Abstract
Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae was investigated in this study. Resveratrol is widely used in medicine, food, and cosmetic because of its pharmacological properties. However, it has a much lower content in plants compared with its glucoside piceid, which has a much lower bioavailability. Traditionally, the aglycone is acquired by acid or enzymatic hydrolysis of its glucoside, but the violent condition and the acid pollution in hydrolytic reaction and the high cost of the enzyme limit their industrial development. In this paper, fermentation of P. cuspidatum by A. oryzae was successfully performed, during which, piceid was converted to resveratrol with the highest yield of trans-resveratrol 1.35%, 3.6 times higher than that obtained from raw herb by microwave-assisted extraction. Scale-up production was also performed and the yield of trans-resveratrol was 3.1 times higher after 24 h incubation. Therefore, biotransformation is a better method to increase the yield of resveratrol because of its high yield and mild conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
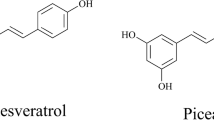
Resveratrol (3, 4′, 5-trihydroxystilbene, Fig. 1) is a phenolic compound produced by many spermatophytes, including grapevines, peanuts, and Polygonum cuspidatum Sieb. et Zucc. As a phytoestrogen, resveratrol possesses a wide range of pharmacological properties that may account for its possible cardioprotective action, including the inhibition of low-density lipoprotein oxidation, inhibition of smooth muscle cell proliferation, and platelet aggregation (Pace-Asciak et al. 1995). Moreover, this compound shows anti-inflammatory and anticancer activities (Bertelli et al. 1999).
Because of its pharmacological functions, resveratrol is now gaining scientific attention as a longevity promoter. At present, resveratrol is widely used in medicine, food, as well as cosmetic. Many chemical syntheses of resveratrol have been carried out, but the cost is high, and they are environmentally unfriendly (Andrus et al. 2003). Genetic engineering has also been performed successfully to obtain a strain of Saccharomyces cerevisiae that produces resveratrol (Becker et al. 2003). However, further research is essential to ensure that such a strain will not compromise the safety and sensory quality of the wine so that the yeast can be harnessed for the production of wine at a commercial scale. For these reasons, resveratrol is mainly obtained by solvent extraction from plants now. Unfortunately, it is present in such small concentrations that it is difficult and expensive to obtain large quantities through large-scale extraction procedures. In fact, the content of piceid (resveratrol-3-O-β-glucoside, Fig. 1) is usually much higher than that of resveratrol. In P. cuspidatum produced from Hanzhong in China, the average content of piceid is six times higher than that of resveratrol (Zhou et al. 2002). However, its bioavailability is much lower compared with that of resveratrol (Meng et al. 2004). If piceid were transformed into resveratrol, the yield of resveratrol would increase dramatically.
Traditionally, aglycone is acquired by hydrolyzation of its glucoside, and acid or alkaline is often used as the hydrolytic reagent. However, the hydrolytic reaction is always carried out under violent conditions and often causes pollution. In contrast, biotransformation only requires mild conditions, simple procedures, lower cost, and results in less pollution (Wendy 2000). At present, it has been well established as a means for the structural modification of natural or synthetic organic compounds. The rich enzymes in microorganisms could be used to transform or synthesize some natural products. Moreover, new pharmaceutically active compounds may be produced during the process. For instance, Protopanaxadiol Rb1 and protopanaxatrial Re were transformed into other saponins by cell extracts from various food-grade edible microorganisms (Chi and Ji 2005). Hydroxylation of 10-deoxoartemisinin was performed by Aspergillus niger (Parshikov et al. 2004a,b). In addition, triptolide was transformed by Cunninghamella blakesleana, and four products were characterized as new compounds and were found to exhibit potent in vitro cytotoxicity against some human tumor cell lines (Ning et al. 2003).
Biotransformation of piceid in P. cuspidatum has been carried out by enzymatic catalysis or microbial fermentation. For example, cellulase was used to transform piceid in extract of P. cuspidatum to resveratrol (Luo and Tong 2005), and composite enzymes were also added into the powder of raw material to improve the yield of resveratrol (Jiang 2000). Microbial fermentations of P. cuspidatum were carried out spontaneously (Zou and Shu 2002) or by a specific unknown strain (Cao 2004). Although these methods were reported to be able to increase the yield of resveratrol, the high cost of the enzymes limits its industrial development, and toxic compounds may be produced during spontaneous fermentation. Moreover, microbial fermentation of raw herb by a specific strain has not been systematically studied. In this paper, the fermentation of raw herb of P. cuspidatum by Aspergillus oryzae was investigated systematically to transform piceid to resveratrol in comparison with hydrolysis by H2SO4 solution and cellulase.
