Abstract
Three structural classes of (1→3)-β-d-glucans are encountered in some important soil-dwelling, plant-associated or human pathogenic bacteria. Linear (1→3)-β-glucans and side-chain-branched (1→3,1→2)-β-glucans are major constituents of capsular materials, with roles in bacterial aggregation, virulence and carbohydrate storage. Cyclic (1→3,1→6)-β-glucans are predominantly periplasmic, serving in osmotic adaptation. Curdlan, the linear (1→3)-β-glucan from Agrobacterium, has unique rheological and thermal gelling properties, with applications in the food industry and other sectors. This review includes information on the structure, properties and molecular genetics of the bacterial (1→3)-β-glucans, together with an overview of the physiology and biotechnology of curdlan production and applications of this biopolymer and its derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The (1→3)-β-glucans from bacteria include the linear glucans, (1→3,1→6)-β-glucans that have branch-on-branch or cyclic structures and the side-chain-branched (1→3,1→2)-β-glucans. These glucans are found both among the prokaryotes (Table 1) and eukaryotes. Functionally, the eukaryote (1→3)-β-glucans may be storage polysaccharides, as in euglenoid protozoa (paramylon), brown algae and diatoms (laminarin-type glucans), or are wall components of yeasts and fungi [branch-on-branch (1→3,1→6)-β-glucans], or fungal surface mucilages [side-chain-branched (1→3,1→6)-β-glucans]. In higher plants, callose, a linear (1→3)-β-glucan, occurs in specialized cell walls of reproductive tissues, as a transient component of the cell plate in dividing cells and as deposits on plasma membranes following wounding or in physiological or pathological stress (Stone and Clarke 1992).
A variety of bacteria, including important pathogens of humans, livestock and plants, produce extracellular and capsular polysaccharides (EPS). Some EPS are major virulence determinants of animal pathogens. Others are required for pathogenic and symbiotic interactions between bacteria and plants, have roles in associations between bacteria and biotic/abiotic surfaces and are matrix components of bacterial biofilms (Sutherland 2001a). Additionally, a number of EPS have industrial applications as gelling and emulsifying agents (Sutherland 2001b). Three bacterial EPS have been approved as food adjuncts by the Food and Drug Administration of the United States. These are xanthan (from Xanthomonas campestris), gellan (from Sphingomonas paucimobilis) and curdlan (from Agrobacterium sp. biovar. 1 or A. radiobacter; Jezequel 1998). In this review, we discuss the occurrence, biology and chemical properties of three members of the (1→3)-β-d-glucan polysaccharide family produced by bacteria: the linear (1→3)-β-glucan, curdlan, the cyclic (1→3,1→6)-β-glucans and a side-chain-branched (1→3,1→2)-β-glucan.
Linear (1→3)-β-d-glucans (curdlan)
Curdlan is a neutral, essentially linear, (1→3)-β-glucan which may have a few intra- or inter-chain (1→6)-linkages (Saito et al. 1968; Fig. 1). It was first detected in Agrobacterium biovar. 1 (formerly Alcaligenes faecalis var. myxogenes strain 10C3; Harada and Harada 1996) and was co-produced with another extracellular polysaccharide, succinoglycan (an acidic heteroglycan) and a periplasmic cyclic (1→2)-β-d-glucan (Hisamatsu et al. 1982). A spontaneous, high curdlan-yielding mutant 10C3K (IFO13714) produced no succinoglycan (Harada et al. 1966; Hisamatsu et al. 1982) and became the progenitor of strains with sustained curdlan-production ability and/or enhanced curdlan yield and gel-forming quality. Notable amongst these strains are NTK-u (ATCC21680, IFO13140; Nakanishi et al. 1992), ATCC31749 (Phillips and Lawford 1983) and ATCC31750 (Kim et al. 2003).
A comprehensive survey of the occurrence of microbial curdlan-like polysaccharides based on the formation of blue-staining colonies on agar medium containing the (1→3)-β-glucan specific dye, aniline blue, was made by Nakanishi et al. (1976). Of 687 strains from 43 genera, only Alcaligenes faecalis (now reclassified as an Agrobacterium) and Agrobacterium spp (six of 17 strains, including A. radiobacter and A. rhizogenes) produce curdlan, whereas none was recoverable from Bacillus spp (five of 158 strains) that stained with the dye. Curdlan production also occurs in a few Rhizobium strains (Footrakul et al. 1981; Ghai et al. 1981) and in species of the Gram-positive Cellulomonas, including C. flavigena KU (Buller 1990; Kenyon and Buller 2002). Of the 144-sequenced bacterial genomes (Ussery 2004), including A. tumefaciens C58, various rhizobia and Gram-positive bacteria, only A. tumefaciens C58 has homologues of the curdlan-production genes (crdASC) identified in Agrobacterium sp. ATCC31749 (Karnezis et al. 2003).
Chemical and physicochemical properties of curdlan
Curdlan and other (1→3)-β-d-glucans specifically bind the triphenylmethane dye, aniline blue (Nakanishi et al. 1974), and a benzophenone fluorochrome found in the dye mixture (Evans et al. 1984). Calcofluor and Congo Red also bind to curdlan and induce fluorescence, but these dyes are not specific for (1→3)-β-glucans (Nakanishi et al. 1974). Curdlan molecules may have as many as 12,000 glucose units (Futatsuyama et al. 1999) and are insoluble in water, alcohols and most organic solvents, but dissolve in dilute bases (0.25 M NaOH), dimethylsulphoxide (DMSO), formic acid and aprotic reagents such as N-methylmorpholino-N-oxide and lithium chloride in dimethylacetamide (Yotsuzuka 2001). When precipitated from NaOH or DMSO solutions, curdlan shows different morphologies, ranging from endless microfibrils to spindle-shaped fibrils of various lengths, depending on the method of preparation (Koreeda et al. 1974).
Three forms of regenerated curdlan have been identified and the structural differences between them as proposed by Kasai and Harada (1980) are shown schematically in Fig. 2. The anhydrous form obtained by vacuum heat annealing shows three inter-twining glucan chains forming a triple helix in which each chain has a right-handed, six-fold conformation, with a diameter of 1.57 nm, a fibre repeat of 0.58 nm and a P63 space group. The three chains in the helix are linked together through triads of strong hydrogen bonds between C(O)2 hydroxyls. When annealing is performed under hydrothermal conditions, a crystalline hydrate containing two molecules of water per glucosyl residue is obtained. The hydrated form consists of triple helices, but symmetry is lost due to the water molecules, and a P1 space group and fibre repeat of 1.8 nm is adopted. A third form, obtained by dialysing alkaline solutions of curdlan against water, has a fibre repeat of ∼2.3 nm and a 71 or 61 helical conformation. Whether this room-temperature form consists of loose inter-twined triple helices with approximately two molecules of water per glucosyl unit, or a mixture of single helices with ∼20 molecules of water per glucosyl unit and perfectly formed and imperfectly formed triple helices, is not clear (Koreeda et al. 1974; Marchessault and Deslandes 1979; Deslandes et al. 1980; Fulton and Atkins 1980; Kasai and Harada 1980; Chuah et al. 1983; Okuyama et al. 1991).
