Abstract
The goal of this investigation was to determine the effect of a xylose transport system on glucose and xylose co-consumption as well as total xylose consumption in Saccharomyces cerevisiae. We expressed two heterologous transporters from Arabidopsis thaliana in recombinant xylose-utilizing S. cerevisiae cells. Strains expressing the heterologous transporters were grown on glucose and xylose mixtures. Sugar consumption rates and ethanol concentrations were determined and compared to an isogenic control strain lacking the A. thaliana transporters. Expression of the transporters increased xylose uptake and xylose consumption up to 46% and 40%, respectively. Xylose co-consumption rates (prior to glucose depletion) were also increased by up to 2.5-fold compared to the control strain. Increased xylose consumption correlated with increased ethanol concentration and productivity. During the xylose/glucose co-consumption phase, strains expressing the transporters had up to a 70% increase in ethanol production rate. It was concluded that in these strains, xylose transport was a limiting factor for xylose utilization and that increasing xylose/glucose co-consumption is a viable strategy for improving xylose fermentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fuel ethanol production in the USA amounted to 6.4 billion gallons for 2007 and is projected to increase (Hartwig 2008). Most of the ethanol produced in the USA is currently from fermentation of corn grain. Although the volume of fuel that can be displaced by corn is substantial, it is limited. To reach some of the ambitious goals for producing renewable fuels, such as the “twenty in ten” goal of replacing 20% of gasoline consumption in 10 years, technologies are being developed for converting non-food biomass to ethanol. It is estimated that current biomass waste could be converted to 50 billion gallons of ethanol with the potential for significant increases (up to 350 billion gallons) using dedicated energy crops (Wyman 1994).
Saccharomyces cerevisiae is currently used to produce ethanol from glucose, but it cannot utilize five-carbon sugars contained in the hemicellulose component of biomass feedstocks. Hemicellulose can make up to 20–30% of biomass and is primarily composed of xylose (Saha 2003). Enzymes from native xylose-assimilating organisms have been transferred to S. cerevisiae allowing fermentation of xylose (Hahn-Hägerdal et al. 2007; Ho et al. 1998; Jeffries 2006; Kuyper et al. 2005a). However, efficient conversion of xylose to ethanol is limited by multiple issues including cellular redox imbalance, low flux of xylose into the pentose phosphate pathway, and lack of efficient xylose transport into the cell.
S. cerevisiae does not express xylose-specific transporters. Xylose enters the cell through hexose transporters, but does so at considerably slower rates than glucose. The highest affinity transport for xylose in S. cerevisiae has been measured at KM ∼190 mM, compared to high-affinity glucose transport with KM values of about 1.5 mM (Kötter and Ciriacy 1993; Lagunas 1993). Native xylose-utilizing yeasts have been shown to use both low-affinity and high-affinity xylose transport systems with KM values for xylose transport ranging from 140 to 0.05 mM, respectively (Gardonyi et al. 2003; Kilian and Uden 1988; Stambuk et al. 2003). It has also been shown that xylose-fermenting yeast such as Pichia stipitis contain high-affinity xylose transport systems which are active transport, coupled to proton symport.
Analyses of strains that have been adapted for enhanced growth on xylose indicate increased expression of hexose transporters as well as altered xylose transport kinetics (Kuyper et al. 2005b; Wahlbom et al. 2003). These studies suggest that xylose transport may be one of the limiting steps in xylose utilization. Since the increase in xylose transport in these strains results from increases in hexose transporters, xylose transport is still competitively inhibited by glucose, resulting in sequential utilization of glucose and xylose, with glucose being the preferred substrate. Additionally, S. cerevisiae does not recognize xylose as a strictly fermentable carbon source. Some glucose-repressible genes are repressed effectively by xylose, while others (including genes for respiration) are derepressed (Belinchon and Gancedo 2003; Jin et al. 2004; Salusjarvi et al. 2008). A recombinant strain expressing a xylose-specific transport system, resulting in co-utilization of glucose and xylose, is likely to metabolize more xylose to ethanol due to glucose-induced repression of respiration that occurs in Crabtree-positive yeasts like S. cerevisiae.
In the present investigation, we constructed S. cerevisiae strains engineered to utilize xylose via reduction/oxidation to xylulose that also expressed two related heterologous transporters from Arabidopsis thaliana. Xylose co-consumption with glucose and product formation was compared to an isogenic control strain without the xylose transporters.
