Abstract
An effective method for purification of nattokinase from fermentation broth using magnetic poly(methyl methacrylate) (PMMA) beads immobilized with p-aminobenzamidine was proposed in this study. Firstly, magnetic PMMA beads with a narrow size distribution were prepared by spraying suspension polymerization. Then, they were highly functionalized via transesterification reaction with polyethylene glycol. The surface hydroxyl-modified magnetic beads obtained were further modified with chloroethylamine to transfer the surface amino-modified magnetic functional beads. The morphology and surface functionality of the magnetic beads were examined by scanning electron microscopy and Fourier transform infrared. An affinity ligand, p-aminobenzamidine was covalently immobilized to the amino-modified magnetic beads by the glutaraldehyde method for nattokinase purification directly from the fermentation broth. The purification factor and the recovery of the enzyme activity were found to be 8.7 and 85%, respectively. The purification of nattokinase from fermentation broth by magnetic beads only took 40 min, which shows a very fast purification of nattokinase compared to traditional purification methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fibrin aggregation in arteries often causes cardiovascular diseases. Thrombolytic therapy is the best way to achieve recanalization in these diseases nowadays. Urokinase and streptokinase were widely used for thrombosis but they have a low specificity to fibrin and also have hemorrhagic side effects. Tissue plasminogen activator (tPA) was used for the treatment of thrombosis because of its strong ability to lyse fibrin. However, the wide application of tPA is limited due to its cost and its short half-life in the body.
Nattokinase was considered as one kind of promising agent for thrombosis therapy, which was first discovered from Bacillus subtilis used in the traditional Japanese food, natto (Sumi et al. 1987). Closely resembling plasmin, nattokinase dissolves fibrin directly. Oral administration of nattokinase showed that this enzyme could enhance fibrinolytic activity in plasma and the production of tPA (Sumi et al. 1990). In some ways, nattokinase is actually superior to conventional clot-dissolving drugs, which has many benefits including convenience of oral administration, confirmed efficacy, prolonged effects, cost-effectiveness, and preventative use. Over the past two decades, several methods were used in the separation and purification of nattokinase from fermentation broth, such as salting out, organic solvent fractionation, chromatography, reverse micelle extraction, and expanded bed adsorption (Urano et al. 2001; Fujita et al. 1993; Chang et al. 2000; Liu et al. 2004; Hu et al. 2000). Nakanishi et al. (1998) separated nattokinase from fermentation broth by a procedure consisting of alcohol or ammonium sulfate precipitation, hydrophobic interaction chromatography, ion exchange, and gel filtration. The conventional purification of nattokinase requires a long period (about 2 days) due to the multistep process. In addition, each step requires expensive equipment and may result in a significant loss of the enzyme activity. Therefore, developing a simple method to purify the nattokinase from fermentation broth is a new challenge nowadays.
Magnetic beads based on separation are fast, gentle, and compatible with complex biological system, such as whole blood, milk, and cell, and have consequently become increasingly popular in bioengineering. Nowadays, the magnetic bioseparation is becoming a very important method in the field of biology, especially for routine handling of large numbers of samples in protein purification, cell sorting, and diagnostics (Furukawa et al. 2003; Kondo et al. 1994; Safarik and Safarikova 2004; Saiyed et al. 2003). There were different monomer copolymerization methods to the preparation of magnetic beads with pros and cons to each approach, such as emulsion polymerization (Noriko et al. 1993), dispersion polymerization (Horák and Shapoval 2000), suspension polymerization (Lee et al. 2003), and two-step swelling method (Ugelstad et al. 1983). However, in the monomer copolymerization, a large amount of functional groups were buried in the beads with only a small part left on the surface. Among these methods, suspension polymerization is simple and easy to scale up, hence, more suitable for mass production. However, the magnetic polymer beads made by conventional suspension copolymerization were mostly in the size of several hundred micrometers with a very broad size distribution. The main disadvantages of these magnetic beads are their large size, broad size distribution, and low density of surface reactive groups. Spraying suspension polymerization (SSP) is a promising technique, which was first proposed by our group (Yang et al. 2005), to prepare magnetic polymer beads with a narrow size distribution. The SSP technique involves the spray of dispersed phase containing magnetite nanoparticles through a nozzle into a continuous phase. The small droplets in continuous phase are directly formed from the nozzle by applying appropriate pressure. Compared with the conventional mechanical stirring method, the SSP technique can provide small droplets with a narrow size distribution.
