Abstract
The ability of biosurfactant obtained from the probiotic bacterium Lactococcus lactis 53 to inhibit adhesion of four bacterial and two yeast strains isolated from explanted voice prostheses to silicone rubber with and without an adsorbed biosurfactant layer was investigated in a parallel-plate flow chamber. The microbial cell surfaces and the silicone rubber with and without an adsorbed biosurfactant layer were characterized using contact-angle measurements. Water contact angles indicated that the silicone-rubber surface with adsorbed biosurfactant was more hydrophilic (48°) than bare silicone rubber (109°). The results showed that the biosurfactant was effective in decreasing the initial deposition rates of Staphylococcus epidermidis GB 9/6 from 2,100 to 220 microorganisms cm−2 s−1, Streptococcus salivarius GB 24/9 from 1,560 to 137 microorganisms cm−2 s−1, and Staphylococcus aureus GB 2/1 from 1,255 to 135 microorganisms cm−2 s−1, allowing for a 90% reduction of the deposition rates. The deposition rates of Rothia dentocariosa GBJ 52/2B, Candida albicans GBJ 13/4A, and Candida tropicalis GB 9/9 were far less reduced in the presence of the biosurfactant as compared with the other strains. This study constitutes a step ahead in developing strategies to prevent microbial colonization of silicone-rubber voice prostheses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicone rubber is used for a wide variety of biomedical applications due to its good mechanical properties, combined with a hydrophobic surface. For instance, silicone rubber has been used for voice prostheses (Neu et al. 1993), urinary catheters (Farber and Wolff 1993), and contact lens materials (Holly and Owen 1983). Patients who have undergone a laryngectomy due to a malignant laryngeal tumor need to breath through a tracheostoma and receive voice prostheses for speech rehabilitation (Van der Mei et al. 1999). The major drawback of silicone-rubber voice prostheses is the concurrent microbial colonization of the device (Neu et al. 1993) which causes leakage of food and liquid or blocking of the valve thereby increasing the airflow resistance (Mahieu et al. 1986), thus forcing replacement of the prosthesis on average every 3–4 months. It has been suggested by laryngectomized patients that the consumption of dairy products influences the lifetime of their prostheses (Van der Mei et al. 1999). There is anecdotal evidence among patients that the consumption of buttermilk, which contains antimycotic-releasing Lactococcus lactis spp., prolongs the lifetime of indwelling voice prostheses. This suggestion has been confirmed in an artificial throat model, in which the effects of daily buttermilk consumption on biofilm formation on silicone-rubber voice prostheses were simulated (Busscher et al. 1998).
The formation of an infectious biofilm on biomaterials appears to involve several sequential steps, including adsorption of host conditioning film onto the device and adhesion of the infectious microorganisms, anchoring by exopolymer production, growth, and spread of the organisms in large clumps separated by water channels (Bos et al. 1999; Busscher et al. 1996; Reid 1999). When microorganisms and biomaterials surfaces are in an aqueous environment in which organic matter is present (e.g., sea water, milk, saliva, urine, or blood), macromolecular components adsorb onto biomaterial surfaces to form a conditioning film and may change the electrical properties and the hydrophobicity of the surface. Therefore, weakening or breaking this link, for example, under the influence of fluctuating shear forces or by using surfactants (Velraeds et al. 1996), would force the entire biofilm to detach, thereby aiding and abetting the erradication of infection.
Biosurfactants are surface-active compounds released by microorganisms that have some influence on interfaces, most notably on the surface tension of liquid–vapor interfaces. With regard to an anti-adhesive effect of biosurfactants, hypotheses have been forwarded in which adsorption of biosurfactants to a substratum surface alters the hydrophobicity of the surface and causes interference in microbial adhesion and desorption processes (Desai and Banat 1997).
The aim of the present study was to compare the extent of adhesion of a large variety of different bacterial and yeast strains isolated from explanted voice prostheses to silicone rubber with and without an adsorbed biosurfactant layer. The tested biosurfactant was obtained from the probiotic bacterium L. lactis 53.