Schematic representation of the structural changes between the three forms of curdlan. In the room-temperature form, single and triple helices and some imperfectly formed triple helices (not shown) are present. W Water. After Kasai and Harada (1980)
Curdlan’s name reflects its ability to form gels with differing characteristics (Yotsuzuka 2001). Heating aqueous suspensions of curdlan above ∼80°C and then cooling it produces a high-set, thermo-irreversible gel, whereas a low-set, thermo-reversible gel is produced on heating to 55°C. Gelation involves aggregation of the rod-like triple helices through non-covalent associations (extended junction zones). At high temperatures, the triple-helical strands may unwind to give single chains that, as the temperature is lowered, anneal to reform triple helices. In high-set gels, single chains involved in more than one complex may interconnect the triple helices. In low-set gels, curdlan molecules are present as single helical chains (Kasai and Harada 1980), but some triple-stranded helices may also occur, as judged by X-ray diffraction (Okuyama et al. 1991) and 13C NMR (Saito et al. 1977). In alkaline solutions, the curdlan triple helix unwinds and, on neutralisation or dialysis against water, a low-set gel is formed without heating. Such neutralised gels are converted to irreversible high-set gels on heating to above ∼80°C. The rheological and thermal behaviour of low- and high-set curdlan gels has been documented by Zhang et al. (2002).
Molecular biology of curdlan production
The molecular genetics of curdlan production have been investigated in Agrobacterium sp. ATCC31749. Studies on transposon-insertion mutants with altered capacity to stain with aniline blue when grown on indicator agar identified four genes (crdA, crdS, crdC, crdR) that are essential for curdlan production (Stasinopoulos et al. 1999) and a fifth gene for phosphatidylserine synthase (pss AG ) that is required for maximal yields of the polymer (Karnezis et al. 2002). Curdlan production also depends on the global nitrogen metabolism genes ntrBC and on several other genes, as yet uncharacterised (S. Aracic, A. Anguillesi, unpublished data).
The crdASC genes occupy a contiguous 4,948-bp region of the genome and are transcribed in the same direction and opposite to that of the flanking genes. A 180-bp AT-rich region precedes crdA. This operon-like organisation is typical of that found in other polysaccharide production systems (Leigh and Coplin 1992). The remaining genes (pss AG , crdR, ntrBC) occur at separate loci not linked to the crdASC cluster.
The crdS product (73 kDa) deduced from the DNA sequence (1,965 bp) is curdlan synthase, based on its sequence and structural homology with β-d-glycan synthases, including bacterial and plant cellulose synthases, and chito-oligosaccharide and hyaluronan synthases, which are members of glycosyltransferase Family GT2 (Coutinho and Henrissat 1999). CrdS shares no homology with the (1→3)-β-glucan synthase-related FSK1 and FSK2 proteins from fungi (e.g. Saccharomyces, Candida, Aspergillus; Dijkgraaf et al. 2001) or the plant callose synthase-related proteins (Li et al. 2003), which are both classified as GT48 glycosyltransferases. In Agrobacterium, CrdS is an integral inner membrane protein with seven transmembrane (TM) helices, one non-membrane-spanning amphipathic helix and a Nout–Cin disposition (Karnezis et al. 2000, 2003). A central large and relatively hydrophilic cytoplasmic region of ∼300 residues situated between TM3 and TM4 carries various conserved motifs, including the UDPGlc substrate-binding and catalytic D,D,D35QxxRW motif. This region shares highest homology (42% similarity) with bacterial cellulose [(1→4)-β-glucan] synthases such as those from A. tumefaciens (CelA) and Gluconacetobacter xylinus (AcsA). Two motifs (FFCGS, RxxFLxxPL) in known or putative bacterial cellulose synthases are proposed to have a role in determining (1→4)-β-linkage specificity (Römling 2002). These motifs are barely recognisable (FxxGx, xxxxLxxPx) in CrdS (Karnezis et al. 2003).
In contrast to crdS, crdA (1,539 bp) and crdC (1,269 bp) encode proteins that have no counterparts in the gene/protein databases. CrdA (Mr 48 kDa) is predicted to be membrane-anchored with a large periplasmic C-terminal portion. CrdC (Mr 42 kDa) is predicted to be periplasmic, since it carries a cleavable signal sequence. The process of polymerisation mediated by CrdS occurs on the cytoplasmic face of the inner membrane (Karnezis et al. 2003) and so is unlikely to involve the periplasmic CrdC, or CrdA directly. Rather, CrdA might assist translocation of the nascent polymer across the cytoplasmic membrane and CrdC, its passage across the periplasm. This sequence of events is suggested by the distinctive ability of crdC mutants, but not crdA or crdS mutants, to produce some curdlan, detectable by the sensitive aniline blue fluorochrome (McIntosh 2004). The possibility that CrdASC forms a membrane-associated, oligomeric, biosynthetic complex is suggested by the detection of native protein aggregates of ∼420 kDa and ∼500 kDa that contain CrdS (Karnezis et al. 2003) and by the critical role played by the phospholipid composition of Agrobacterium membranes in the production of curdlan (Karnezis et al. 2002).
The regulation of curdlan production involves crdR (423 bp, Mr 15 kDa) whose product is probably a transcriptional activator, based on the presence of a helix-turn-helix, DNA-binding motif in the deduced CrdR sequence, and the finding that crdR mutants are curdlan-deficient. CrdR may not be the primary effector of curdlan production, since expression of the gene is not induced under N-limitation, a condition that elicits curdlan production. Rather, crdR is expressed constitutively by chromosomal crdR-lacZ transcriptional fusion mutants and expression declines on N-depletion. CrdR homologues occur in A. tumefaciens C58 and several rhizobial species, but have no known function and are not related to the regulatory proteins for other EPS production systems (e.g. for succinoglycan, galactoglucan or cellulose) of these bacteria (Anguillesi 2003).
The involvement of the ntrBC genes in curdlan production is not surprising, since the encoded sensory kinase-regulator protein is a conserved component of bacterial nitrogen regulatory systems (Merrick and Edwards 1995) and curdlan is produced after cell growth has ceased due to N-exhaustion (Lee 2002). Mutants of ntrBC fail to produce curdlan on yeast extract/glucose medium and cannot use nitrate as the sole N source, features that distinguish them from crd mutants. NtrC is phosphorylated by NtrB under N-depleted conditions, but the possibility that NtrC-P then operates with the RpoN sigma factor directly to activate crdASC (or crdR) is unlikely, since the crd genes are not associated with a recognizable RpoN-dependent promoter consensus sequence (Dombrecht et al. 2002). The manner in which crdR and ntrBC serve to activate curdlan production may be complex and interconnected with the production of other EPS or intracellular carbon reserves (S. Aracic, unpublished data).
Physiology and biochemistry of curdlan production
Agrobacterium NTK-u grown on solid media produces a coherent aniline blue-staining pellicle which may be stripped from the colony surface (Nakanishi et al. 1976). Scanning electron microscopy of the cells in the pellicle of Agrobacterium 10C3K (Kako et al. 1989) revealed that they gradually enlarged during culture and, in the later stages, appeared to break out of the enveloping curdlan matrix.
In liquid culture, curdlan is produced as a capsule on both Agrobacterium sp. ATTC 31749 (McIntosh 2004) and Cellulomonas flavigena KU (Voepel and Buller 1990) and, in each, capsule formation is correlated with cell aggregation (floc formation). Capsule formation occurs when the N source in minimal medium is depleted, suggesting that the capsule and floc formation togther function as protective structures in nutritional stress. In C. flavigena, the capsular curdlan can be depolymerised by a (1→3)-β-glucan hydrolase for use as a C source for growth (Voepel and Buller 1990).
