Abstract
The biomass of an oxygen-limited autotrophic nitrification/denitrification (OLAND) biofilm reactor was preserved in various ways to find a storage method for both aerobic and anaerobic ammonium-oxidizing bacteria (AerAOB and AnAOB). Storage occurred at −20°C with and without glycerol as cryoprotectant and at 4 and 20°C with and without nitrate as redox buffer. After 2 and 5 months, reactivation of AerAOB and AnAOB was achieved with the biomass stored at 4°C with and without nitrate and at 20°C with nitrate. Moreover, the presence of the AerAOB and AnAOB was confirmed with fluorescent in situ hybridization (FISH). Preservation in a nitrate environment resulted in a lag phase for the AnAOB reactivation. The supplied nitrate was denitrified during storage, and a real-time polymerase chain reaction with nitrifying and denitrifying genes allowed to estimate that at least 1.0 to 6.0% of the OLAND biofilm consisted of denitrifiers. It was concluded that reactivation after long-term storage is possible and that preservation at 4°C without nitrate addition is the recommended storage technique. The possibility to store OLAND biomass will facilitate research on AnAOB and can overcome larger-scale start-up and inhibition problems of novel nitrogen processes involving AnAOB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, several new nitrogen-removal processes have been developed to treat wastewaters that are rich in ammonium and poor in organic carbon (Ahn 2006). One of these processes is oxygen-limited autotrophic nitrification/denitrification (OLAND), a process in which ammonium is autotrophically oxidized to dinitrogen gas with nitrite as the electron acceptor under oxygen-limited conditions (Kuai and Verstraete 1998). Similar processes are the aerobic deammonification process (Hippen et al. 1997) and the completely autotrophic nitrogen removal over nitrite (CANON) process (Third et al. 2001). These autotrophic processes consume 63% less oxygen and 100% less biodegradable organic carbon compared to the conventional nitrification/denitrification process and therefore have a lower operating cost (Verstraete and Philips 1998). In its current technical configuration, the OLAND process is operated in a rotating biological contactor (RBC), producing a thick biofilm (Pynaert et al. 2003). The OLAND biofilm consists primarily of two major groups of bacteria responsible for autotrophic nitrogen removal: the aerobic ammonium-oxidizing bacteria (AerAOB, Nitrosomonas spp.) convert ammonium to nitrite with oxygen as the electron acceptor and the anaerobic ammonium-oxidizing bacteria (AnAOB, close relatives of Kuenenia stuttgartiensis, belonging to the Planctomycetales) subsequently oxidize ammonium with nitrite as the electron acceptor (Pynaert et al. 2003; Strous et al. 1998; Wyffels et al. 2003).
A current problem in research and industry is the lack of considerable quantities of biomass capable of initiating this form of autotrophic ammonium to dinitrogen conversion. One of the reasons is that the AnAOB grow very slowly, with an optimal doubling time of 11 days (Strous et al. 1998). In addition, the anammox reaction is easily inhibited by oxygen and nitrite. Very low oxygen levels (<0.04 mg/l) inhibit reversibly (Strous et al. 1997) and high nitrite concentrations (>100 mg N/l) inhibit irreversibly (Strous et al. 1999). By seeding stored and reactivated OLAND biomass into new set-ups, a rapid start-up of lab-scale experiments or even full-scale reactors could be obtained. Moreover, stored biomass would allow to quickly restore the process whenever it is disturbed or inhibited.
Hence, the goal of this study was to find a simple and adequate method to store highly active OLAND biomass. Most conservation techniques have been developed mainly for axenic microbial cultures. To our knowledge, only two studies have reported on the conservation of a complex microbial community. Vogelsang et al. (1999) reported on the storage of a nitrifying culture by freezing and Laurin et al. (2006) investigated the conservation of a denitrifying sludge by freezing and starvation. The results of these conservation studies are compared with the results from our study in “Discussion”.