In this study, we describe an effective process for the preparation of highly functionalized, micron-sized p-aminobenzamidine immobilized superparamagnetic beads and their practical application for nattokinase purification from fermentation broth. Firstly, magnetic poly(methyl methacrylate) (PMMA) beads with a narrow size distribution were prepared by SSP. Then the resulting magnetic PMMA beads were highly functionalized via transesterification reaction with poly(ethylene glycol) in the presence of sodium methylate as a catalyst. The surface hydroxyl-modified magnetic beads obtained were further modified with chloroethylamine to transfer the surface amino-modified magnetic functional beads. The morphology and surface functionality of the magnetic beads were examined by scanning electron microscopy (SEM) and Fourier transform infrared (FTIR). An affinity ligand, p-aminobenzamidine was covalently attached onto the amino-modified magnetic beads by the glutaraldehyde method for the nattokinase purification directly from the fermentation broth.
Materials and methods
Materials
Methyl methacrylate (MMA) and divinylbenzene (DVB) were analytical grade and were distilled under reduced pressure to remove inhibitors before use. Benzoyl peroxide (BPO), polyvinyl alcohol (PVA)-1788, polyethylene glycol (PEG)-400, methyl alcohol, and NH3·H2O were analytical grade and were used without any further purification. 2-chloroethylamine hydrochloride, iminodiacetic acid (IDA), and p-aminobenzamidine were purchased from Sigma Chemical. Water was purified by distillation followed by deionization using ion exchange resins. Other chemicals were reagent grade and used as received.
Bacillus natto NLSSE was obtained from Institute of Process Engineering, the Chinese Academy of Sciences. Bacteria were maintained as spores suspended in 50% (v/v) glycerol, stored at −20 °C.
Preparation of Fe3O4 nanoparticles coated with oleic acid
Fe3O4 nanoparticles coated with oleic acid were prepared by the modified coprecipitation method. At 80 °C, 0.196 mol of FeCl3·6H2O and 0.098 mol of FeCl2·4H2O were dissolved in 600 ml deionized water in a 2.0-l beaker under nitrogen gas with vigorous stirring. Then, 30 ml of NH3·H2O was added to the solution and 20 ml of oleic acid was added dropwise into the suspension within 20 min. After another 20 min, the magnetite nanoparticles were obtained from the solvent by magnet and were washed with deionized water several times.
Preparation of magnetic PMMA beads
Magnetic PMMA beads were prepared by SSP. The SSP apparatus was already presented in our previous work (Yang et al. 2005). Thirty grams of PVA dissolving 2,000 ml of deionized water was used as an aqueous phase. The oil phase, a mixture of 50 ml of MMA, 5 ml of DVB, and 2 g of BPO dissolving 10 g of Fe3O4 magnetic nanoparticles, was stored in the dispersion phase storage tank. By applying nitrogen pressure of 0.15 MPa, the oil phase was sprayed through the spraying nozzle into the aqueous phase to form uniform droplets, which were polymerized for 2 h with gentle stirring under a nitrogen atmosphere. The resulting magnetic PMMA beads were separated by a permanent magnet and washed with deionized water and ethanol several times.
Surface functionalization and activation
Two grams of magnetic PMMA beads were washed with methanol three times, which were mixed with 80 ml of PEG in a 250-ml three-necked flask. The temperature was increased to 100 °C to remove water and 3 h later, 5 ml of 33% sodium methylate solution was added to the solution mixture and maintained at 90 °C for 24 h with mild stirring. The PEG-modified magnetic PMMA beads with plenty of hydroxyl groups on the surface were thoroughly washed with deionized water. Then, the PEG-modified magnetic PMMA beads were added into the 100-ml of 20% 2-chloroethylamine solution at pH 10 and were maintained at 60 °C for 24 h with stirring. The resulting surface amino groups modified magnetic beads were obtained after magnetic separation. To facilitate the covalent attachment of the affinity ligand, the amino groups on the surface of magnetic beads were activated with 5% (v/v) glutaraldehyde solution in 0.1 M of saline phosphate buffer at pH 7.4. After agitating at 30 °C for 12 h, the glutaraldehyde-activated magnetic beads were recovered by magnet. The resulting magnetic beads were then washed three times with deionized water and stored as a suspension.