Agrobacterium NTK-u produces curdlan on defined media containing d-glucose, d-fructose, d-mannose, d-mannitol, d-glucitol, d-arabinose, d-glycerol, inositol, maltose, lactose, sucrose or raffinose, but not d-galactose, d-rhamnose, d-xylose, d-ribose, ethylene glycol, starch or starch dextrins (Nakanishi et al. 1992). This reflects the broad range of carbohydrate substrates utilised by members of the Rhizobiaceae (Stowers 1985). The range of carbohydrate sources is strain-dependent and thus, in contrast to NTK-u, ethylene glycol and d-galactose are substrates for curdlan production by 10C3 (Harada and Yoshimura 1964) and ATTC31750 (Lee et al. 1997a), respectively. Citric acid, but not starch, is a substrate for ATCC31749 (Philips and Lawford 1983).
Agrobacteria have transporters for glucose, galactose, fructose and lactose (Cornish et al. 1988; Kemner et al. 1997). Intracellular glucose is successively converted to Glc-6-P by hexokinase, to Glc-1-P by phosphoglucomutase and to UDPGlc by UDPGlc pyrophosphorylase. In a number of Gram-negative organisms, a phosphoenolpyruvate-dependent sugar phosphotransferase system (PTS) transports and phosphorylates monosaccharides so that a separate hexokinase is not required. PTS-related homologues are found in the A. tumefaciens C58 genome (http://66.93.129.133/transporter/wb/transporter2.php?oOID=atum1). It has been reported that, unusually, both Glc-1-P and UDPGlc can be taken up by A. tumefaciens cells, by an active process (Fukui 1969; Fukui and Miyairi 1970), the former by an inducible carrier that is inhibited by dinitrophenol.
13C-Glucose tracer studies combined with structural determinations by 13C NMR spectroscopy (Kai et al. 1993; 1994) showed that more than 60% of curdlan is synthesized directly from imported glucose, the remainder from fructose 6-phosphate arising from glucose entering the pentose phosphate/Entner–Doudoroff pathways (Arthur et al. 1973). d-Glycerol, d-mannose, d-fructose, d-glucitol and d-mannitol have specific transporters in Agrobacterium and are converted to glucose units from fructose-6-phosphate after phosphorylation. d-Galactose metabolism in A. tumefaciens is through the De Ley–Doudoroff oxidative pathway and not the Leloir galactokinase, galactose-1-phosphate uridylyltransferase pathway (Uttaro et al. 1993). Pentose substrates presumably enter the carbohydrate metabolic pool through the pentose phosphate pathway. Agrobacterium ATCC31749 insertion mutants, in which the genes for glycogen synthesis have been inactivated, do not synthesize curdlan from exogenous d-glucose, but both d-galactose and d-mannitol support curdlan production (McIntosh 2004). This may be due to feed-back inhibition of glucokinase and/or the PTS system by accumulated glucose-6-phosphate.
Membrane preparations from Agrobacterium sp. incorporate glucose from UDP[14C]-α-glucose into ethanol-insoluble (1→3)-β-glucan, albeit at a low efficiency (McIntosh 2004). It is not known whether the polymerisation of the glucose units involves a direct transfer from UDPGlc or whether it occurs through an intermediate, e.g. a carrier lipid as appears to be the case for cellulose synthesis in A. tumefaciens C58 strain A1045 (Matthysse et al. 1995).
Biotechnology of curdlan production by fermentation
The production of curdlan by Agrobacterium is strain-dependent (Nakanishi et al. 1992; Kim et al. 2003) and is typical of a secondary metabolite in that its biosynthesis occurs in the post-stationary growth phase during conditions of N-starvation (Phillips and Lawford 1983; Nakanishi et al. 1992; Harada and Harada 1996). The optimisation of curdlan yield thus depends on the formation of a cell mass in the pre-stationary phase and on the biosynthetic capability of cells in the post-stationary phase.
Curdlan yield is optimal at 30–32°C and is affected by nutritional factors, prime amongst which are carbohydrate and N sources (Phillips and Lawford 1983; Nakanishi et al. 1992; Lee et al. 1997a). Glucose is used for the commercial production of curdlan (Yotsuzuka 2001) but more cost-effective C sources, such as sucrose and sugar cane molasses, have also been used in pilot-plant production (Lee et al. 1997a). Ammonium and nitrate and organic (urea) N sources all support the growth of Agrobacterium (Nakanishi et al. 1992), although the bacterial cell mass produced may differ. For some N sources, there is a significant decrease in the pH of the culture medium as growth proceeds (Nakanishi et al. 1992). In batch fermentations, the cell growth rate in the pre-stationary phase is optimal at pH 7.0, whereas curdlan production is optimal at pH 5.5 (Nakanishi et al. 1992; Lee et al. 1999b; Lee and Park 2001). As glucose concentrations are increased, higher N concentrations are required to produce cell mass thereby maximizing yields of curdlan in the post-stationary, N-limited production phase (Nakanishi et al. 1992).
Curdlan production under N-limitation is further dependent on an optimum concentration of phosphate (Kim et al. 2000) and sulphate (Phillips and Lawford 1983) and on the cation composition of the medium (Phillips and Lawford 1983; Nakanishi et al. 1992). Curdlan production is stimulated by the addition of uracil, a precursor of UDPGlc, when added to cells after curdlan synthesis has commenced (Lee and Lee 2001). This may reflect a relationship between levels of uridine and adenine nucleotides and curdlan production. Increases in intracellular UMP levels caused by N-limitation enhance curdlan synthesis by promoting cellular UDPGlc synthesis (Kim et al. 1999).
The observation that different conditions are required to produce optimal yields of cells on the one hand and curdlan on the other, led to the design of a two-stage continuous process (Phillips and Lawford 1983; Phillips et al. 1983). Agrobacterium is grown aerobically in a medium containing C and minimal N and the effluent is fed into a fermenter and mixed with N-free medium for curdlan production. Such continuous cultures are, in general, not commercially viable for the large-scale production of microbial products, due mainly to the limitations on culture volume and the high risk of contamination.
Batch cultures of ATCC21680 in a medium with 8% glucose and 0.07% uracil have been successful at a 6,000-l scale, yielding 38 g curdlan l−1 in 90 h (Nakanishi et al. 1992). By comparison, batch fermentation with ATCC31750 in a 300-l stirred tank, using a medium containing 14% sucrose and in which the culture pH was changed at the end of the growth phase from pH 7.0 to 5.5 by the addition of acid, resulted in a curdlan yield of 64 g l−1 (Lee et al. 1999a). Under similar conditions, the yield from a chemically induced mutant of ATCC31750 was 76 g l−1 (Kim et al. 2003). Even higher yields (93 g l−1) were obtained in laboratory-scale fermentations with the same strain in 14% sucrose medium supplemented with uracil, after N-depletion (Lee and Lee 2001).
In the production of curdlan, Agrobacterium is grown aerobically and, since diffusion of oxygen is limited in submerged culture and may be further slowed by the presence of curdlan on the cell surface, reactor design is important (Phillips and Lawford, 1983; Phillips et al. 1983; Lawford et al. 1986; Orts et al. 1987; Lawford and Rousseau 1991, 1992). Aeration by sparging or stirring using radial-flow, flat-bed and axial-flow impellers has been used (Lawford et al. 1986; Lawford and Rousseau, 1991). Radial-flow stirring causes shearing and leads to curdlan of lowered tensile strength. The effect of agitation speed and aeration rate has also been investigated (Lawford and Rousseau 1992; Lee et al. 1999a).