In the view of practical applicability on semi-industrial scale, the storage of OLAND biomass was investigated at three commonly available temperatures: deep-frozen at −20°C and starved at 4°C and at 20°C. At these three temperatures, the effect of a suitable additive was investigated. For the frozen samples, the use of glycerol as a cryoprotectant was tested, and at positive temperatures, the addition of nitrate as redox buffer was examined. For the nitrate treatments, nitrate concentration during storage was monitored, and nitrate was re-added when necessary to prevent the occurrence of sulphate reduction leading to anaerobic conditions. The origin of the nitrate removal was investigated by screening the original biomass with real-time polymerase chain reaction (PCR) for denitrification genes. After 2 months of storage, the activity of the AnAOB as well as the AerAOB was tested in batch. The treatments that allowed for reactivation were re-investigated after 5 months, and fluorescent in situ hybridization (FISH) was used to confirm the presence of the AerAOB and AnAOB.
Materials and methods
Origin of biomass
The biomass was harvested from the lab-scale RBC described by Pynaert et al. (2003). The reactor was operated in a climate-controlled room at 34 ± 1°C. At the moment of the biomass harvesting, the reactor had a loading rate of 0.52 ± 0.02 g NH4 +–N l−1 day−1 and a nitrogen removal of 0.47 ± 0.03 g N l−1 day−1. Dissolved oxygen concentration and pH in the reactor were 0.56 ± 0.05 mg O2/l and 7.73 ± 0.09, respectively. Means and standard deviations were calculated from 13 data points over a period of 2 months of stable reactor operation. Biomass used for storage and reference activity tests was harvested from the reactor by scraping off parts of the biofilm (in the whole depth) of several discs. The biomass had a dry matter content of 4.4 ± 0.4%, and the volatile suspended solids (VSS)-to-total suspended solids (TSS) ratio was 91.4 ± 0.9%. Microscopic investigations showed that the biomass aggregate diameter varied from about 250 to 1,000 μm.
Storage of biomass
To remove all dissolved nitrogen compounds originating from the reactor liquid, the harvested biofilm biomass was washed with tap water in a sieve (pore size 250 μm). For storage in the freezer, plastic tubes of 50 ml were used. In the glycerol treatment, 25 ml of glycerol solution was added to 25 g of washed biomass, resulting in a final glycerol concentration of 20%. For storage at 4 and 20°C, 60-ml serum flasks (55-ml active volume) were used, each containing 35 g of biomass. As such, the final biomass concentration was about 26 g VSS/l. In the nitrate treatment, initially 20 ml of a NaNO3 solution (80 mg N/l) was added to the biomass. The flasks were closed with butyl rubber stoppers and aluminium caps. Three flasks per temperature were reserved for frequent sampling through the septum to monitor nitrate, nitrite and ammonium in the liquor. New nitrate was added whenever the nitrate depletion was near. Both at 4 and 20°C, nitrous oxide in the gas phase was measured for the nitrate treatments after 5 months of storage. For the samples stored without nitrate, 20 ml of tap water was added to the biomass instead of the nitrate solution.
Aerobic and anaerobic batch tests
The aerobic and anaerobic ammonium removal was tested at the moment of biomass harvesting and after preservation of the biomass. Aerobic and anaerobic batch experiments were performed in triplicate as described by Windey et al. (2005). In short, aerobic batch experiments were performed in 250-ml Erlenmeyer flasks with 100-ml working volume. Per Erlenmeyer, 0.1 g NH4Cl–N/l and a buffering solution (final concentrations 1 g NaHCO3/l, 3.4 g KH2PO4/l and 4.4 g K2HPO4/l) were supplied to the biomass (0.22 ± 0.02 g VSS). The flasks were incubated on a shaker at 28 ± 2°C. During the experiment, pH and dissolved oxygen concentration were monitored, and samples were taken for ammonium, nitrite and nitrate analyses. For the anaerobic batch tests, 120-ml serum flasks were used, containing 80 ml of mixed liquor. Once the biomass (0.22 ± 0.02 g VSS) and a buffering solution (final concentrations 1 g NaHCO3/l and 0.04 g KH2PO4/l) were added, the flasks were closed with rubber stops and flushed with N2 gas. Then, flushed substrate solutions of NH4Cl and NaNO2 were supplemented by means of needled syringes. Final concentrations were 0.1 g NH4 +–N/l and 0.1 g NO2 −–N/l. The flasks were incubated at 34 ± 1°C, and liquid samples were taken for ammonium, nitrite and nitrate analyses.