Materials and methods
Yeast strains and cultivation conditions
Escherichia coli strains DH10B, TOP10 (Invitrogen, Carlsbad, CA, USA), NEB5α and NEB10β (NEB, Beverly, MA, USA) were used for routine maintenance and preparation of plasmids and were grown in Luria–Bertani medium (Sambrook and Russell 2001). Yeast strains and plasmids used in this study are listed in Table 1. DNA was transformed into yeast cells using a standard lithium acetate method (Gietz and Schiestl 2007).
Synthetic medium consisted of 6.7 g/l Difco yeast nitrogen base (YNB; United States Biological, Marblehead, MA, USA), 50 mM potassium phthalate (pH 5.5), and was supplemented with amino acids (Amberg et al. 2005). For maintenance of plasmids, media were made without uracil, tryptophan, and histidine as necessary. Synthetic medium was filter-sterilized, and sterile glucose and xylose were added separately at 20 and 50 g/l, respectively.
Xylose utilization was investigated by inoculating 50-ml cultures at a starting OD660 of 1.0 in 125-ml flasks grown aerobically at 30°C, shaking at 225 rpm. Exponentially growing cells were used for the inoculum. At various time points after inoculation, samples were taken to determine cell biomass (by OD660) and analyze remaining sugars and products formed (by high-performance liquid chromatography, HPLC). All xylose utilization experiments were performed three to four times.
Cloning the XYL1, XYL2, and XKS1 genes
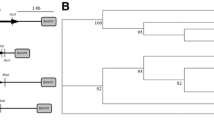
DNA fragments for cloning were amplified using PfuTurbo Hotstart polymerase (Stratagene, La Jolla, CA, USA). The XYL1 and XYL2 genes were amplified from P. stipitis (NRRL Y-7124, obtained from the USDA-ARS Culture Collection, Peoria, IL, USA) genomic DNA using primer pairs RHFBT 31/32 and RHFBT 33/34. The XKS1 gene was amplified from S. cerevisiae (BY4727, ATCC, Manassas, VA, USA) genomic DNA using primers RHFBT 61 and 62. All DNA oligos (Sigma-Genosys, St. Louis, MO, USA) used in this study are listed in Table 2. Each DNA fragment was cloned into pCR2.1-TOPO (Invitrogen) for DNA sequencing. Error-free DNA fragments were sub-cloned into yeast expression vectors using restriction endonuclease sites designed into the oligos that were used for amplification. Figure 1 shows the XYL1-, XYL2-, and XKS1-containing plasmids used in this study.
Plasmids used in this study. Each strain contains pRH195 and pRH209, providing the P. stipitis xylose reductase (XYL1) and xylitol dehydrogenase (XYL2) genes as well as the S. cerevisiae xylulokinase (XKS1) gene allowing metabolism of xylose. The open reading frames for the A. thaliana transporters At5g59250 and At5g17010 were sub-cloned separately into the high-copy S. cerevisiae expression vector pRH145 containing the S. cerevisiae PDC1 promoter and ADH1 terminator
Cloning the A. thaliana transporter genes
The predicted A. thaliana xylose transporter genes were amplified using PfuTurbo Hotstart polymerase from full-length complementary DNA (cDNA) clone S67376 (accession #BT015354, locus At5g59250) and clone S67257 (accession #BT015128, locus At5g17010) obtained from the Arabidopsis Biological Resource Center at The Ohio State University (ABRC, Columbus, OH, USA). The full-length cDNAs were amplified using primer pairs RHFBT 125/126 and 127/128 for clones S67376 and S67257, respectively. Each DNA fragment was cloned into pCR2.1-TOPO for DNA sequencing. Error-free DNA fragments were identified and sub-cloned into yeast expression vectors pRH145 using restriction endonuclease sites designed into the oligos used for amplification, generating plasmids pRH192 and 224 (Table 1). These untagged versions of the xylose transporter genes were used for determining xylose utilization (Fig. 1).