Curdlan is recovered from commercial-scale cultures by dissolution in 0.85 M NaOH and removal of the bacterial cells by filtration through diatomaceous earth. The curdlan is then precipitated by neutralization, collected by centrifugation, washed to remove salts, concentrated again by centrifugation and finally converted to a powder by spray-drying (Nakanishi et al. 1992).
Current and prospective applications for curdlan and its derivatives
Curdlan’s “conspicuously unusual” rheological properties among natural and synthetic polymers underlie its use as a biothickening and gelling agent in foods (Harada and Harada 1996; Lee 2002). Apart from being tasteless, colourless and odourless, its advantages are that, in contrast to cold-set gels (e.g. gelatin, gellan, carrageenan) and heat-set gels (e.g. konjac glucomannan, methylcellulose), the heating process alone produces different forms of curdlan gel with different textural qualities, physical stabilities and water-holding capacities. Moreover, gels of differing strength are formed depending on the heating temperature, time of heat-treatment and curdlan concentration. Gelation is possible over a wide pH range (2–10), in the presence of sugars (sucrose, glucose, fructose), starches and salt (at typical food levels) and with the incorporation of fats and oils. In most food applications, curdlan is used in the high-set, thermo-irreversible, gel form and is stable during retorting, deep-fat frying and cycles of freeze-thawing. Curdlan gels have been used to develop new food products (e.g. freezable tofu noodles) and calorie-reduced food, since there are no digestive enzymes for curdlan in the upper alimentary tract, and curdlan can be used as a fat substitute (Nishinari and Zhang 2000; Yotsuzuka 2001). The safety of curdlan has been assessed in animal studies and in vitro tests (Spicer et al. 1999; Anonymous 2000) and it is approved for food use in Korea, Taiwan and Japan as an inert dietary fibre and is registered in the United States as a food additive (Anonymous 1996a).
Curdlan has also found applications in non-food sectors. Its water-holding capacity is applied in the formulation of “superworkable” concrete, where its enhanced fluidity prevents cement and small stones from segregating (Anonymous 1996b). It has also been proposed as an organic binding agent for ceramics (Harada 1992). In addition to applications based on its physicochemical properties, e.g. in drug delivery through sustained and diffusion-controlled release from curdlan gels (Kanke et al. 1995), curdlan (Janeway and Medzhitov 2002), other (1→3)-β-glucans and their derivatives have medical and pharmacological potential. They are members of a class of compounds known as biological response modifiers that enhance or restore normal immune defences. These effects are manifested through interactions with soluble or cell-bound (Toll-like) receptors of the innate immune system. Binding to these receptors activates signalling cascades which regulate specific genes concerned with the removal of foreign materials and micro-organisms in both invertebrate and vertebrates and further, through the induction of co-stimulatory molecules, and increased antigen presenting activity, helps to direct adaptive immune responses against antigens derived from the foreign source (Janeway and Medzhitov 2002).
The reported immunomodulating and pharmacological responses include anti-tumorigenicity, anti-infective activities against bacterial, fungal, viral and protozoal agents, anti-inflammatory activity, wound repair, protection against radiation and anti-coagulant activity (Stone and Clarke 1992; Bohn and BeMiller 1995; Ross et al. 1999). The effectiveness of curdlan and other (1→3)-β-d-glucans in eliciting these responses depends on their chemical structure, molecular mass and conformation. Structure/activity relationships of (1→3)-β-glucans show that the intactness of the triple helical structure is of importance in receptor binding (Mueller et al. 2000; Kataoka et al. 2002). However, the partially opened triple helix is reported to be the biologically active form of curdlan that induces inflammatory responses in rats (Young et al. 2003); other studies suggest that the curdlan single helix is more potent than the triple helix as an anti-tumour agent (Saito et al. 1991).
Hydrolysed curdlans with a degree of polymerisation (DP) <50 are not effective anti-tumour agents (Sasaki et al. 1978). The carboxymethyl ether (Honda et al. 1986) and the sulphate and phosphate esters of curdlan (Koumoto et al. 2004) with increased water solubility, show enhanced biological activity (Toida et al. 2003) and, moreover, a water-soluble aminated curdlan derivative has tumorigenic properties (Seljelid 1986). Curdlan sulphates with varying chain-lengths, degrees and position of sulphation show anticoagulant (antithrombotic) activity by interfering with the coagulation-dependent cascade at several points (Alban and Franz 2001). Curdlan sulphate has anti-HIV activity (Jagodzinski et al. 1994) and inhibitory effects on the development of malarial parasites in vitro (Evans et al. 1998). Based on these immunomodulating responses, curdlan is proposed for use in cosmetic formulations (Davis 1992) and as a protective agent for farmed fish (Lee 2002).
The full potential of curdlan in existing and proposed applications would be enhanced by reducing the cost of production. This may involve the use of cheaper C sources, e.g. molasses to replace glucose (Lee et al. 1997a), optimisation of fermentation conditions, development of higher curdlan-yielding strains by mutagenesis (Kim et al. 2003), or manipulation of curdlan synthesis and/or regulatory genes. New curdlan-based polysaccharides have been produced by the direct in vivo incorporation of non-standard sugars as alternative building blocks for the polymer. One such derivative with 8–12 mol% incorporation of 3-O-methyl-d-glucose into the curdlan chain has been obtained using ATCC31749 (Lee et al. 1997b). Curdlan also has potential for exploitation as a new biomaterial based on the self-assembling ability of (1→3)-β-glucan-megalosaccharides (DP 30–45) to form single, hexagonal, lamellar nanocrystalline structures (∼8–9 nm thick) containing water of crystallization after heating to 90°C (Harada et al. 1979; Chanzy and Vuong 1985). Manipulation of the conditions for self-assembly may allow the engineering of new materials.
Cyclic (1→3)- and (1→3,1→6)-β-d-glucans
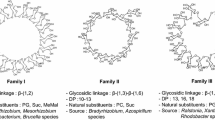
Water-soluble cyclic (1→3,1→6)-β-glucans are found in the periplasm of the legume symbionts Bradyrhizobium japonicum, Rhizobium loti and Azorhizobium caulinodans and in the free-living, root-colonising diazotroph, Azospirillum brasilense. The cyclic glucan from B. japonicum (Miller et al. 1990) and R. loti (Estrella et al. 2000) is composed of two blocks of three (1→3)-linked glucose units, each separated by a block of three (1→6)-linked glucose units, and has a single-branch glucose unit at C(O)6 (Miller et al. 1990). Some molecules are substituted at C(O)6 by phosphocholine (Rolin et al. 1992; Fig. 3). Similar but unbranched and unsubstituted cyclic glucans are produced by Azorhizobium caulinodans (Komaniecka and Choma 2003) and Azospirillum brasilense (Altabe et al. 1998). Cyclic (1→3)-β-glucans that have one of their ten residues substituted at a C(O)6 position by a β-laminaribiose residue are produced by a ndvC∷Tn5 mutant of B. japonicum and a ndvB mutant of Sinorhizobium meliloti carrying the B. japonicum β-glucan synthesis locus (Bhagwat et al. 1999).