Chemical analyses
Ammonium was determined colorimetrically with Nessler reagent according to standard methods (Greenberg et al. 1992). Both nitrite and nitrate were determined using a Metrohm 761 Compact Ion Chromatograph equipped with a conductivity detector. The operational parameters were as follows: column metrosep A supp 5; eluent 1.06 g Na2CO3/l; flow 0.7 ml/min; sample loop 20 μL. VSS and TSS were determined by drying and weighing according to standard methods (Greenberg et al. 1992). The pH was determined potentiometrically with a portable Consort C532 pH meter. The dissolved oxygen (DO) concentration was measured with a portable Endress–Hauser COM381 DO meter. Nitrous oxide was measured on a Shimadzu GC-14B gas chromatograph fitted with an electron capture detector. A 1-m Porapack Q column separated N2O from the other gases, and the temperature of the detector, injector and oven were 250, 100 and 35°C, respectively. The carrier gas helium was flowing at 55 ml/min. Nitrous oxide dissolved in the aqueous phase was accounted for (Moraghan and Buresh 1977).
Real-time PCR
Real-time PCR was used to quantify the denitrifiers in OLAND biomass. DNA was extracted from 2-ml samples according to Boon et al. (2000) and purified over a Promega Wizard Minicolumn DNA purification kit. DNA was resuspended in 100 μl H2O, and the DNA concentration was adjusted to 100 ng/μl, measured with a NanoDrop ND-1000 spectrophotometer. Quantitative PCR was performed on an ABI Prism® SDS 7000 (PE Applied Biosystems). Amplification reactions were carried out with the SYBR Green PCR master mix (PE Applied Biosystems). Primer names, references and target genes are listed in Table 1. An oligonucleotide concentration of 300 nM and DNA template volume of 1 μl was added to 24 μl PCR master mix in MicroAmp Optical 96-well reaction plates (PE Applied Biosystems). Standard curves were constructed using reference organisms Nitrosomonas europaea ATCC 19718 (for 16S rRNA AerAOB and amoA genes), Paracoccus denitrificans LMG 4049T (for nirS and nosZ genes) and Alcaligenes faecalis LMG 1229T (for nirK and nosZ genes), obtained from the American Type Culture Collection and the BCCM/LMG Bacteria Collection (Ghent, Belgium). The standard curves had an R 2 value of 0.98, 0.99, 0.98, 0.99 and 0.98 and a slope of −3.1, −3.3, −3.8, −3.6 and −3.6 for 16S rRNA AerAOB, amoA, nirK, nirS and nosZ, respectively.
FISH
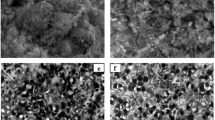
FISH was used to investigate the presence of β-proteobacterial aerobic ammonia oxidizers and Planctomycetales in stored and reactivated biomass. Biomass samples were fixed in a 4% paraformaldehyde solution, washed with phosphate-buffered saline and stored in phosphate-buffered saline–ethanol (1:1) at −20°C until further processing (Amann et al. 1990a). The following oligonucleotide probes were used: (1) Cy3 labeled Nso1225, targeting β-Proteobacteria ammonia-oxidizers (Mobarry et al. 1996), (2) Cy3 labeled Pla46, targeting Planctomycetales (Neef et al. 1998) and (3) a 1:1:1 mixture of FLUO labelled EUB338I, EUB338II and EUB338III, targeting all bacteria (Amann et al. 1990b; Daims et al. 1999). Image acquisition was done on a Zeiss Axioskop 2 Plus epifluorescence microscope using a Hamamatsu Orca IIIm camera.
Results
-
1.