Materials and methods
Chemicals and materials
Dried root of P. cuspidatum was obtained from Xiancaotang Medicine Cooperation (Dalian, P. R. China), ground into powder, and passed through a 60 mesh sieve. Cellulase was obtained from Shanghai Lizhu biotechnological (Shanghai, P. R. China), trans-piceid was purchased from the National Institute for Control of Biological and Pharmaceutical Drugs (Beijing, P. R. China), trans-resveratrol from Sigma Chemical (USA), and salicin from Shanghai Kangjiu Chemical (Shanghai, P. R. China).
Hydrolysis of raw herb by H2SO4 solution
Powder of P. cuspidatum (2 g) was mixed with 20 ml H2SO4 (1% w/w) and hydrolyzed in a sterilizer under the pressure of 1.1 MPa. After hydrolyzation, 20 ml ethanol was added into the mixture, and microwave-assisted extraction was performed in a modified household microwave oven for 2 min. Extracts were centrifuged and diluted in methanol.
Enzymatic hydrolysis of raw herb
Enzymatic hydrolysis of raw herb was performed in 150-ml flasks by incubating 2 g of raw herb with 18 mg cellulase (75 U/g of β-glucosidase) in 25 ml acetate buffer, pH 4.8 at 50°C. The reactions were stopped by adding 25 ml ethanol, and then microwave-assisted extraction was performed.
Microorganism
A. oryzae (CICC 2436) was purchased from China Center of Industrial Culture Collection (Beijing, P. R. China).
Shake-flask cultivation
Well-developed fungal spores were removed from agar slants and used to inoculate 30 ml wheat bran extract medium in a 250-ml shake flask. The shake flasks with media were autoclaved at 121°C for 20 min. Microbial cells were grown for 24 h on a rotary shaker at 25°C and 150 rpm. The resulting biomass was used as inocula for 50 ml fermentation medium contained in 250-ml shake flasks that were again incubated at 25°C. The fermentation medium was composed of 20 g/l wheat bran, 5 g/l NH4NO3, and 80 g/l powder of P. cuspidatum. Microwave sterilization of the medium was carried out for 2 min. After incubation, the culture was mixed with 50 ml ethanol, and microwave-assisted extraction was performed for 2 min. Extracts were centrifuged and diluted in methanol.
Batch fermentation
Wheat bran medium with 6% powder of P. cuspidatum was used as growth medium. Seed culture was incubated in 250-ml shake flasks with 30 ml medium at 30°C and 150 rpm for 24 h. Batch fermentation was carried out in a 5-l fermentator (BIOTECH-5JG, Shanghai Baoxing Bioengineering Equipment, Shanghai, China) with a working volume of 2.5 l. The reactor was equipped with pH, pO2, temperature, and agitation controls. Temperature, airflow rate, and agitation rate were regulated at 37°C, 1.5 vvm and 250 rpm, respectively. Ten milliliters of the culture in triplicate was harvested for each sampling between 8 and 32 h.
Analytical methods
Diluted extracts were analyzed by high-performance liquid chromatography, using a Jasco separation module with a Kromasil C18 column (5 μm, 4.6 × 250 mm) as the stationary phase. The mobile phase consisted of water (A) and acetonitrile (B). Trans-piceid and trans-resveratrol were eluted with a gradient time programme, 0–7 min: 31% B; 7–15 min: 31–60% B at room temperature and a flow rate of 0.7 ml/min. The eluate was monitored at 306 nm, where both trans-piceid and trans-resveratrol have absorbance maxima. In the present work, the percent conversion of piceid was defined as follows:
Enzyme assays
β-glucosidase activity in culture supernatant were assayed at 50°C by using 0.5% salicin as the substrate in 0.2 M acetate buffer at pH 4.8 (Mandeds et al. 1976). Glucose was analyzed by a glucose analyzer (Biosensor SBA-50, Institute of Biology, Shandong Academy of Sciences, Shandong, China). One unit of enzyme is defined as the amount of enzyme by which 1 μmol of glucose is liberated in 1 min. The volumetric activity of enzyme was expressed as U/ml.
Results
Hydrolysis of raw herb by H2SO4 solution
Resveratrol is unstable in alkaline solution, so hydrolytic reaction was performed in H2SO4 solution. The effect of hydrolysis time on the yield of trans-resveratrol and trans-piceid was illustrated in Fig. 2. The results showed that the yield of trans-piceid decreased dramatically with the increasing hydrolysis time. Simultaneously, the yield of trans-resveratrol first increased slightly and then declined after 20 min, which accounted for the fact that trans-piceid can be hydrolyzed to trans-resveratrol in acid condition under 1.1 Mpa, while trans-resveratrol also decomposes during hydrolysis. Therefore, acid hydrolyzation is not a feasible method to increase the yield of trans-resveratrol.