Structure of the cyclic (1→3,1→6)-β-d-glucan from B. japonicum. From Rolin et al. (1992)
Cyclic (1→3,1→6)-β-glucans are synthesised by inner membrane preparations from B. japonicum (de Iannino and Ugalde 1993) and A. brasilense (Altabe et al. 1994; 1998) using UDP[14C]Glc as the monosaccharide donor. In B. japonicum USDA 110, three adjacent, monocistronically transcribed genes (ndvBDC) are required for in vivo production of the glucan but only two (ndvBC) for in vitro production (Chen et al. 2002). Sequence analysis of the deduced products indicates that all are membrane proteins and that NdvB (102 kDa) and NdvC (62 kDa) are probably (1→3)-β- and (1→6)-β-glucosyltransferases, respectively, since ndvB mutants synthesise no glucan and ndvC mutants produce a mainly (1→3)-β-linked cyclic product. The function of NdvD (26.4 kDa), which shares no homology with proteins in databases, is unknown. It may assist NdvB–NdvC during β-glucan synthesis or have a role in glucan transport to the periplasm (Chen et al. 2002). The mechanism of cyclisation is not known but, for analogous cyclic (1→2)-β-glucans from rhizobia, it has been proposed that non-repetitive glucosylation of a high-molecular-mass membrane protein produces an oligoglucan whose terminal non-reducing residue is able to accept the “reducing” glucose of the growing chain on the enzyme to release the cyclic molecule (Williamson et al. 1992).
Cyclic (1→3,1→6)-β-glucans have a role in osmotic adaptation comparable with that of cyclic (1→2)-β-glucans (Miller and Gore, 1992) and, like them, are most abundant in the periplasm of hypo-osmotically grown bacteria (Gore and Miller 1993). In the B. japonicum symbiotic interaction with soybean roots, cyclic (1→3,1→6)-β-d-glucan apparently suppresses plant defence responses by binding to the (1→3,1→6)-β-oligoglucoside receptor site on the plasma membrane, thereby allowing nodulation to occur (Mithöfer et al. 1996, 2001; Bhagwat et al. 1999). There may also be other roles in the later stages of legume nodule development, since B. japonicum bacteroids have the same content of the cyclic (1→3,1→6)-β-glucan as cells in culture (Gore and Miller 1993). B. japonicum mutants that lack (1→3,1→6)-β-glucan, or synthesise only cyclic (1→3)-β-glucan form small ineffective nodules containing no bacteroids or a significantly reduced number (Bhagwat et al. 1999; Chen et al. 2002).
(1→3,1→2)-β-d-glucan
The type 37 capsule of Streptococcus pneumoniae (Knecht et al. 1970) is the only homopolysaccharide and one of two neutral polysaccharides amongst the 90 pneumococcal capsular types (Henrichsen 1995). The S37 polymer has a (1→3)-β-glucan backbone with (1→2)-linked β-glucopyranosyl side-branches at each glucosyl residue, giving a crowded, comb-like molecular organisation (Fig. 4). This glucan is soluble in water and DMSO (Adeyeye et al. 1988) and is the main virulence factor of type 37 strains. Synthesis of the S37 polysaccharide is determined by a single gene (tts) located distant from the cap locus responsible for capsular formation in all other pneumococal types. The tts gene encodes a GT2 glycosyltransferase that is an integral membrane protein with a potentially cleavable signal sequence (Llull et al. 1999). Cell-free membrane preparations support the synthesis of the S37 polysaccharide from UDP[14C]-glucose without the participation of a lipid intermediate (Llull et al. 2001). The synthase has a dual-specificity, synthesising both (1→3)- and (1→2)-linkages in this branched polymer, a feature also shared with the synthases producing the type 3 pneumococcal polysaccharide (Arrecubieta et al. 1996), hyaluronan in S. pyogenes and S. equisimilis (DeAngelis 1999), heparosan in Escherichia coli K5 (Griffiths et al. 1998) and Pasteurella multocida (DeAngelis and White 2004), and chondroitin in P. multocida (DeAngelis and Pagett-McCue 2000) and E. coli K4 (Ninomiya et al. 2002). These enzymes are different from the previously discussed NdvB and NdvC synthases for the cyclic (1→3,1→6)-β-glucan, which respectively synthesise (1→3)- or (1→6)-linkages only.
The amino acid sequences of the synthases producing β-glucans with (1→3)-linkages (CrdS, NdvB, Tts) all carry the UDPGlc substrate-binding and catalytic D,D,D35QxxRW motifs, except that the QxxRW sequence, believed to be important in repetitive action (Karnezis et al. 2000), is poorly conserved (RHSKW) in Tts (Llull et al. 1999).
Envoy
Of the three bacterial (1→3)-β-glucan types discussed in this review, only curdlan has well defined applications, especially in the food sector, and, unexpectedly, as an additive in concrete. Curdlan, the linear (1→3)-β-glucan, has potential in the medical and pharmacological sector but possible applications that derive from the nanocrystalline structure of curdlan megalosaccharides are yet to be explored. The cyclic-(1→3,1→6)-β-glucans and the side-chain-branched (1→3,1→2)-β-glucans have not been evaluated, for example, as immunomodulators, since they are not widely available. For all three glucans, there is now a body of recent information on the molecular genetic basis for their production, including regulatory genes in the case of curdlan. This makes possible, for the first time, directed genetic manipulation of the producing organism to affect the yield and perhaps even molecular mass of the linear polymer. Already, a cyclic-(1→3)-β-glucan lacking (1→6)-linkages has been generated by mutagenesis.
References
Adeyeye A, Jansson P-E, Lindberg B, Henrichsen J (1988) Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 37. Carbohydr Res 180:295–299
Alban S, Franz G (2001) Partial synthetic glucan sulphates as potential new antithrombotics: a review. Biomacromolecules 2:354–361
Altabe SG, Inon de Iannino N, Mendoza D de, Ugalde RA (1994) New osmoregulated β (1–3), β(1–6) glucosyltransferase(s) in Azospirillum brasilense. J Bacteriol 176:4890–4898
Altabe SG, Talaga P, Wieruszeski J-M, Lippens G, Ugalde R, Bohin J-P (1998) Periplasmic glucans of Azospirillum brasilense. In Elmerich C, Kondorosi A, Newton WE (eds) Biological nitrogen fixation for the 21st century. Kluwer, Dordrecht, p. 390
Anguillesi A (2003) Molecular biology of curdlan biosynthesis by Agrobacterium sp. ATTC 31749. MSc thesis, La Trobe University, Melbourne
Anonymous (1996a) 21 CFR 172. Food additives permitted for direct addition to food for human consumption: curdlan. Federal Register 61:65941
Anonymous (1996b) Bioproducts: bio-concrete. BioIndustry 13:56–57
Anonymous (2000) WHO food additives series. In: WHO (ed) 53rd Meeting of the joint FAO/WHO expert committee on food additives. JEFCA/WHO, Geneva