Nitrogen removal during storage of OLAND biomass
For the nitrate supplemented biomass, nitrate and nitrite concentrations were monitored over time. Nitrate was consumed during storage (Fig. 1), and to prevent anaerobiosis, new pulses were given when nitrate depletion was near. At 4 and 20°C, mean nitrate consumption rates were 0.08 ± 0.01 and 0.59 ± 0.14 mg NO3 −–N g−1 VSS day−1, respectively. No significant amounts of ammonium were detected in the liquid, and the largest observed nitrite concentrations were 3.7 and 9.8 mg N/l at 4 and 20°C, respectively. To elucidate the origin and biochemical nature of the nitrate removal, a real-time PCR with nitrification and denitrification primers was done on OLAND biomass from the reactor. This revealed the presence of the nitrite reductase encoding nirS and nirK and the nitrous oxide reductase gene nosZ (Table 1), demonstrating the presence of denitrifiers in OLAND biomass. In addition, the nitrous oxide concentration in the gas phases was measured. From this, it was calculated that 3.2 ± 1.0 and 3.5 ± 2.2 μg N2O– N g−1 VSS day−1 were formed at 4 and 20°C, respectively. Thus, the majority of consumed nitrate was denitrified into dinitrogen gas, with 3.1 ± 11% (at 4°C) and 0.68 ± 0.43% (at 20°C) of the removed NO3 −–N remaining as N2O–N.
-
2.
Activity tests on preserved OLAND biomass
After 2 months of biomass storage, the aerobic and anaerobic ammonium-oxidizing capacity of the biomass was investigated for the first time. The treatments that enabled for reactivation of both AerAOB and AnAOB were also examined after 5 months of storage. The maximum ammonium removal rate was calculated from the steepest decrease in ammonium concentration between two sampling points. A comparison of the resulting removal rates relative to the activity of reference OLAND biomass is presented in Fig. 2.
Nitrate (open square) and nitrite (filled square) concentration (mg N/l) during anoxic storage of OLAND biomass with nitrate addition. Whenever nitrate was near to depletion, a new nitrate pulse was given (arrows). a Storage at 4°C. b Storage at 20°C. Means and standard deviations were calculated from a triplicate test. Standard deviations for the nitrite concentrations were too small to see on the graph
Maximum specific aerobic and anaerobic ammonium-oxidizing capacity of stored OLAND biomass relative to reference biomass activity. Reference biomass converted 49 ± 10 mg N g−1 VSS day−1 aerobically and 65 ± 8 mg N g−1 VSS day−1 anaerobically. Results are grouped per storage treatment; glyc and nitr stand respectively for the addition of glycerol (20%) and nitrate during storage. Tests for reactivation were done after 2 (2m) and 5 (5m) months of preservation, except for the storage at −20°C, which was not tested after 5 months (filled diamond). AerAOB aerobic ammonium-oxidizing bacteria, AnAOB anaerobic ammonium-oxidizing bacteria. All experiments were performed in triplicate
Biomass stored at −20°C could be reactivated aerobically, both with and without glycerol addition. The aerobic ammonium removal was about three times higher with glycerol added during storage. Reactivation of the AnAOB was not successful: anaerobically, no ammonium was removed, regardless of glycerol addition. Nitrite consumption was observed, though, at a rate of 34 ± 4 and 18 ± 7 mg N g−1 VSS day−1 for the treatments with and without glycerol, respectively. As there was no anaerobic ammonium removal after 2 months, no further tests were performed after 5 months of storage at −20°C. Biomass stored at 4°C showed an aerobic and anaerobic ammonium removal after 2 and 5 months of storage. This was the case for storage with and without nitrate. Finally, biomass stored at 20°C without added nitrate turned black after 1 week; therefore, no further work was done on this particular biomass. For the nitrate-supplied biomass, both aerobic and anaerobic activity could be partially restored after 2 and 5 months of storage at 20°C.
Apart from the ammonium removal rate, the lag phase is another reactivation aspect. The lag phase was defined as the last sampling time without significant ammonium removal. For the aerobic tests, no lag phases were observed for biomass stored at −20, 4 and 20°C. For the anaerobic tests, however, lag phases were seen for the nitrate treatments (Fig. 3). This was not the case for storage without nitrate at 4°C.
Lag phases observed during AnAOB reactivation of stored OLAND biomass. Results are grouped per treatment; nitr stands for the addition of nitrate. Grey bars show lag phases after 2 months of storage, black bars after 5 months. No observed AnAOB lag phase is indicated by filled diamond. Reported lag phases were the same for each flask of the triplicate experiment, so standard deviations are zero
The presence of both AerAOB and AnAOB after 5 months of storage was confirmed with FISH. A probe for all bacteria and a specific probe for either β-proteobacterial aerobic ammonia oxidizers or Planctomycetales was added to both stored and reactivated biomass. The results for all treatments indicate that the aerobic ammonia oxidizers and the Planctomycetales were present before and after reactivation (Fig. 4).