Plasmids containing tagged versions of the transporters were used separately to verify protein stability and expression levels in S. cerevisiae. To generate plasmids pRH241and 243, DNA coding for the V5-6xHis epitope was polymerase chain reaction (PCR)-amplified from pYES-DEST52 (Invitrogen) using PfuTurbo Hotstart polymerase and primers RHFBT 173/175 and 174/175 for clones S67376 and S67257. The amplified DNA fragments (100 ng) were used to co-transform strain BY4727 with 25 ng of SalI-linearized pRH192 or pRH224. Targeting sequence for homologous recombination-based repair of the SalI-linearized plasmids was provided by the oligos used to amplify the V5-6xHis fusion. His+ transformants were isolated and the presence of a C-terminal V5-6xHis fusion verified by PCR. Western blots also confirmed the expression of the heterologous transporters.
Total protein, Western blot analysis, and cellular immunofluorescence
Protein from log-phase cells was isolated using standard glass bead lysis in trichloroacetic acid. The protein pellet was resuspended in 2X Laemmli buffer (Bio-Rad Laboratories, Hercules, CA, USA) and incubated on ice for 30 min, followed by 37°C for 30 min to allow solubilization of membrane proteins. Equal amounts of protein were loaded in each lane and resolved on sodium dodecyl sulfate, 12% polyacrylamide gels (Bio-Rad Laboratories). For total protein analysis, the proteins were visualized using Bio-Safe Coomassie Stain (Bio-Rad Laboratories). For Western analysis, proteins were transferred to a nitrocellulose membrane and probed with a 1:5,000 dilution of anti-V5 antibody (Invitrogen). Reactive protein bands were visualized by following the WesternBreeze® Chromogenic Immunodetection Protocol (Invitrogen).
Cell immunofluorescence was performed as described (Amberg et al. 2005) using anti-V5-FITC antibodies (Invitrogen) at a 1:500 dilution. DNA staining was achieved using mounting media with DAPI (Prolong Gold with DAPI, Invitrogen). Images were obtained using a digital camera (Olympus DP70) mounted to an Olympus BX60 fluorescence microscope equipped with a 100× objective.
Intracellular accumulation of xylose
Intracellular accumulation of xylose in strains YRH117, YRH307, and YRH309 was determined essentially as described (Sedlak and Ho 2004). Cells were grown in YNB-His plus 20 g/l glucose for 40 h at 30°C (glucose was completely exhausted at this point). Xylose was added to a final concentration of 20 g/l to start the uptake, and the cells were incubated at 30°C with shaking (200 rpm). Samples were collected at 30, 60, and 120 min. Cells were washed twice with ice-cold sterile water, resuspended in 2 ml of sterile water, and incubated at 37°C for 1 h with shaking. One milliliter of the sample was centrifuged at 12,000×g for 5 min at 4°C. The amount of xylose in the supernatant was determined using HPLC. Results were plotted as milligram of xylose per gram of cell dry weight. Cell dry weight was determined by drying cells in pre-weighed glass tubes at 100°C for 24 h.
Analytical methods
Substrates and products were analyzed using HPLC. Culture samples were clarified by centrifugation (12,000×g for 15 min) to remove cells. Supernatant solutions were maintained at −20°C before analysis. The separation system consisted of a solvent delivery system (P2000 pump, Thermo Scientific, Waltham, MA, USA) equipped with an autosampler (717 plus, Waters Chromatography Division, Millipore, Milford, MA, USA), a refractive index detector (410 differential refractometer, Waters), and a computer software based integration system (Chromquest 4.0, Thermo Scientific). The ion-oderated partition chromatography column (Aminex HPX-87H with cation H micro-guard cartridge from Bio-Rad Laboratories) was used. The Aminex HPX-87H column was maintained at 65°C, and the sugars were eluted with 10 mM HNO3 prepared in Milli-Q filtered water at a flow rate of 0.6 ml/min. Peaks were detected by refractive index and were identified and quantified by comparison to retention times of authentic standards (glucose, xylose, glycerol, ethanol, and xylitol).
For calculations involving biomass (grams of cell dry weight), OD660 values were converted to gram cells (dry weight) per liter according to standards derived for the BY4727 strain (0.66 g of cell dry weight per liter of culture at an OD660 = 1.0). Specific ethanol productivity was calculated as gram ethanol produced per gram cells (dry weight) per hour (g/g cells/h). Specific consumption rates were also calculated as gram sugar consumed per gram cells (dry weight) per hour (g/g cells/h). Volumetric rates were calculated as gram substrate consumed or product formed per liter per hour (g l−1 h−1). Ethanol yield was determined as gram ethanol per gram substrate consumed (g/g). Sugar and product concentrations were reported as grams per liter.