Arrecubieta C, López R, García E (1996) Type 3-specific synthase of Streptococcus pneumoniae (Cap3B) directs type 3 polysaccharide biosynthesis in Escherichia coli and in pneumococcal strains of different serotypes. J Exp Med 184:449–455
Arthur LO, Bulla LA, Grant SJ, Lawrence KN (1973) Carbohydrate metabolism in Agrobacterium tumefaciens. J Bacteriol 116:304–313
Bhagwat AA, Mithöfer A, Pfeffer PE, Kraus C, Spickers N, Hotchkiss A, Ebel J, Keister DL (1999) Further studies of the role of cyclic β-glucans in symbiosis. An ndvC mutant of Bradyrhizobium japonicum synthesizes cyclodecakis-(1→3)-β-glucosyl. Plant Physiol 119:1057–1064
Bohn JA, Bemiller JN (1995) (1→3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Polym 28:1–14
Buller CS (1990) Water insoluble polysaccharide polymer and method thereof. US patent 4,908,310
Chanzy H, Vuong R (1985) Ultrastructure and morphology of crystalline polysaccharides. In Atkins EDT (ed) Polysaccharides: topics in structure and morphology. Macmillan, London, pp 41–71
Chen R, Bhagwat AA, Yaklich R, Keister DL (2002) Characterisation of nvdD, the third gene involved in the synthesis of cyclic-β-(1–3),(1,6)-d-glucans in Bradyrhizobium japonicum. Can J Microbiol 48:1008–1016
Chuah CT, Sarko A, Deslandes Y, Marchessault RH (1983) Packing analysis of carbohydrates and polysaccharides. Part 14. Triple-helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 16:1375–1382
Cornish A, Greenwood JA, Jones CW (1988) Binding-protein-dependent glucose transport by Agrobacterium radiobacter grown in glucose-limited continuous culture. J Gen Microbiol 134:3099–3110
Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies G, Henrissat B, Svensson B (eds) Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge
Davis WB (1992) Unique bacterial polysaccharide polymer gel in cosmetics, pharmaceuticals and foods. US patent 5,158,772
Iannino NI de, Ugalde RA (1993) Biosynthesis of cyclic β-(1–3),β-(1–6) glucan in Bradyrhizobium spp. Arch Microbiol 159:30–38
DeAngelis PL (1999) Hyaluronan synthases: fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses. Cell Mol Life Sci 56:670–682
DeAngelis PL, Padgett-McCue AJ (2000) Identification and molecular cloning of a chondroitin synthase from Pasteurella multocida type F. J Biol Chem 275:24124–24129
DeAngelis PL, White CL (2004) Identification of a distinct, cryptic heparosan synthase from Pasteurella multocida types A, D, and F. J Bacteriol 186:8529–8532
Deslandes Y, Marchessault RH, Sarko A (1980) Triple-helical structure of (1→3)-β-d-glucan. Macromolecules 13:1466–1471
Dijkraaf GJP, Li H, Bussey H (2001) Cell wall glucans of Saccharomyces cerevisiae. In: De Baets S, Vandamme EJ, Steinbüchel A (eds) Polysaccharides II: polysaccharides from eukaryotes. (Biopolymers vol 5) Wiley, pp 179–213
Dombrecht B, Marchal K, Vanderleyden J, Michelis J (2002) Prediction and overview of the RpoN-regulon in closely related species of the Rhizobiales. Genome Biol 3:1–11
Estrella MJ, Pfeffer PE, Brouillette JN, Ugalde RA, Iannino NI de (2000) Biosynthesis and structure of cell associated glucans in slow growing Rhizobium loti strain NVP2309. Symbiosis 29:173–199
Evans NA, Hoyne PA, Stone BA (1984) Characteristics and specificity of the interaction of a fluorochrome from aniline blue (Sirofluor) with polysaccharides. Carbohydr Polym 4:215–230
Evans SG, Morrison D, Kaneko Y, Havlik I (1998) The effect of curdlan sulphate on development in vitro of Plasmodium falciparum. Trans R Soc Trop Med Hyg 92:87–89
Footrakul K, Suyanandana P, Amemura A, Harada T (1981) Extracellular polysaccharides of Rhizobium from Bangkok MIRCEN collection. J Ferment Technol 59:9–14
Fukui S (1969) Active transport of uridine diphosphate glucose in Agrobacterium tumefaciens. J Biochem 66:873–876
Fukui S, Miyairi S (1970) Active transport of glucose-1-phosphate in Agrobacterium tumefaciens. J Bacteriol 101:685–691
Fulton WS, Atkins EDT (1980) The gelling mechanism and relationship to molecular structure of microbial polysaccharide curdlan. In: French AD, Gardner KH, (eds) Fibre diffraction methods. American Chemical Society, Washington, D.C., pp 385–410
Futatsuyama H, Yui T, Ogawa K (1999) Viscometry of curdlan, a linear (1→3)-β-d-glucan, in DMSO or alkaline solutions. Biosci Biotechnol Biochem 63:1481–1483
Ghai SK, Hisamatsu A, Amemura A, Harada T (1981) Production and chemical composition of extracellular polysaccharides of Rhizobium. J Gen Microbiol 122:33–40
Gore RS, Miller KJ (1993) Cyclic β-1,6 -1,3 glucans are synthesised by Bradyrhizobium japonicum bacteroids within soybean (Glycine max) root nodules. Plant Physiol 102:191–194
Griffiths G, Cook NJ, Gottfridson E, Lind T, Lidholt K, Roberts IS (1998) Characterization of the glycosyltransferase enzyme from the Escherichia coli K5 capsule gene cluster and identification and characterization of the glucuronyl active site. J Biol Chem 273:11752–11757
Harada T (1992) The story of research into curdlan and the bacteria producing it. Trends Glycosci Glycotechnol 4:309–317
Harada T, Harada A (1996) Curdlan and succinoglycan. In Dumitriu S (ed) Polysaccharides in medical applications. Dekker, New York, pp 21–57
Harada T, Yoshimura T (1964) Production of a new acidic polysaccharide containing succinic acid by a soil bacterium. Biochim Biophys Acta 83:374–376
Harada T, Fujimori K, Hirose S, Masada M (1966) Growth and β-glucan 10C3K production by a mutant of Alcaligenes faecalis var. myxogenes in defined medium. Agric Biol Chem 30:764–769
Harada T, Koreeda A, Sato S, Kasai N (1979) Electron microscopic study on the ultrastructure of curdlan gel: assembly and dissociation of fibrils by heating. J Electron Microsc 28:147–153
Henrichsen J (1995) Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol 33:2759–2762
Hisamatsu M, Amemura A, Matsuo T, Matsuda H, Harada T (1982) Cyclic (1,2)-β-d-glucans and the octasaccharide repeating-unit of succinoglycan produced by Agrobacterium. J Gen Microbiol 128:1873–1879
Honda S, Sugino H, Asano T, Kakinuma A (1986) Activation of the alternative pathway of complement by an antitumour (1,3)-β-d-glucan from Alkaligenes faecalis var. myxogenes IFO13140 and its lower molecular weight and carboxymethylated derivatives. Immunopharmacology 11:29–37
Jagodzinski PP, Wiaderkiewicz R, Kurawski G, Kloczewiak M, Nakashima H, Hyjek E, Yamamoto N, Uryu T, Kaneko Y, Posner MR, Kozbor D (1994) Mechanism of the inhibitory effect of curdlan sulphate on HIV-1 infection in vitro. Virology 202:735–745
Janeway CA, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Jezequel V (1998) Curdlan: a new functional β-glucan. Cereal Foods World 43:361–364