Enzymatic hydrolysis of raw herb
The effect of enzymatic hydrolysis time on the yield of trans-piceid and trans-resveratrol was illustrated in Fig. 3. The results demonstrated that 90% of trans-piceid was converted to trans-resveratrol, while the percent conversion reached more than 100% after 12 h incubation, indicating that cellulase hydrolyzed the cellulose of the raw herb and promotes the hydrolysis and extraction of the products.
Shake-flask cultivation of P. cuspidatum by A. oryzae
The time courses of enzyme activities, concentration of glucose, and pH were depicted in Fig. 4. The results indicated that β-glucosidase activity decreased to its lowest value at 40 h and then reached its highest at 44 h. At the same time, glucose concentration peaked at 40 h and then dropped sharply. The lower β-glucosidase activity is coupled with the higher concentration of glucose, because glucose is an inhibitor of β-glucosidase. During the cultivation, pH decreased gradually because of cell growth and fermentation. At 60 h, it reached its lowest value of 3.91.
Figure 5 showed the time courses of the yield of trans-peceid and trans-resveratrol in shake-flask cultivation. When cultivated at 25°C, the yield of both trans-peceid and trans-resveratrol remained nearly constant before 40 h. After 40 h incubation, the yield of trans-piceid decreased markedly with the increase in fermentation time, whereas that of trans-resveratrol first increased and reached its highest value at 44 h, when the percent conversion got its highest, and then declined obviously.
Considering the higher β-glucosidase activity and the lower glucose concentration at 44 h, the incubation temperature was increased to 50°C; because the optimum temperature for β-glucosidase from Aspergillus strains ranges from 50 to 65°C (Yan and Lin 1997; Riou et al. 1998; Decker et al. 2001; James et al. 2006), the enzyme is very stable at 50°C and the condition is easy to control. High temperature accelerated the hydrolysis of trans-piceid so that it decreased dramatically and disappeared after 46 h. Simultaneously, the yield of trans-resveratrol peaked at 46 h, when percent conversion reached its highest, and then declined slightly.
Batch fermentation of P. cuspidatum by A. oryzae
Taking the industrial application into account, scale-up fermentations were conducted in a 5-l fermentor, and the fermentation temperature was increased to 37°C. To shorten the fermentation time, 6% raw herb was added into the seed medium, and it was cultivated at 30°C. Dissolved oxygen (DO) decreased significantly at the first 15 h and remained a low value afterwards (Fig. 6), indicating the increasing cell growth rate. After 22 h incubation, it increased obviously. The increase and decrease in pH value was in accordance with that of DO, suggesting that the insufficient oxygen causes the low pH value. Concentration of glucose declined slightly from 8 to 16 h and increased afterwards, whereas β-glucosidase activity was not detected until 20 h, and then it increased and peaked at 28 h, as shown in Fig. 6. The yield of the two compounds did not change obviously until 16 h, and then the yield of trans-piceid declined significantly and that of trans-resveratrol increased dramatically and got its highest at 24 h, when percent conversion reached nearly 100%, and then declined (Fig. 7).
Discussion
Fermentation of P. cuspidatum was successfully performed by A. oryzae, and trans-piceid was transformed to trans-resveratrol with the highest yield of trans-resveratrol 1.35%, 3.6 times higher than that obtained from raw herbs by microwave-assisted extraction, as shown in Fig. 8. Enzymatic hydrolysis of raw herb was also carried out successfully, and the yield of trans-resveratrol was equal to that from biotransformation, but the high cost limits its industrial application. Hydrolysis of raw herb by H2SO4 solution was proved to be a failure because trans-resveratrol was seriously destroyed in the process. Scale-up production was also performed, and the yield of trans-resveratrol was 3.1 times higher after 24 h incubation.
In shake-flask cultivation, when temperature was regulated at 25°C, the optimum temperature for the growth of the strain, the yield of trans-resveratrol peaked at 44 h, whereas in batch fermentation, when 6% powder of P. cuspidatum was added in the seed medium and the incubation temperature was increased to 37°C, 24 h was enough for the highest yield of trans-resveratrol, indicating that the raw herb in the seed culture significantly induced the growth of the strain and the secretion of enzyme in fermentation culture.
It should be emphasized that in enzymatic hydrolysis, cellulase with high β-glucosidase activity promoted the hydrolysis of trans-piceid to trans-resveratrol, whereas in both shake-flask cultivation and batch fermentation, the much lower β-glucosidase activity did not influence the rapid transformation of trans-piceid to trans-resveratrol. As concerns β-glucosidase, it catalyzes the hydrolysis of β-O-glucosidic linkages between β-d-glucose and an aglycone or another sugar. It exhibits similar specificity for a β-glucoside substrate, but distinct specificities for the aglycone linked to the glucosyl group (Esen 1993). A piceid-β-d-glucosidase was purified and characterized by Zhang et al. (2007), and it was proved to have higher activity to hydrolyze piceid and much lower activity to hydrolyze pNPG. Therefore, whether the β-glucosidase in our culture had the specificity to hydrolyze β-(1→4)-d-glucoside of piceid is worthwhile for further study.