Attachment of affinity ligand
Magnetic affinity beads were prepared by the covalent attachment of p-aminobenzamidine onto the surface of the glutaraldehyde-activated magnetic beads. The glutaraldehyde-activated magnetic beads (1.0 g) were dispersed in 50 ml of 0.1 M p-aminobenzamidine solution at pH 7.4. The mixture was incubated at room temperature for 5 h. Then the benzamidine-attached magnetic beads were recovered by magnet and the supernatant was retained for the residual p-aminobenzamidine concentration assay. To remove the physically adsorbed p-aminobenzamidine, the mixture was washed carefully by phosphate-buffered saline four times. The desorbed amount of p-aminobenzamidine was determined from absorbance at 295 nm of supernatants. The equilibrium adsorption capacity based on those chemically bound onto the magnetic beads was obtained from calculation. The p-aminobenzamidine-attached magnetic beads were stored in the adsorption buffer (0.1 M of Tris–HCl buffer at pH 8.0) until further use.
Nattokinase production
A 5% (v/v) spore suspension of Bacillus natto was added to seed medium composed of 10 g/l of glucose, 10 g/l of yeast extract, 1 g/l of K2HPO4·3H2O, and 0.5 g/l of MgSO4·7H2O with a pH value of 7.0. The subsequent cultivation lasted for 12 h at 30 °C in an orbital shaker to obtain seed culture with an OD600 of 7 nm. Then, a 5% (v/v) seed culture was added to the production medium consisting of 8.0 g/l of soy peptone, 0.6 g/l of calcium chloride, 0.8 g/l of yeast extract, 20 g/l of maltose, 2 g/l K2HPO4·3H2O, and 1 g/l of MgSO4·7H2O. Shake flask cultures were carried out at 30 °C in an orbital shaker. After 48 h of fermentation, the fermentation broth of nattokinase was stored at −4 °C for further use in affinity purification by magnetic beads.
Purification of nattokinase using magnetic affinity beads
Nattokinase was directly purified by the p-aminobenzamidine-attached magnetic beads from the fermentation broth. The p-aminobenzamidine-attached magnetic beads (100 mg) were added to a 50-ml flask and washed twice with adsorption buffer by magnet. The washed magnetic beads were resuspended in 10 ml of 0.1 M Tris–HCl buffer at pH 8.0 and then 10 ml of fermentation broth was added. The mixture was incubated with slow rotation mixing for 30 min at room temperature. The resulting magnetic beads were washed three times with adsorption buffer by magnet and the supernatant was removed. To elute the nattokinase, the above magnetic beads were resuspended in 10 ml of 0.1 M ammonium acetate buffer at pH 4.0 with slow rotation for 10 min. Then the magnetic beads were magnetically sedimented and the supernatant, containing purified nattokinase, was transferred to a clean flask for nattokinase activity assay. After this step, the supernatant obtained was concentrated with ethanol and freeze-dried.
Analysis and measurement
The morphology and structure of magnetic beads were investigated by SEM (JSM-6700F, JEOL, Japan). The magnetization curves of the samples were measured at room temperature with a vibrating sample magnetometer (VSM, model-155, Digital Measurement System). The size distribution of magnetic beads was measured by laser diffraction using a Coulter LS 230 (Coulter Electronics, USA). Surface functional groups were characterized with FTIR (Bruker, Vector 22).
Fibrinolytic activity of nattokinase was measured by the method of fibrin hydrolysis (Liu et al. 2004). The incubation mixture contained 2.5 ml of 12 g/l of fibrin suspension (pH 7.8), 6.5 ml of 0.1 M Tris–HCl buffer (containing 10 mM CaCl2, pH 7.8), and 1 ml enzyme solution with suitable dilution. The incubation was carried out at 37 °C for 15 min and was stopped by adding 5 ml of 0.11 M of trichloroacetic acid containing 0.22 M of sodium acetate and 0.33 M of acetic acid. The absorbency at 275 nm of the supernatant obtained after centrifugation was determined. A fibrinolytic unit (U) was defined as the amount of enzyme that gave an increase in absorbency at 275 nm, equivalent to 1 μg of tyrosine/min at 37 °C.