Kai A, Ishino T, Arashida T, Hatanaka K, Akaike T, Matsuzaki K, Kaneko Y, Mimura T (1993) Biosynthesis of curdlan from culture media containing 13C-labeled glucose as the carbon source. Carbohydr Res 240:153–159
Kai A, Arashida T, Hatanaka K, Akaike T, Matsuzaki K, Mimura T, Kaneko Y (1994) Analysis of the biosynthetic process of cellulose and curdlan using 13C-labelled glucoses. Carbohydr Polym 23:235–239
Kako K, Koreeda A, Harada T (1989) Electron microscopic studies on curdlan formation by bacteria in solid culture medium. Bull Kobe Women’s Univ 22:183–189
Kanke M, Tanabe E, Katayama H, Koda Y, Yoshitomi H (1995) Application of curdlan to controlled drug delivery. III. Drug release from sustained release suppositories in vitro. Biol Pharm Bull 18:1154–1158
Karnezis T, McIntosh M, Wardak AZ, Stanisich VA, Stone BA (2000) The biosynthesis of β-glycans. Trends Glycosci Glycotechnol 12:211–227
Karnezis T, Fisher HC, Neumann GM, Stone BA, Stanisich VA (2002) Cloning and characterization of the phosphatidylserine synthase gene of Agrobacterium sp. strain ATTC31749 and effect of its inactivation on production of high molecular-mass (1→3)-β-d-glucan (curdlan). J Bacteriol 184:4114–4123
Karnezis T, Epa VC, Stone BA, Stanisich VA (2003) Topological characterization of an inner membrane (1→3)-β-d-glucan (curdlan) synthase from Agrobacterium sp. strain ATCC31749. Glycobiology 13:693–706
Kasai N, Harada T (1980) Ultrastructure of curdlan. In: French AD, Gardner KH (eds) Fiber diffraction methods. Am Chem Soc Symp 141:363–383
Kataoka K, Muta T, Yamazaki S, Takeshige K (2002) Activation of macrophages by linear (1→3)-β-d-glucans. J Biol Chem 277:36825–36851
Kemner JM, Liang X, Nester EW (1997) The Agrobacterium tumefaciens virulence gene chvE is part of a putative ABC-type sugar transport operon. J Bacteriol 179:2452–2458
Kenyon WJ, Buller CS (2002) Structural analysis of the curdlan-like exopolysaccharide produced by Cellulomonas flavigena KU. J Ind Microbiol Biotechnol 29:200–203
Kim M-K, Lee I-Y, Ko J-H, Rhee Y-H, Park Y-H (1999) Higher intracellular levels of uridinemonophosphate under nitrogen-limited conditions enhance metabolic flux of curdlan synthesis in Agrobacterium species. Biotechnol Bioeng 62:317–323
Kim M-K, Lee I-Y, Lee J-H, Kim K-T, Rhee Y-H, Park Y-H (2000) Residual phosphate concentration under nitrogen-limiting conditions regulates curdlan production in Agrobacterium species. J Ind Microbiol Biotechnol 25:180–183
Kim MK, Ryu KE, Choi WA, Rhee YH, Lee IY (2003) Enhanced production of (1→3)-β-d-glucan by a mutant strain of Agrobacterium species. Biochem Eng J 16:163–168
Knecht JC, Schiffman G, Austrian R (1970) Some biological properties of Pneumococcus type 37 and the chemistry of its capsular polysaccharide. J Exp Med 132:475–487
Komaniecka I, Choma A (2003) Isolation and characterisation of periplasmic cyclic β-glucans of Azorhizobium caulinodans. FEMS Microbiol Lett 227:263–269
Koreeda A, Harada T, Ogawa K, Sato S, Kasai N (1974) Study of the ultrastructure of gel-forming (1→3)-β-d-glucan (curdlan-type polysaccharide) by electron microscopy. Carbohydr Res 33:396–399
Koumoto K, Umeda M, Numata M, Matsumoto T, Sakurai K, Kunitake T, Shinkai S (2004) Low MW sulphated curdlan with improved water solubility forms macromolecular complexes with polycytidylic acid. Carbohydr Res 339:161–167
Lawford HG, Rousseau JD (1991) Bioreactor design considerations in the production of high quality microbial exopolysaccharides. Appl Biochem Biotechnol 28/29:667–684
Lawford HG, Rousseau JD (1992) Production of β-1,3-glucan exopolysaccharide in low shear systems: the requirement for high oxygen tension. Appl Biochem Biotechnol 34/35:597–612
Lawford H, Keenan J, Phillips K, Orts W (1986) Influence of bioreactor design on the rate and amount of curdlan-type exopolysaccharide product ion by Alcaligenes faecalis. Biotechnol Lett 8:145–150
Lee I-Y (2002) Curdlan. In: Vandamme EJ, De Baets S, Steinbüchel A (eds) Polysaccharides I: Polysaccharides from prokaryotes. (Biopolymers vol 5) Wiley, pp 135–158
Lee J-H, Lee IY (2001) Optimization of uracil addition for curdlan (β-1→3-glucan) production by Agrobacterium sp. Biotechnol Lett 23:1131–1134
Lee J-H, Park Y-H (2001) Optimal production of curdlan by Agrobacterium sp. with feedback inferential control of optimal pH profile. Biotechnol Lett 23:525–530
Lee I-Y, Seo WT, Kim GJ, Kim MK, Park CS, Park YH (1997a) Production of curdlan using sucrose or sugar cane molasses by two-step fed-batch cultivation of Agrobacterium species. J Indust Microbiol Biotechnol 18:255–259
Lee JW, Yeomans WG, Allen AL, Kaplan DL, Deng S, Gross RA (1997b) Exopolymers from curdlan production: incorporation of glucose-related sugars by Agrobacterium sp. strain ATTC 31749. Can J Microbiol 43:149–156
Lee IY, Kim MK, Lee JH, Seo WT, Jung JK, Lee HW, Park YH (1999a) Influence of agitation speed on production of curdlan by Agrobacterium species. Bioprocess Eng 20:283–287
Lee J-H, Lee I-Y, Kim M-K, Park Y-H (1999b ) Optimal pH control of batch processes for production of curdlan by Agrobacterium species. J Ind Microbiol Biotechnol 23:143–148
Leigh JA, Coplin DL (1992) Exopolysaccharides in plant–bacterial interactions. Annu Rev Microbiol 46:307–346
Li J, Burton RA, Harvey AJ, Hrmova M, Wardak AZ, Stone BA, Fincher GB (2003) Biochemical evidence linking a putative callose synthase gene with (1→3)-β-d-glucan biosynthesis in barley. Plant Mol Biol 53:213–225
Llull D, Muñoz R, López R, García E (1999) A single gene (tts) located outside the cap locus directs the formation of Streptococcus pneumoniae type 37 capsular polysaccharide: type 37 pneumococci are natural, genetically binary strains. J Exp Med 190:241–252
Llull D, García E, López R (2001) Tts, a processive β-glucosyltransferase of Streptococcus pneumoniae, directs the synthesis of branched Type 37 capsular polysaccharide in pneumococcus and other Gram-positive species. J Biol Chem 276:21053–21061
Marchessault RH, Deslandes Y (1979) Fine structure of (1→3)-β-d-glucans: curdlan and paramylon. Carbohydr Res 75:231–242
Matthysse AG, Thomas DL, White AR (1995) Mechanism of cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 177:1076–1081
McIntosh M (2004) An investigation of the production of curdlan, a (1→3)-β-glucan, by an Agrobacterium sp. PhD thesis, La Trobe University, Melbourne
Merrick MJ, Edwards RA (1995) Nitrogen control in bacteria. Microbiol Rev 59:604–622
Miller KJ, Gore RS, (1992) Cyclic β-(1,6)(1,3)-glucan of Bradyrhizobium japonicum: functional analogs of the cyclic β-(1,2)-glucan of Rhizobium? Curr Microbiol 24:101–104
Miller KJ, Gore RS, Johnson R, Benesi AJ, Reinhold VN (1990) Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol 172:136–142