FISH pictures of reactivated OLAND biomass after 5 months of storage at 4°C without nitrate supply. All bacteria are coloured with the EUB probe mixture (green). a Aerobically reactivated biomass hybridized with Nso1225 probe (red) highlighting ammonia oxidizers. b Anaerobically reactivated biomass hybridized with PLA46 probe (red) highlighting planctomycetes
Discussion
A problem in novel nitrogen removal processes involving the use of AnAOB is the lack of considerable biomass quantities. The goal of this study was, therefore, to provide a simple technique for storage of a microbial community containing AnAOB in casu OLAND biomass. Three of the examined treatments have been found suitable for adequate preservation of a biomass containing both AerAOB and AnAOB: biomass stored without nitrate at 4°C and with nitrate supplement at 4 or 20°C. All three treatments allowed for both aerobic and anaerobic ammonium conversion after a biomass storage period of 5 months. However, the initial ammonium conversion capacity of the biomass was not restored. Inactivation or die-off of AerAOB or AnAOB as a function of storage time was not likely, as removal rates after 5 storage months were higher than or equal to those after 2 months. A possible explanation of the partial reactivation is that the biomass was not fully reactivated within the time frame of the reactivation test. This was verified in an additional test: during reactivation of biomass stored at 4°C with nitrate, a second substrate pulse was given after AnAOB depletion of the nitrite. This resulted in an ammonium removal of 36 ± 2 mg N g−1 VSS day−1, about twice as fast as the removal after the first pulse (17 ± 5 mg N g−1 VSS day−1).
During storage of the nitrate-added treatments, a steady consumption of nitrate was measured. Therefore, nitrate had to be re-added several times at both 4 and 20°C. As preliminary tests with Fe3+ or Mn4+ as redox stabilizers could not prevent the blackening of the biomass at 20°C, nitrate was chosen as redox buffer in spite of its consumption. The presence of the denitrification genes nirS, nirK and nosZ, as well as the production of some N2O in the absence of ammonium, prompts to conventional heterotrophic denitrification as nitrate-removing process. Heterotrophs have been found previously in autotrophic nitrifying biofilms (Kindaichi et al. 2004). In reactor systems, active nitrifiers release organic molecules from substrate metabolism and biomass decay (Rittmann et al. 1994), which are then degraded and utilized by heterotrophs (Okabe et al. 2005). Yet, in the biomass storage test, the origin of the electron donor for denitrification is unclear. Supposedly, organic molecules, which were steadily released from decaying biomass, fuelled the nitrate consumption.
The reference anaerobic ammonium-removal rate of freshly harvested OLAND biomass (65 mg N g−1 VSS day−1) allows quantification of the AnAOB. The responsible AnAOB (close relatives of K. stuttgartiensis; Pynaert et al. 2003) was reported to remove 320 mg N g−1 VSS day−1 at a culture purity of 88% (Egli et al. 2001). Based on these values, 18% of the OLAND biomass would consist of AnAOB. The percentage nitrifiers and denitrifiers in the OLAND biomass can be calculated using the real-time PCR results (Table 1) and current genomic knowledge. The redundancy of the amoA operon in nitrifier cells is species-dependent and varies from two to three copies in β-proteobacterial AerAOB (Norton et al. 2002). The N. europaea genome has two amoA copies and one copy of the 16S rRNA gene (Chain et al. 2003). These numbers of N. europaea are in correspondence with the OLAND biomass analyses, showing an amoA/16S rRNA AerAOB ratio of almost 2 (Table 1). Therefore, the value of a single 16S rRNA AerAOB gene copy per OLAND nitrifying cell (Nitrosomonas spp.; Pynaert et al. 2003) was assumed, implying that at least 54% (Table 1) of the OLAND biofilm consisted of AerAOB. Together with 18% AnAOB, the maximum possible percentage of denitrifiers was 28%. With one 16S rRNA copy per AnAOB cell (Strous et al. 2006), the non-AerAOB and non-AnAOB cells cannot have more than six 16S rRNA copies per cell, on average. As the nitrite reductase encoding nirK is also present in the nitrifier N. europaea (Casciotti and Ward 2001), the denitrifier percentage was calculated with the nitrous oxide reductase encoding nosZ copy number, although not all denitrifiers possess nosZ (Zumft 1997). As a nosZ-containing denitrifying cell contains one nosZ gene copy (Philippot 2002), at least 1.0 to 6.0% (Table 1) of the OLAND bacterial community consisted of denitrifiers.