The percentage of total activity recovery and the purification factor are defined as follows:
-
Percentage of total activity recovery:
$$TA{\left( \% \right)} = \frac{{A_{a} }} {{A_{i} }} \times 100$$ -
Purification factor:
$$PF{\left( \% \right)}\frac{{\frac{{A_{a} }} {{C_{a} V_{a} }}}} {{\frac{{A_{i} }} {{C_{i} V_{i} }}}} \times 100$$
where V, C, and A are the volume, concentration of protein, and total nattokinase activity, respectively, and the subscripts a and i mean the desorption solution from magnetic beads and initial fermentation broth, respectively.
The protein concentration in supernatant of fermentation broth and separated solution were determined by Coomassie brilliant blue method (Bradford 1976) using bovine serum albumin as the standard protein.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with 12% gel was carried out according to the method of Laemmli (1970). Detection was done by Coomassie brilliant blue.
Results
Characterization of magnetic beads
In conventional suspension polymerization, the size and size distribution of droplets are only controlled by the mechanical stirring method, so it is very difficult to control the size and size distribution of the droplets during the polymerization. Therefore, the magnetic polymer particles are mostly in the size range of several hundred micrometers with very broad size distribution. In this work, we used the spraying method to uniformly disperse the droplets. As expected, micron-sized magnetic PMMA beads with a relatively narrow size distribution were obtained by SSP. The morphology and size of the resulting magnetic beads were measured by SEM as shown in Fig. 1. It can be seen that the magnetic beads have a relatively narrow size distribution with the mean size of about 8 μm.
In addition, the surface functionalization of magnetic PMMA beads was carried out based on the transesterification reaction in the presence of sodium methylate as a catalyst. The –OCH3 groups on the magnetic PMMA beads were replaced with PEG as follows:
The fact was proven by the comparison of FTIR spectra of magnetic PMMA beads before (Fig. 2a) and after (Fig. 2b) transesterification. Before transesterification, no distinct band appears at around 3,400 cm−1, which shows that no hydroxyl group exists on the surface of PMMA beads. After transesterification, the hydroxyl (–OH) characteristic vibration bands appear obviously at 3,400 cm−1 as shown in Fig. 2b, owing to the plenty of PEG coupling to the surface of magnetic beads.
To realize affinity ligand attachment, the PEG-modified magnetic beads must be further modified. In the present work, 2-chloroethylamine was covalently coupled to the PEG-modified beads via a nucleophilic reaction between the chloride of 2-chloroethylamine and the hydroxyl groups on the surface of the PEG-modified magnetic beads. The hydroxyl (–OH) groups onto the magnetic beads were replaced with 2-chloroethylamine as follows:
The amount of available surface amino groups was determined by surface chemical reaction method. The amino groups of magnetic beads could be carboxymethylated by sodium salt of chloroacetic acid. This reaction resulted in the transformation of amino groups to IDA groups, which is a tridentate chelator and can coordinate with many metal ions such as Cu2+, Ni2+, and Zn2+. Cu2+ was selected in this study for its high coordinate capacity with IDA groups. To remove the physically adsorbed Cu2+, the Cu2+-charged magnetic beads were washed carefully by phosphate-buffered saline five times, which indicated that the Cu2+ was immobilized onto the magnetic beads only through chemical binding. The bound Cu2+ could be stripped easily from the magnetic beads by using 0.5 M of EDTA. The immobilized Cu2+ capacity is equal to the amount released, so amino groups coordinating with Cu2+ could be quantitatively proven, and their capacity was found to be 0.26 mmol/g of magnetic beads.