Materials and methods
Biosurfactant production
The lactic acid strain L. lactis 53 was cultured in 600 ml optimized MRS broth and grown overnight (18 h) in ambient air. The growth medium used for the production of this biosurfactant was previously optimized to obtain higher yields of biosurfactant production (Rodrigues et al. 2003). Cells were harvested by centrifugation (10,000 g, 5 min, 10°C), washed twice in demineralized water, and resuspended in 100 ml phosphate-buffered saline (PBS: 10-mM KH2PO4/K2HPO4 and 150-mM NaCl with pH adjusted to 7.0). The bacteria were left at room temperature for 2 h with gentle stirring for biosurfactant release. Subsequently, the bacteria were removed by centrifugation and the remaining supernatant liquid was filtered through a 0.22-μm pore-size filter (Millipore). The supernatant was dialyzed against demineralized water at 4°C in a Spectrapor membrane tube (molecular weight cut-off 6,000–8,000, Spectrum Medical Industries, Calif., USA) and freeze-dried, and the biosurfactant was used for further studies.
Microbial strains and growth conditions
Four bacterial strains, Staphylococcus epidermidis GB 9/6, Streptococcus salivarius GB 24/9, Staphylococcus aureus GB 2/1, and Rothia dentocariosa GBJ 52/2B, and two yeast strains, Candida albicans GBJ 13/4A and Candida tropicalis GB 9/9, isolated from explanted voice prostheses were used in this study. All strains were first grown overnight at 37°C in ambient air on an agar plate from a frozen stock; this agar plate was kept at 4°C, never longer than 2 weeks. Several colonies were used to inoculate 10 ml brain heart infusion broth (BHI, OXOID, Basingstoke, UK). This pre-culture was incubated at 37°C in ambient air for 24 h and used to inoculate a second culture of 200 ml that was grown for 18 h. The microorganisms from the second culture were harvested by centrifugation for 5 min at 10,000 g and washed twice with demineralized water. Subsequently, bacterial cells were suspended in 200 ml PBS, after sonication on ice (10 s), to a concentration of 3×108 ml−1. The sonication procedure did not promote cell lysis. Yeasts were suspended in PBS to a concentration of 3×106 ml−1. Cells were counted in a Bürker-Türk counting chamber.
Contact-angle measurements and surface free energy calculation
Advancing-type contact angles with three different standard liquids (Millipore water, formamide, and diiodomethane) on silicone rubber with and without an adsorbed biosurfactant layer were measured with a homemade contour monitor using the sessile-drop technique. On each sample, at least five droplets were placed at different positions and the results of three separately prepared surfaces with adsorbed biosurfactant were averaged. For the microorganisms, the contact angles were measured on lawns of yeasts and bacteria. Briefly, microorganisms were layered onto 0.45-μm (bacteria) or 3-μm (yeasts) pore-sized filters (Millipore) using negative pressure. The yeasts were layered from a suspension in PBS buffer and then washed with demineralized water, while the bacteria were suspended in water. The filters were left to air-dry in ambient air until so-called plateau contact angles could be measured (Van Oss and Gillman 1972). At least three different filters from separate cultures were used for each strain tested.
The acid–base nature of surfaces can be directly determined in terms of the surface free-energy components γsvLW (LW, Lifshitz–van der Waals) and γsvAB (AB, acid–base) (Van Oss 1989; Van Oss et al. 1988; Volpe and Siboni 1997),
in which the AB component equals
where γsv− and γsv+ are the electron-donating and electron-accepting surface free-energy parameters. Note that s stands for solid, v for vapor, and l for liquid. Proper diagnostic liquids with known surface free-energy components (γlvLW, γlvAB, γlv+, and γlv−) should be selected and the most commonly applied are diiodomethane, water, and formamide. Diiodomethane is apolar (\(\gamma ^{{{\text{AB}}}}_{{{\text{lv}}}} = 0\)) and consequently its contact angle on a surface can be employed to calculate the γsvLW component of the surface free-energy
Both water and formamide are polar liquids and combined use of their contact angles on a surface yields γsv− and γsv+ from the Young equation
Parallel-plate flow chamber and image analysis
A parallel-plate flow chamber (Sjollema et al. 1989) was used to study deposition of the bacterial and yeast strains on silicone rubber with and without an adsorbed biosurfactant layer. The parallel-plate flow chamber consists of a polymethylmethacrylate bottom plate with implant-grade silicone rubber affixed to it with double-sided adhesive tape, and a glass top plate, both with dimensions 5.5×3.8 cm. The top and bottom plates were cleaned by sonicating for 3 min in a commercially available surfactant cleaning solution (2% RBS35 in water, Societé des Traitements Chimiques de Surface, Lambersat, France), rinsed thoroughly with tap water, and then rinsed with demineralized water. Top and bottom plates were subsequently mounted in the housing of the flow chamber, separated by 0.06-cm Delrin spacers. Images were taken from the silicone rubber with or without an adsorbed biosurfactant layer on the bottom plate. Deposition was observed with a CCD-MXRi camera (High Technology, Eindhoven, The Netherlands) mounted on a phase-contrast microscope (Olympus BH-2) equipped with a 40 × ultra-long working distance objective (Olympus ULWD-CD Plan 40 PL) for experiments with bacteria and with a 10 × objective for experiments with yeasts. The camera was coupled to an image analyzer (TEA, Difa, Breda, The Netherlands). Each live image (512×512 pixels with 8-bit resolution) was obtained after summation of 15 consecutive images (time interval 1 s) in order to enhance the signal to noise ratio and to eliminate moving microorganisms from the analysis.