Laurin et al. (2006) described the 17-month storage of a denitrifying biofilm at 4 and at −20°C with and without glycerol. Under starvation conditions at 4°C, inactivation or die-off of the denitrifiers was suggested by the steady decrease in the denitrification rate as a function of the storage time. As a possible explanation, exposure to H2S was put forward. In our study, nitrate addition effectively prevented sulphate reduction at 20°C. At 4°C however, the lower temperature restrained sulphate reduction for a period of at least 5 months, rendering nitrate addition redundant. The addition of nitrate at 4°C had even a small negative effect on the AnAOB removal rates, which were significantly lower compared to the treatment without nitrate (Fig. 2). In the freezing experiments of Laurin et al. (2006), glycerol had a beneficial effect on the survival of denitrifiers. This is in correspondence with the difference we observed in anoxic nitrite consumption rates after biomass freezing with and without glycerol. As no organic carbon was added in the anoxic batch tests, the observed denitrification rates suggest that part of the biomass had died during storage and, as such, released organic carbon, which became available for surviving heterotrophic denitrifiers.
Vogelsang et al. (1999) described the 2 to 3 months storage of a gel-entrapped nitrifying culture at −80°C with and without glycerol. Biomass frozen with glycerol maintained 40% of its activity, versus 60% without glycerol. The findings of our study (Fig. 2), on the contrary, indicate a better AerAOB survival after freezing at −20°C with added glycerol (70%) than without glycerol (23%). Possibly, the freezing temperature, the presence of nitrite oxidizers or the biomass form (biofilm, suspended, gel entrapped...) influences AerAOB survival after freezing. For storage of AerAOB at positive temperatures, all treatments allowed for reactivation at more than 50% of the original ammonium oxidizing rate (Fig. 2). Recently, Geets et al. (2006) reviewed the physiological traits of AerAOB that are advantageous for their survival under conditions of substrate limitation, starvation and fluctuation. Three strategies in particular are suitable for survival during long-term ammonium and/or oxygen starvation. First of all, AerAOB have a stable set of catabolic cellular components such as energy-generating enzymes. This is in correspondence with our observation that the aerobic ammonium oxidation started very quickly upon reactivation of the stored biomass. As a second physiological advantage, AerAOB have low decay rates and a low maintenance-energy demand. Finally, cell-to-cell communication by AerAOB might initiate signalling pathways involved in starvation survival. However, the AerAOB production of signalling molecules during starvation has not been demonstrated yet.
Concerning AnAOB, this is the first report on possible biomass storage conditions. In nature, anammox was found to be co-responsible for N2 production in sea ice (Rysgaard and Glud 2004). In our results however, AnAOB seemed not able to survive freezing at −20°C, regardless of glycerol addition (Fig. 2). Further research in this area could reveal the possible influence of freezing rate, freezing temperature or salinity on cryotolerance or cryosurvival of specific AnAOB. From the three storage treatments that allowed for AnAOB reactivation, lag phases of at least 24 h were observed when storage occurred in a nitrate environment (Fig. 3). This suggests the de novo synthesis of the necessary RNA and enzymes for combining ammonium and nitrite. Possibly, the AnAOB switch to a different metabolism in the presence of nitrate. It is, for instance, known that nitrate can act as electron acceptor for the AnAOB oxidation of ammonium (Vandegraaf et al. 1995), propionate (Guven et al. 2005) and iron (Strous et al. 2006). Another possible factor influencing the AerAOB and AnAOB lag phases is that the growth rates differ a factor of 10: AerAOB have a growth rate of about 0.04 h−1 (Jetten et al. 2001), whereas this is only 0.003 h−1 for AnAOB (Strous et al. 1998).