The p-aminobenzamidine is a competitive inhibitor of serine protease and is widely used as a ligand for affinity separation of serine protease such as trypsin (Hubbuch and Thomas 2002). Nattokinase also belongs to the family of serine protease. Therefore, the p-aminobenzamidine-coupled magnetic beads will be the potential targets to be developed as affinity carrier for nattokinase purification. To facilitate the covalent attachment of affinity ligand, the amino groups on magnetic PMMA beads were transferred to aldehyde groups. For some biological applications, the aldehyde groups on the surface are more reactive groups and ready for attachment of proteins or amino groups containing affinity ligand. In this work, affinity ligand, p-aminobenzamidine was covalently attached onto the glutaraldehyde-modified magnetic beads by Schiff base linkage between aldehyde and primary amino groups. To make the unstable Schiff base become the stable covalent bond, the reducing agent such as NaBH4 was added after the affinity ligand immobilization. Therefore, the magnetic beads have the following structure and reaction equation:
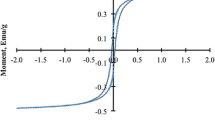
To evaluate the covalent binding of affinity ligand to magnetic beads, the PMMA, PMMA–OH, PMMA–NH2, and PMMA–CHO magnetic beads were used in batch attachments. The attached amount of p-aminobenzamidine onto the different magnetic beads is shown in Fig. 3. The former three magnetic beads showed very little attachment of ligand, while the aldehyde group containing magnetic beads were significantly increased due to the easier reaction between aldehyde groups on the magnetic beads and primary amino groups of the ligand. The attachment capacity is about 61.2 mg/g of magnetic beads under the experimental conditions used, as judged by three independent tests. The high attachment capacity can be explained that the magnetic PMMA beads prepared have some small pores, which shows a large special area. After the magnetic PMMA beads were modified by the long space-arm PEG, they would have plenty of functional groups. The magnetic properties of the unmodified and modified magnetic beads were measured by VSM at room temperature (Yang et al. 2004). The results showed that these magnetic beads were typically superparamagnetic and the specific saturation magnetization of unmodified, PEG-modified, amino-modified, glutaraldehyde-modified, and ligand-attached magnetic beads were found to be 17.6, 16.6, 15.5, 14.9, and 14.2 emu/g, respectively.
Affinity purification of nattokinase from fermentation broth
The results of the present study aimed at identifying conditions for the affinity purification of nattokinase from fermentation broth by p-aminobenzamidine-attached magnetic beads are presented in Fig. 4. It shows the change of nattokinase affinity adsorption against the pH at the equilibrium concentration. The pH value was varied from 6 to 11. The maximum adsorption capacity (Q max) of nattokinase on p-aminobenzamidine-attached magnetic beads was found to be 908 U/g at around pH 9.0, which is the isoelectric point (pH 8.7) of nattokinase (Takashi et al. 1992). With the change of pH above or below the isoelectric point, the nattokinase adsorption capacity was decreased. The reasons can be attributed to electrostatic repulsion effects between the identically charged groups. As can be seen from Table 1, the equilibrium constant (K d) at pH 9.0 was found to be 290 U/ml. The value of the equilibrium constant is very low, which shows that the fast adsorption of nattokinase on the magnetic beads.
The desorption of nattokinase from magnetic beads were accomplished using dissociation agents such as ammonium acetate buffer (pH 4.0) in a matter of minutes. It was found that more than 95% nattokinase would be desorbed from the magnetic beads. The enzyme recovery of nattokinase was more than 85% and the purification factor of the nattokinase was about 8.7. The magnetic beads were reused five times and they maintained the same results. It is also evident from Fig. 5 (lane 3) that this proved to be the case with nattokinase being successfully isolated from fermentation broth in a one-step batch procedure, which took just 40 min to accomplish.
Discussion
We have succeeded in developing magnetic poly(methyl methacrylate) beads with p-aminobenzamidine as a ligand that could quickly purify nattokinase from fermentation broth by external magnetic field. Compared with the traditional suspension copolymerization method, the magnetic beads made by this process have the advantages of smaller size (8 μm in mean diameter) with a relatively narrow size distribution, and higher density of surface functional groups. Due to the successful surface functionalization of the magnetic beads, they have an extremely high adsorption capacity and can bind a large amount of nattokinase (908 U/g of magnetic beads).
There were several reports of purification of nattokinase from fermentation broth. The traditional purification of nattokinase requires a long period (about 2 days) due to the multistep process. In addition, each step requires expensive equipment and may result in a significant loss of the enzyme activity. For the broader application of nattokinase, development of a simple, convenient, and highly efficient affinity selection procedure based on a magnetic separation technique is very important.
Compared with the traditional purification methods, the purification of nattokinase from fermentation broth by magnetic beads only took 40 min and the purification factor and the recovery of the enzyme activity were found to be 8.7 and 85%, respectively. Using magnetic beads therefore, not only saves time in purification of nattokinase, but also significantly simplifies the selection process. As far as we know, this method provides the best results in terms of efficiency and required time.