Statistical analyses
All data are reported as the mean value from three to four independent cultures. Xylose consumption analyses were performed from four independent cultures for YRH269 and YRH289. The analysis for YRH185 was performed in triplicate. Probability analyses were performed using the Student’s t test with a two-tailed distribution. Data for each transporter-expressing strain were compared to the appropriate control strain. Values with p < 0.05 were considered significant for this study and are included with data tables.
Results
Expression of the A. thaliana At5g59250 and At5g17010 Genes in S. cerevisiae
The A. thaliana genome contains multiple sugar and sugar alcohol transporters. Some of these transporters have been analyzed in S. cerevisiae, indicating that transporter genes from A. thaliana are expressed and folded properly for activity in S. cerevisiae (Sauer and Stolz 1994; Schneidereit et al. 2003; Stolz et al. 1994; Truernit et al. 1999). Two sugar transport genes from A. thaliana, At5g59250 and At5g17010, are classified as xylose transporter homologs based on sequence similarity to known xylose–H+ transporters (Johnson and Thomas 2007). The open reading frames for the At5g59250 and At5g17010 genes were cloned into high-copy yeast expression vectors. The A. thaliana transporter plasmids were transformed into recombinant S. cerevisiae stains that also expressed the genes necessary for xylose utilization (Fig. 1). To confirm that the proteins were expressed, the genes were also cloned in-frame with a C-terminal V5 epitope. The At5g59250 and At5g17010 genes code for proteins with predicted molecular weights of 59.8 and 53.4 kDa, respectively. Western analysis (Fig. 2b) showed that proteins of the correct size were expressed in S. cerevisiae. To determine if the transporters were transported to the plasma membrane, we used anti-V5-FITC antibodies to detect the V5-tagged A. thaliana transporters. Fluorescence could be seen at the cell periphery for both strains expressing the transporters (Fig. 2c). Cells expressing the V5-tagged At5g59250 transporter showed diminished fluorescence in daughter cells and less uniform fluorescence at the cell periphery. In most fluorescing cells, fluorescence was also seen internally and may represent inefficient export of the transporters from the endoplasmic reticulum or increased endocytosis due to elevated expression of the transporters. Fluorescence was not seen in all of the cells and indicates that further improvements may come from stable integration of the transporters. No fluorescence was seen in cells lacking the transporters (data not shown). These data show that the A. thaliana transporters were expressed and could localize to the plasma membrane.
Western analysis and cellular localization for the A. thaliana xylose transporters expressed in S. cerevisiae. a Coomassie-stained gel showing equal loading of protein for each strain. Total protein isolated from an equal quantity of cells was loaded for strains containing the empty vector or the A. thaliana transporters expressed from a high-copy (2 μ-based) expression vector. b Western analysis showing expression of the A. thaliana transporters encoded by the At5g59250 and At5g17010 genes. The transporters contained an in-frame C-terminal V5 epitope fusion and were detected using an anti-V5 antibody. The proteins encoded by the At5g59250 and At5g17010 genes have predicted molecular weights of 59.8 and 53.4 kDa, respectively. c Fluorescence microscopy of V5-tagged A. thaliana transporters. Panels in the top row show localization of the transporter by conventional immunofluorescence using FITC-conjugated anti-V5 antibodies (bar = 2 μm). Fluorescence seen at the cell periphery indicates that A. thaliana transporters At5g59250 and At5g17010 can localize to the plasma membrane, although some may be retained in the endoplasmic reticulum. Panels in the bottom row show the DNA stained by DAPI
Xylose uptake and consumption for strains expressing the A. thaliana transporters
Expression and correct localization of a heterologous protein in yeast does not confirm that the protein is functional. To determine if expression of the A. thaliana transporters increased xylose uptake in our strains, we measured the intracellular concentration of xylose in cells expressing the transporters but lacking the genes for xylose utilization. The control strain lacking the A. thaliana transporters accumulated a maximum of 26.8 mg xylose per gram cells (dry weight). Strains expressing the transporters accumulated up to 46% more xylose compared to the control strain (Fig. 3). Both transporter-expressing strains showed significant increases in xylose accumulation (p < 0.05 at 30 and 120 min), suggesting that xylose uptake is increased in cells expressing the A. thaliana transporters.