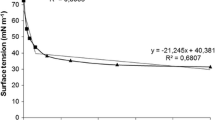
Silicone rubber was immersed overnight in a 5 g biosurfactant l−1 solution at 4°C for the experiments done with conditioned silicone rubber. The concentration was estimated by axisymmetric drop-shape analysis by profile (ADSA-P) as it allowed for a reasonable reduction in the surface tension (12 mJ m−2), which means that at this concentration the biosurfactant has good surface activity . The results are presented in Fig. 1 (Van der Vegt et al. 1991).
Prior to each experiment, all tubes and the flow chamber were filled with PBS, while care was taken to remove air bubbles from the system. Flasks, containing microbial suspension and buffer, were positioned at the same height with respect to the chamber to ensure that, immediately after the flows were started, all fluids would circulate by hydrostatic pressure through the chamber at the desired shear rate of 10 s−1 (0.025 ml s−1), which yields a laminar flow (Reynolds number 0.6). The microbial suspension was circulated through the system for 4 h and images were obtained from silicone rubber with or without the adsorbed biosurfactant layer. The initial increase in the number of adhering microorganisms with time was expressed as a so-called initial deposition rate j0 (microorganisms cm−2 s−1), i.e., the number of adhering microorganisms per unit area and time. The number of adhering microorganisms after 4 h was also determined (n4 h). All values presented in this work are the averages of experiments on three separate silicone-rubber surfaces with or without the adsorbed biosurfactant layer, and were carried out with separately grown microorganisms.
Results
Microbial cell surface and silicone-rubber characterization
Table 1 summarizes the contact angles measured with water, formamide, and diiodomethane on silicone rubber with and without adsorbed biosurfactant, as well as the contact angles measured on the microorganisms. The water contact angle on bare silicone rubber decreased from 109° to 48° after adsorption of the biosurfactant. A similar behavior was obtained with formamide. The diiodomethane contact angle decreased to a much lesser extent, from 86° to 64°.
The water contact angles obtained for all the microorganisms tested were between 24° and 35°, except that for R. dentocariosa GBJ 52/2B, which was found to be 54°, thus being the most hydrophobic bacterial strain studied. The formamide contact angle of all microorganisms was higher than their water contact angle, except for R. dentocariosa GBJ 52/2B. Diiodomethane contact angles were similar for all microorganisms, between 26° and 33°, except that for R. dentocariosa GBJ 52/2B, which was 40°.
The surface free-energies calculated from the contact angles are also given in Table 1 and show that silicone rubber with the adsorbed biosurfactant layer is strongly electron-donating compared with bare silicone rubber. The Lifshitz–van der Waals component is higher for the conditioned silicone rubber than for bare silicone rubber.
The Lifshitz–van der Waals component of the surface free-energies determined for all the microorganisms were similar and were found to be between 40 mJ m−2 and 46 mJ m−2, the lowest value being that for R. dentocariosa GBJ 52/2B and the highest that for C. albicans GBJ 13/4A. Major differences were observed in the electron-donating component, with R. dentocariosa GBJ 52/2B having the lowest electron-donating component. The electron-accepting component of all the microorganisms was zero, except that of R. dentocariosa GBJ 52/2B, being 1.0 mJ m−2.