Three techniques were found in this study to preserve a biomass containing both AerAOB and AnAOB for at least 5 months. Both treatments with nitrate addition were suitable and could possibly be improved by using a higher nitrate concentration (e.g. 2 g N/l) to avoid repeated changes in redox conditions due to frequent pulsing at low nitrate concentrations. From the successful techniques, we recommend storage of the biomass at 4°C without nitrate addition. This treatment allowed for the best AnAOB reactivation (55% of the original activity), no lag phase was present upon AnAOB reactivation, and there was no need for nitrate addition and monitoring. This study provides a simple biomass storage method, which is most likely applicable for any active biomass containing AnAOB, as from the Anammox (deGraaf et al. 1996), CANON (Third et al. 2001) or deammonification process (Hippen et al. 1997). In a broader context, this study differs from most conservation or long-term starvation studies that not a single species or functionality was investigated but a complex bacterial community with two concrete functions, i.e. aerobic and anaerobic ammonium removal.
References
Ahn YH (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721
Amann RI, Krumholz L, Stahl DA (1990a) Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172:762–770
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990b) Combination of 16s ribosomal-RNA-targeted oligonucleotide probes with flow-cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Boon N, Goris J, De Vos P, Verstraete W, Top EM (2000) Bioaugmentation of activated sludge by an indigenous 3-chloroaniline-degrading Comamonas testosteroni strain, I2gfp. Appl Environ Microbiol 66:2906–2913
Braker G, Fesefeldt A, Witzel KP (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64:3769–3775
Casciotti KL, Ward BB (2001) Dissimilatory nitrite reductase genes from autotrophic ammonia-oxidizing bacteria. Appl Environ Microbiol 67:2213–2221
Chain P, Lamerdin J, Larimer F, Regala W, Lao V, Land M, Hauser L, Hooper A, Klotz M, Norton J, Sayavedra-Soto L, Arciero D, Hommes N, Whittaker M, Arp D (2003) Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol 185:2759–2773
Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
deGraaf AAV, deBruijn P, Robertson LA, Jetten MSM, Kuenen JG (1996) Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology-UK 142:2187–2196
Egli K, Fanger U, Alvarez PJJ, Siegrist H, van der Meer JR, Zehnder AJB (2001) Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch Microbiol 175:198–207
Geets J, Boon N, Verstraete W (2006) Strategies of aerobic ammonia-oxidizing bacteria for coping with nutrient and oxygen fluctuations. FEMS Microbiol Ecol 58:1–13
Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Guven D, Dapena A, Kartal B, Schmid MC, Maas B, van de Pas-Schoonen K, Sozen S, Mendez R, Op den Camp HJM, Jetten MSM, Strous M, Schmidt I (2005) Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl Environ Microbiol 71:1066–1071
Hippen A, Rosenwinkel KH, Baumgarten G, Seyfried CF (1997) Aerobic deammonification: a new experience in the treatment of wastewaters. Water Sci Technol 35:111–120
Jetten MSM, Wagner M, Fuerst J, van Loosdrecht M, Kuenen G, Strous M (2001) Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr Opin Biotechnol 12:283–288
Kindaichi T, Ito T, Okabe S (2004) Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl Environ Microbiol 70:1641–1650
Kloos K, Mergel A, Rosch C, Bothe H (2001) Denitrification within the genus Azospirillum and other associative bacteria. Aust J Plant Physiol 28:991–998
Kowalchuk GA, Stephen JR, DeBoer W, Prosser JI, Embley TM, Woldendorp JW (1997) Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol 63:1489–1497
Kuai LP, Verstraete W (1998) Ammonium removal by the oxygen-limited autotrophic nitrification–denitrification system. Appl Environ Microbiol 64:4500–4506
Laurin V, Labbe V, Juteau P, Parent S, Villemur R (2006) Long-term storage conditions for carriers with denitrifying biomass of the fluidized, methanol-fed denitrification reactor of the Montreal Biodome, and the impact on denitrifying activity and bacterial population. Water Res 40:1836–1840
Mobarry BK, Wagner M, Urbain V, Rittmann BE, Stahl DA (1996) Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol 62:2156–2162