It is also worth pointing out that the yield of trans-resveratrol first increased and then decreased after it got its highest value in both shake-flask fermentation and batch fermentation, and that in shake-flask fermentation at 25°C, the highest percent conversion is only 64% after a long-time incubation. All of the above may be because of a certain enzyme that can transform trans-resveratrol in the culture. When the temperature was increased to 50°C in shake-flask cultivation, the yield of trans-resveratrol decreased slightly, suggesting that high temperature inhibits the enzyme activity. However, other properties of this enzyme are unknown, and no new products were detected in the culture. Therefore, it deserves further investigation how to control the enzyme activities and optimize the bioprocess to increase the yield of trans-resveratrol.
References
Andrus MB, Liu J, Meredith EL, Nartey E (2003) Synthesis of resveratrol using a direct decarbonylative Heck approach from resorcylic acid. Tetrahedron Lett 44:4819–4822
Becker JVW, Armstrong GO, Merwe MJ, Lambrechts MG, Vivier MA, Pretorius IS (2003) Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res 4:79–85
Bertelli AA, Ferrara F, Diana G, Fulgenzi A, Corsi M, Ponti W, Ferrero ME, Bertelli A (1999) Resveratrol, a natural stilbene in grapes and wine, enhances intraphagocytosis in human promonocytes: a co-factor in antiinflammatory and anticancer chemopreventive activity. Int J Tissue React 21:93–104
Cao Y (2004) Techniques for the extraction of high purity resveratrol from Polygonum cuspidatum. Chinese Patent CN 1513822A
Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27:765–771
Decker CH, Visser J, Schreier P (2001) β-glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Appl Microbiol Biotechnol 55:157–163
Esen A (1993) Glucosidases: overview. Chemtracts Biochem Mol Biol 1–14
James L, Neil S, Feng X (2006) Substrate specificity of Aspergillus oryzae family 3 β-glucosidase. Biochim Biophys Acta 1764:972–978
Jiang H-W (2000) A technique for the extraction of resveratrol from Polygonum cuspidatum. Chinese Patent CN 1251361A
Luo H-S, Tong W-Y (2005) A method for the preparation of resveratrol from Polygonum cuspidatum. Chinese Patent CN 1566349A
Mandeds M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulose. Biotechnol Bioeng Symp 6:21–33
Meng X-F, Maliakal P, Lu H, Lee M-J, Yang C-S (2004) Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem 52:935–942
Ning L-L, Zhan J-X, Qu G-Q, Zhong L, Guo H-Z, Bi K-S, Guo D-A (2003) Biotransformation of triptolide by Cunninghamella blakesleana. Tetrahedron 59:4209–4213
Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM (1995) The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin Chim Acta 235:207–219
Parshikov IA, Miriyala B, Avery MA, Williamson JS (2004a) Hydroxylation of 10-deoxoartemisinin to 15-hydroxy-10-deoxoartemisinin by Aspergillus niger. Biotechnol Lett 26:607–610
Parshikov IA, Muraleedharan KM, Avery MA, Williamson JS (2004b) Transformation of artemisinin by Cunninghamella elegans. Appl Microbiol Biotechnol 64:782–786
Riou C, Salmon JM, Vallier MJ, Günata Z, Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerantβ-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64:3607–3614
Wendy AL (2000) Biotransformations in organic synthesis. Bioresour Technol 74:49–62
Yan TR, Lin CL (1997) Purification and characterization of a glucose-tolerant beta-glucosidase from Aspergillus niger CCRC 31494. Biosci Biotechnol Biochem 61:965–970
Zhang C-Z, Li D, Yu H-S, Zhang B, Jin F-X (2007) Purification and characterization of piceid-β-d-glucosidase from Aspergillus oryzae. Process Biochem 42:83–88
Zhou J-J, Zhang H-J, Yang P-J, Li H-N (2002) Determination of resveratrol glucoside and resveratrol in radix and rhizome of Polygonum cuspidatum yielded in Hanzhong Region (in Chinese). Chinese Traditional and Herbal Drugs (Zhong Cao Yao) 33:414–416
Zou X-D, Shu X (2002) Method to improve content of resveratrol in Polygonum cuspidatum. Chinese Patent CN 1385535A
Acknowledgements
This work was supported by the Chinese “973” plan project (2003CB716000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Liu, L., Guo, YX. et al. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae . Appl Microbiol Biotechnol 75, 763–768 (2007). https://doi.org/10.1007/s00253-007-0874-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0874-3