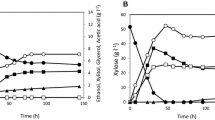
We next analyzed xylose consumption for recombinant xylose-utilizing strains expressing the A. thaliana transporters. Aerobic batch cultures containing glucose and xylose were inoculated at an OD660 of 1.0 and incubated at 30°C. At various time points after inoculation, residual glucose and xylose concentrations were assayed as well as biomass (OD660) and product formation (Fig. 4a–d). Figure 4b–d shows the graphical representation of residual sugar levels and product concentrations. These data showed that strains expressing the A. thaliana transporters consumed 25% and 40% more xylose at the end of the culture than the control strain (Table 3). Interestingly, strains expressing the transporters also displayed increased glucose consumption rates (Table 4).
Growth, sugar, and ethanol concentrations for aerobic batch cultivation of recombinant S. cerevisiae strain YRH269 (empty vector) and strains expressing A. thaliana xylose transporter genes At5g59250 (YRH185) and At5g17010 (YRH289). Cells were grown in YNB with glucose (20 g/l) and xylose (50 g/l) as carbon sources. a Growth was determined by OD660. b–d Glucose, xylose, and ethanol concentrations for each strain. For c, the vertical dotted line at 14 h indicates the end of the co-consumption phase and start of the xylose only phase. The results shown are the average values for three to four independent cultures. Differences between replicates were less than 10%
To determine if expression of the A. thaliana transporters increased co-utilization of xylose and glucose, xylose consumption rates were determined for the growth phase prior to glucose depletion. Some xylose co-consumption with glucose was seen in the control strain. We attribute this result to the fact that our pre-culture medium contained xylose. It has been reported that cells pre-grown in xylose can show continued xylose consumption when inoculated into media containing both glucose and xylose (Govindaswamy and Vane 2007). Compared to the control strain, cells expressing the transporters showed up to a 50% increase in the amount of xylose consumed prior to glucose depletion (Fig. 4c). Xylose co-consumption rates for the transporter expressing strains were increased up to 2.5-fold (Table 4), indicating that expression of the A. thaliana transporters increased co-utilization of xylose and glucose.
Analysis of product formation
Ethanol was produced during the aerobic batch cultivation, and maximum ethanol concentrations were attained shortly after glucose depletion (Fig. 4d). A statistically significant difference was not seen in the maximum ethanol concentration (Table 5). Ethanol yield was also not changed in the transporter-containing strains (Table 5), and there were no significant differences in xylitol or glycerol production (Table 3). However, because the maximum ethanol concentration was reached sooner in stains expressing the transporters, specific ethanol productivity was increased by up to 30%, and volumetric ethanol production rates were increased by up to 70% (Table 5). Part of the increase in volumetric ethanol production rate for YRH185 is most likely due to the increased glucose consumption rate as well as increased xylose consumption rate.
Xylose consumption for the control strain slowed significantly at the end of the cultures (Fig. 4c). During the xylose-only phase, ethanol was consumed by the control strain (Fig. 4d). The fact that the transporter-expressing strains were able to maintain elevated ethanol concentrations throughout the xylose-only phase (25% more ethanol at 96 h compared to YRH269) suggests that increased xylose consumption in these strains was able to offset ethanol losses due to respiration that were seen in the control strain.
Discussion
Strains of S. cerevisiae engineered to consume xylose, either by reduction/oxidation or isomerization, utilize xylose at suboptimal rates for industrial use. Limitations to xylose consumption have been identified in xylose flux through the pentose phosphate pathway, co-factor specificity differences leading to NADH accumulation, and the inefficient transport of xylose into the cell. The S. cerevisiae genome contains 20 genes that encode for hexose transporters but does not contain genes for xylose-specific transport systems like natural xylose-utilizing yeasts. Xylose transport does occur in S. cerevisiae, but xylose enters the cell via hexose transporters at significantly slower rates than glucose (Hamacher et al. 2002).
Heterologous xylose transporters have been expressed in S. cerevisiae and analyzed for their ability to restore growth on xylose to a S. cerevisiae strain lacking all hexose transporters (TMB3201). In one of these studies, GSX1, a glucose–xylose–H+ symporter from Candida intermedia, was expressed from high-copy vectors. Expression of GSX1 failed to restore growth on xylose (Leandro et al. 2006). Heterologous xylose transporters from A. thaliana (At5g59250) and E. coli (xylE) were also expressed in TMB3201 and failed to support growth on xylose (Hamacher et al. 2002).
