (I). Power requirement. J. Ferment Technol 50:100–104
Moshaverinia A, Roohpour N, Darr JA, Rehman IU (2009) Synthesis and characterization of a novel N-vinylcarrolactam-containing acrylic acid terpolymer for application in glass-ionomer dental cements. Acta Biomater 5:2101–2108
Moser A (1991) Tubular bioreactor: case study of bioreactor performance for industrial production and scientific research. Biotechnol Bioeng 37:1054–1065
Nagaraja UP, Kishore G (2005) Glass ionomer cement—the difference generation. Trends Biomater Artif Organs 18:158–165
Naihu J, Wang SS (1986) Continuous itaconic acid production by Aspergillus terreus immobilized in a porous disk bioreactor. Appl Microbiol Biotechnol 23:311–314
Nubel RC, Ratajak ED (1964) Process for producing itaconic acid. US Patent 3,044,941 (to Pfizer)
Okabe M, Ohta N, Park Y (1993) Itaconic acid production in an air-lift bioreactor using a modified draft tube. J Ferment Bioeng 76:117–122
Onken U, Jostmann Th (1984) Influence of pressure on growth of pseudomonas fluorescens. Biotechnol Lett 6:413–418
Park Y, Ohta M, Okabe M (1993) Effect of dissolved oxygen concentration and agitation rate on itaconic acid production by Aspergillus terreus. Biotechnol Lett 15:583–586
Park Y, Itida M, Ohta N, Okabe M (1994) Itaconic acid production using an air-lift bioreactor in repeated batch culture of Aspergillus terreus. J Ferment Bioeng 77:329–331
Pfeifer VF, Vojnovich C, Heger EN (1952) Itaconic acid by fermentation with Aspergillus terreus. Ind Eng Chem 44:2975–2980
Pitzl G (1951) US Patent 2,570,478 (to Du Pont)
Reddy CS, Singh RP (2002) Enhanced production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour Technol 85:69–71
Rober M, Kubicek C (1996) Production of primary metabolism. In: Rehm HJ, Reed G (eds) Biotechnology. VCHmbH, Weinhelm, pp 364–379
Saitoh Y, Kanda K, Fukuda K (1993) Dental adhesive comprising an itaconic acid monoester compound. US Patent 5,234,972 (to Ube Ind. Ltd. Japan)
Sakai A, Kusumoto A, Kiso Y, Furuya E (2004) Itaconate reduces visceral fat by inhibiting fructose 2, 6-bisphosphate synthesis in rat liver. Nutrition 20:997–1002
Sen M, Yakar A (2001) Controlled release oof antifungal drug terbinafine hydrocholode from poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. Int J Pharm 228:33–41
Siegel MH, Merchuk JC, Schugerl K (1986) Air-lift reactor analysis: Interrelationships between riser, downcomer, and gas–liquid separator behavior, including gas recirculation effects. AIChE J 32:1585–1596
Shimi IR, Nour EL, Dein MS (1962) Biosynthesis of itaconic acid by Aspergillus terreus. Arch Mikrobiol 44:181–188
Smith JE, Nowakowska-Waszczuk A, Anderson JG (1974) Organic acid production by mycelial fungi. In: Spencer B (ed) Industrial aspects of biochemistry. Elsevier, Amsterdam, pp 297–317
Stanojević M, Krušić MK, Filipović J, Parojći J, Stupar M (2006) An investigation into the influence of hydrogel composition on swelling behavior and drug release from poly(acrylamide-co-itaconic acid) hydrogels in various media. Informa Pharm Sci 13:1–7
Tabuchi T (1981) Itaconic acid production by a yeast belonging to the group Candida. Agric Biol Chem 45:475–479
Tabuchi T, Nakahara T (1980) Preparation of itaconic acid. JP Patent 55 034 017 (to Mitsubishi)
Tasdelen B, Kayaman-Apohan N, Güven O, Baysal BM (2004) Preparation of poly(N-isopropylacrylamide/itaconic acid) copolymeric hydrogels and their drug release behavior. Int J Pharm 278:343–351
Tate BE (1981) Itaconic acid and derivatives. In: Grayson M, Eckroth E (eds) Kirk–Othmer encyclopedia of chemical technology, vol 3. Wiley, New York, pp 865–873
Träger M, Qazi GN, Onken U, Chopra CL (1989) Comparison of airlift and stirred reactors for fermentation with Aspergillus niger. J Ferment Bioeng 68:112–116
Träger M, Qazi GN, Buse R, Onken U (1992) Comparison of direct glucose oxidation by Gluconobacter oxydans subsp. suboxydans and Aspergillus niger in a pilot scale airlift reactor. J Ferment Bioeng 74:274–281
Tsao GT, Cao NJ, Du J, Gong CS (1999) Production of multifunctional organic acids from renewable resources. Adv Biochem Eng Biotechnol 65:243–280
Walinsky SW (1984) (Meth) acrylic acid/itaconic acid copolymers their preparation and use as antiscalants. US Patent 4,485,223 (to Pfizer)
Willke T, Vorlop KD (2001) Biotechnological production of itaconic acid. Appl Microbiol Biotechnol 56(3–4):289–295
Wu JY, Wu WT (1991) Fed-batch culture of Saccharomyces cerevisiae in an airlift reactor with net draft tube. Biotechnol Prog 7:230–233
Xing Y, Fikes JD, Guarente L (1993) Mutations in yeast HAP2/HAP3 define a hybrid CCAAT box binding domain. EMBO J 12:4647–4655
Yahiro K, Takahama T, Park Y, Okabe M (1995) Breeding of Aspergillus terreus Mutant TN-484 for an itaconic acid production with high yield. J Ferm Bioeng 79:506–508
Yahiro K, Takahama T, Jia S, Park Y, Okabe M (1997a) Comparison of air-lift and stirred tank reactors for itaconic acid production by Aspergillus terreus. Biotechnol Lett 19:619–621
Yahiro K, Takahama T, Park Y, Okabe M (1997b) Efficient itaconic acid production from raw corn starch. J Ferm Bioeng 84:375–377
Yoshida F (1988) Bubble column research in Japan. Chem Eng Technol 11:205–212
Zhao CL, Roser J, Dersch R, Baunstark R (1999) Dispersion resins containing itaconic acid for improving wet abrasion resistance. WO Patent 9 947 611 (to BASF)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okabe, M., Lies, D., Kanamasa, S. et al. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus . Appl Microbiol Biotechnol 84, 597–606 (2009). https://doi.org/10.1007/s00253-009-2132-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2132-3