In conclusion, the present study demonstrates the high potential of a new technology that can purify nattokinase from fermentation broth using p-aminobenzamidine immobilized magnetic poly(methyl methacrylate) beads. Because the SSP can make the droplets uniformly disperse, the magnetic beads prepared have a relatively narrow size distribution. After they were highly surface functionalized, a ligand, p-aminobenzamidine, was easily immobilized onto the magnetic beads and was effective for affinity purification of nattokinase directly from the fermentation broth. The novel magnetic separation process based on the p-aminobenzamidine immobilized magnetic beads is effective and applicable to the nattokinase purification from the fermentation broth.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang CT, Fan MH, Kuo FC, Sung HY (2000) Potent fibrinolytic enzyme from a mutant of bacillus subtilis IMR-NK1. J Agric Food Chem 48:3210–3216
Fujita M, Nomura K, Hong K, Ito Y, Asada A, Nishimuro S (1993) Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun 197:1340–1347
Furukawa H, Shimojyo R, Ohnishi N, Fukuda H, Kondo A (2003) Affinity selection of target cells from cell surface displayed libraries: a novel procedure using thermo-responsive magnetic nanoparticles. Appl Microbiol Biotechnol 62:478–483
Horák D, Shapoval P (2000) Reactive poly(glycidyl methacrylate) microspheres prepared by dispersion polymerization. J Polym Sci A Polym Chem 38:3855–3863
Hu HB, Yao SJ, Mei LH (2000) Partial purification of nattokinase from Bacillus subtilis by expanded bed adsorption. Biotechnol Lett 22:1383–1387
Hubbuch JJ, Thomas ORT (2002) High-gradient magnetic affinity separation of trypsin from porcine pancreatin. Biotechnol Bioeng 79:301–313
Kondo A, Kamura H, Higashitani K (1994) Development and application of thermo-sensitive magnetic immunomicrospheres for antibody purification. Appl Microbiol Biotechnol 41:99–105
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Lee Y, Rho J, Jung B (2003) Preparation of magnetic ion-exchange resins by the suspension polymerization of styrene with magnetite. J Appl Polym Sci 89:2058–2067
Liu JG, Xing JM, Shen R, Yang CL, Liu HZ (2004) Reverse micelles extraction of nattokinase from fermentation broth. Biochem Eng J 21:273–278
Nakanishi K, Nomura K, Tajima K, Hiratani H (1998) Fibrinolytic protein and production method thereof. US Patent 5,750,650
Noriko Y, Hiromichi N, Hideki A, Tatsuo S (1993) Preparation of magnetic latex particles by emulsion polymerization of styrene in the presence of a ferrofluid. J Appl Polym Sci 50:765–776
Safarik I, Safarikova M (2004) Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn Res Technol 2:7–10
Saiyed ZM, Telang SD, Ramchand CN (2003) Application of magnetic techniques in the field of drug discovery and biomedicine. Biomagn Res Technol 1:2–6
Sumi H, Hamado H, Tsushima H, Mihara H, Murica H (1987) A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto: a typical and popular soybean food in the Japanese diet. Experientia 43:1110–1111
Sumi H, Hamada H, Nakanishi K, Hiratani H (1990) Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol 84:139–143
Takashi N, Yamagata Y, Ichishima E (1992) Nucleotide sequence of the subtilisin NAT gene, aprN of bacillus subtilis (natto). Biosci Biotechnol Biochem 56:1869–1871
Ugelstad J, Ellingsen T, Berge A, Helgee B (1983) Magnetic polymer particles. PCT Int Appl, WO Patent 8303920
Urano T, Ihara H, Umemura K, Suzuki Y, Oike M, Akita S, Tskamoto Y, Suzuki I, Takada A (2001) The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem 276:24690–24696
Yang CL, Xing JM, Guan YP, Liu JG, Liu HZ (2004) Synthesis and characterization of superparamagnetic iron nanocomposites by hydrazine reduction. J Alloys Compd 385:283–287
Yang CL, Guan YP, Xing JM, Liu JG, An ZT, Liu HZ (2005) Preparation of magnetic polystyrene microspheres with a narrow size distribution. AIChE J 51:2011–2015
Acknowledgement
This work was supported by the National High Technology and Development Program of China (no. 2002AA302211).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, C., Xing, J., Guan, Y. et al. Superparamagnetic poly(methyl methacrylate) beads for nattokinase purification from fermentation broth. Appl Microbiol Biotechnol 72, 616–622 (2006). https://doi.org/10.1007/s00253-006-0484-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0484-5