Mithöfer A, Bhagwat AA, Feger M, Ebel J (1996) Suppresion of fungal β-glucan induced plant defence in soybean (Glycine max L.) by cyclic 1,3-1-6-β-glucans from the symbiont Bradyrhizobium japonicum. Planta 199:270–275
Mithöfer A, Bhagwat AA, Keister DL, Ebel J (2001) Bradyrhizobium japonicum mutants defective in cyclic β-glucan synthesis show enhanced sensitivity to plant defense responses. Z Naturforsch C 56:581–584
Mueller A, Raptis J, Rice PJ, Kalbfleisch JH, Stout RD, Ensley HE, Browder W, Williams DL (2000) The influence of glucan polymer structure and solution conformation on binding to (1→3)-β-d-glucan receptors in a human monocyte-like cell line. Glycobiology 10:339–346
Nakanishi I, Kimura K, Kusui S, Yamazaki E (1974) Complex formation of gel-forming bacterial (1–3)-β-d-glucans (curdlan-type polysaccharides) with dyes in aqueous solution. Carbohydr Res 32:47–52
Nakanishi I, Kimura K, Suzuki T, Ishikawa M, Banno I, Sakane T, Harada T (1976) Demonstration of curdlan-type polysaccharide and some other β-1,3-glucan in microorganisms with aniline blue. J Gen Appl Microbiol 22:1–11
Nakanishi I, Kimura K, Kanamaru T (1992) Studies on curdlan-type polysaccharide. I. Industrial production of curdlan-type polysaccharide. J Takeda Res Lab 51:99–108
Ninomiya T, Sugiura N, Tawada A, Sugimoto K, Watanabe H, Kimata K (2002) Molecular cloning and characterization of chondroitin polymerase from Escherichia coli strain K4. J Biol Chem 277:21567–21575
Nishinari K, Zhang H (2000) Curdlan. In: Phillips GO, Williams PA (eds) Handbook of hydrocolloids. CRC, Boca Raton, pp 269–286
Okuyama K, Otsubo A, Fukuzawa Y, Ozawa M, Harada T, Kasai N (1991) Single-helical structure of native curdlan and its aggregation state. J Carbohydr Chem 10:645–656
Orts WJ, Rouseau JD, Lawford HG (1987) Improved microbial production of curdlan-type polysaccharide. In: Stivala SS, Crescenzi V, Dea ICM (eds) Industrial polysaccharide: the impact of biotechnology and advanced methodologies. Gordon and Breach, New York, pp 459–469
Phillips KR, Lawford HG (1983) Curdlan: its properties and production in batch and continuous fermentations. In: Bushell DE (ed) Progress in industrial microbiology, vol 18. Elsevier, Amsterdam, pp 201–229
Phillips KR, Pik J, Lawford HG, Lavers B, Kligerman A, Lawford GR (1983) Production of curdlan-type polysaccharide by Alcaligenes faecalis in batch and continuous culture. Can J Microbiol 29:1331–1338
Postma PW, Lengeler JW, Jacobson GR (1993) Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594
Rolin DB, Pfeffer PE, Osman SF, Szwergold BS, Kappler F, Benesi AJ (1992) Structural studies of a phosphocholine substituted β-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim Biophys Acta 1116:215–225
Römling U (2002) Molecular biology of cellulose production in bacteria. Res Microbiol 153:205–212
Ross GD, Vetvicka V, Yan J, Xia,Y, Vetviková J (1999) Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacology 42:61–74
Saito H, Misaki A, Harada T (1968) A comparison of the structure of curdlan and pachyman. Agric Biol Chem 32:1261–1269
Saito H, Ohki T, Sasaki T (1977) A 13C nuclear magnetic resonance study of gel-forming (1→3)-β-d-glucans. Evidence of the presence of single-helical conformation in a resilient gel of a curdlan-type polysaccharide 13140 from Alcaligenes faecalis var. myxogenes IFO 13140. Biochemistry 16:908–914
Saito H, Yoshioka Y, Uehara N, Aketagawa J, Tanaka S, Shibata Y (1991) Relationship between conformation and biological response for (1,3)-β-glucans in the activation of coagulation Factor G from limulus amebocyte lysate and host-mediated antitumor activity. Demonstration of single-helix conformation as a stimulant. Carbohydr Res 217:181–190
Sasaki T, Abiko N, Sugino Y, Nitta K (1978) Dependence on chain length of antitumour activity of (1,3)-β-d-glucan from Alcaligenes faecalis var. myxogenes IFO13140 and its acid-degraded products. Cancer Res 379–383
Seljelid R (1986) A water-soluble aminated beta 1–3d-glucan derivative causes regression of solid tumors in mice. Biosci Rep 6:845–851
Spicer EJF, Goldenthal EI, Ikeda T (1999) A toxicological assessment of curdlan. Food Chem Toxicol 37:455–479
Stasinopoulos SJ, Fischer PR, Stone BA, Stanisich VA (1999) Detection of two loci involved in (1→3)-β-glucan (curdlan) biosynthesis by Agrobacterium sp. ATCC31749, and comparative sequence analysis of the putative curdlan synthase gene. Glycobiology 9:31–41
Stone BA, Clarke AE (1992) Chemistry and biology of (1→3)-β-glucans. La Trobe University, Melbourne
Stowers MD (1985) Carbon metabolism in Rhizobium species. Annu Rev Microbiol 39:89–108
Sutherland IW (2001a) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Sutherland IW (2001b) Microbial polysaccharides from Gram-negative bacteria. Int Dairy Res11:663–674
Toida T, Chaidedgumjorn A, Linhardt RJ (2003) Structure and bioactivity of sulphated polysaccharides. Trends Glycosci Glycotechnol 15:29–46
Ussery DW (2004) Genome update: 161 prokaryote genomes sequenced, and counting. Microbiology 150:261–263
Uttaro AD, Ielpi L, Ugalde RA (1993) Galactose metabolism in Rhizobiaceae: characterization of Agrobacterium tumefaciens exoB mutants. J Gen Microbiol 139:1055–1062
Voepel KC, Buller CS (1990) Formation of an extracellular energy reserve by Cellulomonas flavigena strain KU. J Ind Microbiol 5:131–138
Williamson G, Damani K, Devenney P, Faulds CB, Morris VJ, Stevens BJ (1992) Mechanism of action of cyclic β-1,2-glucan synthetase from Agrobacterium tumefaciens: competition between cyclization and elongation reactions. J Bacteriol 174:7941–7947
Yotsuzuka F (2001) Curdlan. In: Cho SS, Dreher ML (eds) Handbook of dietary fiber. Dekker, New York, pp 737–757
Young S-H, Robinson VA, Barger M, Frazer DG, Castranova V (2003) Partially opened triple helix is the biologically active conformation of 1,3-β-glucans that induces pulmonary inflammation in rats. J Toxicol Environ Health A 66:551–563
Zhang HB, Nishinari K, Williams MAK, Foster TJ, Norton IT (2002) A molecular description of the gelation mechanism of curdlan. Int J Biol Macromol 30:7–16
Acknowledgements
We thank Ann Matthysse for critically reading the manuscript. Work in the laboratories of B.A.S. and V.A.S. was supported, in part, by Australian Research Council Grants (AO 9925079, LX 0211339). M.M. was the recipient of an Australian Postgraduate Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McIntosh, M., Stone, B.A. & Stanisich, V.A. Curdlan and other bacterial (1→3)-β-d-glucans. Appl Microbiol Biotechnol 68, 163–173 (2005). https://doi.org/10.1007/s00253-005-1959-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1959-5