The A. thaliana gene At5g59250 was investigated in this study, as well as a related gene, At5g17010, which possessed greater sequence similarity to xylE from E. coli. A key difference between these and other studies is that the strains used in this investigation contained all of the hexose transporters. In the previous studies with cells lacking all of the hexose transporters (hxtΔ), the only transport mechanism for xylose was active H+ symport, and the net ATP gain would be too low to support growth using xylose as the only carbon source. Indeed, in two separate studies, expression of passive (facilitated diffusion) hexose transporters capable of transporting xylose restored growth of hxtΔ cells on xylose (Hamacher et al. 2002; Sedlak and Ho 2004). For these reasons, the analyses in this investigation were performed using glucose and xylose in strains capable of hexose transport.
Two additional issues arise due to the lack of a xylose-specific transport system. First, the K M values for xylose (100–190 mM) transport through hexose transporters are considerably higher than for glucose (K M = 1–20 mM; Ozcan and Johnston 1999), indicating that high concentrations of xylose are required for transport. Second, in mixtures of glucose and xylose such as found in lignocellulosic feedstocks, xylose will not be consumed until glucose is substantially depleted. The effect of poor xylose transport was seen in a recent investigation using S. cerevisiae (TMB3400) with pre-treated bagasse (Rudolf et al. 2008). The authors reported that during simultaneous saccharification and fermentation, xylose was consumed only when glucose levels were low. In a separate report, a recombinant S. cerevisiae strain identified on the basis of increased mixed sugar utilization showed increased capacity for xylose transport and an improved K M for xylose transport (Kuyper et al. 2005b). However, this strain still consumed glucose prior to xylose.
Additionally, S. cerevisiae does not recognize xylose as a strictly fermentable carbon source (Jin et al. 2004). Rather, xylose is capable of repressing some genes that are catabolite-repressed, while others that are typically repressed on glucose show higher levels when grown on xylose (Belinchon and Gancedo 2003; Salusjarvi et al. 2008). During our studies, after glucose was consumed, cells started to respire (as evidenced by ethanol consumption for the control strain) even though xylose was present. One main benefit from a xylose-specific transport system would be increased xylose utilization when cells are actively fermenting due to the presence of glucose.
In the present study, we show that expression of the A. thaliana xylose transporters in S. cerevisiae strains engineered to utilize xylose resulted in increased xylose consumption prior to glucose depletion. Ethanol production rates were as much as 70% higher when xylose was co-consumed with glucose. The fact that xylose consumption rates prior to glucose depletion increased by up to 2.5-fold in the engineered strains compared to the control strain suggests that the increase in ethanol production seen in these strains is the result of increased xylose/glucose co-consumption. Consistent with increased fermentation of xylose in strains expressing heterologous xylose transporters, the highest specific ethanol productivity and volumetric ethanol production rates were seen for the strain with the highest rate of xylose/glucose co-consumption. All of these data indicate that expression of the A. thaliana transporters increased xylose/glucose co-consumption and that more xylose is fermented to ethanol when it is co-utilized with glucose.