Moraghan JT, Buresh R (1977) Correction for dissolved nitrous oxide in nitrogen studies. Soil Sci Soc Am J 41:1201–1202
Neef A, Amann R, Schlesner H, Schleifer KH (1998) Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology-UK 144:3257–3266
Norton JM, Alzerreca JJ, Suwa Y, Klotz MG (2002) Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol 177:139–149
Okabe S, Kindaichi T, Ito T (2005) Fate of C-14-labeled microbial products derived from nitrifying bacteria in autotrophic nitrifying biofilms. Appl Environ Microbiol 71:3987–3994
Ovreas L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol 63:3367–3373
Philippot L (2002) Denitrifying genes in bacterial and archaeal genomes. Biochim Biophys Acta N Gene Struct Expr 1577:355–376
Pynaert K, Smets BF, Wyffels S, Beheydt D, Siciliano SD, Verstraete W (2003) Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Appl Environ Microbiol 69:3626–3635
Rittmann BE, Regan JM, Stahl DA (1994) Nitrification as a source of soluble organic substrate in biological treatment. Water Sci Technol 30:1–8
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Rysgaard S, Glud RN (2004) Anaerobic N-2 production in Arctic sea ice. Limnol Oceanogr 49:86–94
Strous M, vanGerven E, Kuenen JG, Jetten M (1997) Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (Anammox) sludge. Appl Environ Microbiol 63:2446–2448
Strous M, Heijnen JJ, Kuenen JG, Jetten MSM (1998) The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl Microbiol Biotechnol 50:589–596
Strous M, Kuenen JG, Jetten MSM (1999) Key physiology of anaerobic ammonium oxidation. Appl Environ Microbiol 65:3248–3250
Strous M, Pelletier E, Mangenot S, Rattei T, Lehner A, Taylor MW, Horn M, Daims H, Bartol-Mavel D, Wincker P, Barbe V, Fonknechten N, Vallenet D, Segurens B, Schenowitz-Truong C, Medigue C, Collingro A, Snel B, Dutilh BE, Op den Camp HJM, van der Drift C, Cirpus I, van de Pas-Schoonen KT, Harhangi HR, van Niftrik L, Schmid M, Keltjens J, van de Vossenberg J, Kartal B, Meier H, Frishman D, Huynen MA, Mewes HW, Weissenbach J, Jetten MSM, Wagner M, Le Paslier D (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790–794
Third KA, Sliekers AO, Kuenen JG, Jetten MSM (2001) The CANON system (completely autotrophic nitrogen removal over nitrite) under ammonium limitation: interaction and competition between three groups of bacteria. Syst Appl Microbiol 24:588–596
Throback IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417
Vandegraaf AA, Mulder A, Debruijn P, Jetten MSM, Robertson LA, Kuenen JG (1995) Anaerobic oxidation of ammonium is a biologically mediated process. Appl Environ Microbiol 61:1246–1251
Verstraete W, Philips S (1998) Nitrification–denitrification processes and technologies in new contexts. Environ Pollut 102:717–726
Vogelsang C, Gollembiewski K, Ostgaard K (1999) Effect of preservation techniques on the regeneration of gel entrapped nitrifying sludge. Water Res 33:164–168
Windey K, De Bo I, Verstraete W (2005) Oxygen-limited autotrophic nitrification–denitrification (OLAND) in a rotating biological contactor treating high-salinity wastewater. Water Res 39:4512–4520
Wyffels S, Pynaert K, Boeckx P, Verstraete W, Van Cleemput O (2003) Identification and quantification of nitrogen removal in a rotating biological contactor by N-15 tracer techniques. Water Res 37:1252–1259
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgment
This research was funded by a Ph.D. grant (aspirant) for Siegfried E. Vlaeminck from the Fund of Scientific Research-Flanders (Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen). Part of the work also derives from project grant GOA 1205073 (2003–2008) of the Ministerie van de Vlaamse Gemeenschap, Bestuur Wetenschappelijk Onderzoek (Belgium).
The authors gratefully thank Greet Van de Velde for technical support, Pascal Boeckx for kind assistance with N2O analyses and Loïs Maignien, Kim Heylen, Peter Deschryver, Ilse Forrez, Lieven Wittebolle and the anonymous reviewers for critically reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlaeminck, S.E., Geets, J., Vervaeren, H. et al. Reactivation of aerobic and anaerobic ammonium oxidizers in OLAND biomass after long-term storage. Appl Microbiol Biotechnol 74, 1376–1384 (2007). https://doi.org/10.1007/s00253-006-0770-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0770-2