References
Amberg DC, Burke DJ, Strathern JN (2005) Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 149–152, 199–202
Belinchon MM, Gancedo JM (2003) Xylose and some non-sugar carbon sources cause catabolite repression in Saccharomyces cerevisiae. Arch Microbiol 180:293–297
Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132
Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119–122
Gardonyi M, Osterberg M, Rodrigues C, Spencer-Martins I, Hahn-Hägerdal B (2003) High capacity xylose transport in Candida intermedia PYCC 4715. FEMS Yeast Res 3:45–52
Gietz RD, Schiestl RH (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2:31–34
Govindaswamy S, Vane LM (2007) Kinetics of growth and ethanol production on different carbon substrates using genetically engineered xylose-fermenting yeast. Bioresour Technol 98:677–685
Hahn-Hägerdasl B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953
Hamacher T, Becker J, Gardonyi M, Hahn-Hägerdal B, Boles E (2002) Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology 148:2783–2788
Hartwig M (2008) News release: 2007 final ethanol production and demand. Renewable Fuels Association
Ho NW, Chen Z, Brainard AP (1998) Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl Environ Microbiol 64:1852–1859
Jeffries TW (2006) Engineering yeasts for xylose metabolism. Curr Opin Biotechnol 17:320–326
Jin YS, Laplaza JM, Jeffries TW (2004) Saccharomyces cerevisiae engineered for xylose metabolism exhibits a respiratory response. Appl Environ Microbiol 70:6816–6825
Johnson DA, Thomas MA (2007) The monosaccharide transporter gene family in Arabidopsis and rice: a history of duplications, adaptive evolution, and functional divergence. Mol Biol Evol 24:2412–2423
Kilian SG, Uden N (1988) Transport of xylose and glucose in the xylose-fermenting yeast Pichia stipitis. Appl Microbiol Biotechnol 27:545–548
Kötter P, Ciriacy M (1993) Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 38:776–783
Kuyper M, Hartog MM, Toirkens MJ, Almering MJ, Winkler AA, van Dijken JP, Pronk JT (2005a) Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res 5:399–409
Kuyper M, Toirkens MJ, Diderich JA, Winkler AA, van Dijken JP, Pronk JT (2005b) Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res 5:925–934
Lagunas R (1993) Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol Rev 10:229–242
Leandro MJ, Goncalves P, Spencer-Martins I (2006) Two glucose/xylose transporter genes from the yeast Candida intermedia: first molecular characterization of a yeast xylose–H+ symporter. Biochem J 395:543–549
Ozcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63:554–569
Rudolf A, Baudel H, Zacchi G, Hahn-Hägerdal B, Liden G (2008) Simultaneous saccharification and fermentation of steam-pretreated bagasse using Saccharomyces cerevisiae TMB3400 and Pichia stipitis CBS6054. Biotechnol and Bioeng 99:783–790
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Salusjarvi L, Kankainen M, Soliymani R, Pitkanen JP, Penttila M, Ruohonen L (2008) Regulation of xylose metabolism in recombinant Saccharomyces cerevisiae. Microb Cell Fact 7:18
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sauer N, Stolz J (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker’s yeast and identification of the histidine-tagged protein. Plant J 6:67–77
Schneidereit A, Scholz-Starke J, Buttner M (2003) Functional characterization and expression analyses of the glucose-specific AtSTP9 monosaccharide transporter in pollen of Arabidopsis. Plant Physiol 133:182–190
Sedlak M, Ho NW (2004) Characterization of the effectiveness of hexose transporters for transporting xylose during glucose and xylose co-fermentation by a recombinant Saccharomyces yeast. Yeast 21:671–684
Stambuk BU, Franden MA, Singh A, Zhang M (2003) D-Xylose transport by Candida succiphila and Kluyveromyces marxianus. Appl Biochem Biotechnol 105–108:255–263
Stolz J, Stadler R, Opekarova M, Sauer N (1994) Functional reconstitution of the solubilized Arabidopsis thaliana STP1 monosaccharide–H+ symporter in lipid vesicles and purification of the histidine tagged protein from transgenic Saccharomyces cerevisiae. Plant J 6:225–233
Truernit E, Stadler R, Baier K, Sauer N (1999) A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J 17:191–201
Wahlbom CF, Cordero Otero RR, van Zyl WH, Hahn-Hägerdal B, Jonsson LJ (2003) Molecular analysis of a Saccharomyces cerevisiae mutant with improved ability to utilize xylose shows enhanced expression of proteins involved in transport, initial xylose metabolism, and the pentose phosphate pathway. Appl Environ Microbiol 69:740–746
Wyman CE (1994) Ethanol from lignocellulosic biomass: technology, economics, and opportunities. Bioresour Technol 50:3–15
Acknowledgments
We thank Katherine Card and Nicole Maroon for generating the strains used and technical assistance for this study. We would also like to thank Greg Kennedy and Mike Henderson for their help evaluating residual sugar and product concentrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Rights and permissions
About this article
Cite this article
Hector, R.E., Qureshi, N., Hughes, S.R. et al. Expression of a heterologous xylose transporter in a Saccharomyces cerevisiae strain engineered to utilize xylose improves aerobic xylose consumption. Appl Microbiol Biotechnol 80, 675–684 (2008). https://doi.org/10.1007/s00253-008-1583-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1583-2