Microbial adhesion
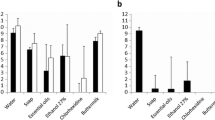
The initial deposition rates (Fig. 2) of S. epidermidis GB 9/6, R. dentocariosa GBJ 52/2B, and C. tropicalis GB 9/9 on silicone rubber were relatively high, between 2,000 and 2,600 microorganisms cm−2 s−1, while S. salivarius GB 24/9 and S. aureus GB 2/1 deposited more slowly onto silicone rubber (1,200–1,600 microorganisms cm−2 s−1). C. albicans GBJ 13/4A had by far the lowest initial deposition rate (170 microorganisms cm−2 s−1). Adsorption of biosurfactant on the silicone rubber reduced the deposition rate about 90% for S. epidermidis GB 9/6, S. salivarius GB 24/9, and S. aureus GB 2/1. The deposition rates of R. dentocariosa GBJ 52/2B, C. albicans GBJ 13/4A, and C. tropicalis GB 9/9 were far less reduced in the presence of the biosurfactant compared to the deposition rates of the other strains.
The initial deposition rates (j0) of the bacterial (Staphylococcus epidermidis GB 9/6, Streptococcus salivarius GB 24/9, Staphylococcus aureus GB 2/1, and Rothia dentocariosa GBJ 52/2B) and yeast (Candida albicans GBJ 13/4A and Candida tropicalis GB 9/9) strains isolated from explanted voice prostheses on silicone rubber with and without an adsorbed biosurfactant layer. Experiments were carried out in triplicate and correspond within 10–15%
The number of microorganisms adhering to silicone rubber after 4 h (n4 h, Fig. 3) was between 15×106 cm−2 and 7×106 cm−2 for all microorganisms studied, except C. albicans GBJ 13/4A which exhibited the lowest number of adhering microorganisms (0.6×106 cm−2). The number of cells adhering after 4 h on the silicone rubber conditioned with biosurfactant was reduced to 90% for S. epidermidis GB 9/6, S. salivarius GB 24/9, and S. aureus GB 2/1. The yeast strains C. albicans GBJ 13/4A and C. tropicalis GB 9/9 as well as the bacterial strain R. dentocariosa GBJ 52/2B showed a smaller decrease in the number of attached cells after 4 h, between 56% and 78%.
The number of microorganisms adhering after 4 h (n4 h) on silicone rubber with and without an adsorbed biosurfactant layer. The codification of the microorganisms is presented in Fig. 2. Experiments were carried out in triplicate and correspond within 10–15%
Discussion
In this study, biosurfactant obtained from the probiotic bacteria L. lactis 53 was investigated for its ability to inhibit the adhesion of several microbial strains involved in the biofilm formation on voice prostheses. Continuous exposure to saliva, food, drinks, and the oropharyngeal microflora contributes to rapid colonization by a mixed biofilm of bacteria and yeasts, leading to valve failure and the need for frequent exchange of the implant (Neu et al. 1993). In this context, anti-fouling improvement of the silicone-rubber material is desirable. In laryngectomized patients with voice prostheses lasting less than 2 months, there is need for employing “antibiofilm” therapy from the time of insertion of the voice prostheses, preferably without using antimycotics or antibiotics because of the risk of inducing resistant strains (Foley and Gilbert 1996; Mahieu et al. 1986; Van Weissenbruch et al. 1997). Therefore, research into the development of new methods for preventing or retarding biofilm formation on voice prostheses is worthwhile. The use of biosurfactants as antimicrobial agents seems to be a promising method of prolonging the lifetimes of voice prostheses.
We have demonstrated that bacterial adhesion in a parallel-plate flow chamber to silicone rubber conditioned with a biosurfactant produced by L. lactis 53 is greatly decreased compared to adhesion to bare silicone rubber. Similarly, adhesion of yeasts was also decreased by the presence of the biosurfactant, but not to the same extent as observed for bacteria. The mechanisms by which a biosurfactant discourages microbial adhesion have not been fully elucidated, but the main physiological role of biosurfactants is to facilitate the uptake of water-immiscible substrates by lowering the surface tension at the phase boundary (Fiechter 1992; Hommel and Ratledge 1993). Involvement of biosurfactants in microbial adhesion, as demonstrated in this study, has been described previously. Dairy Streptococcus thermophilus strains, for example, produce biosurfactants that cause their own desorption (Busscher et al. 1994), the reduction of yeast adhesion to silicone rubber in a parallel-plate flow chamber system (Busscher et al. 1997), and interference with the formation of a mixed fungal/bacterial biofilm on silicone-rubber voice prostheses in the modified Robbins device (Busscher et al. 1999). In addition, oral Streptococcus mitis strains secrete biosurfactants inhibiting the adhesion of Streptococcus mutans (Pratt-Terpstra et al. 1989).
In the DLVO approach (theory advanced independently by Derjaguin and Landau and by Vervey and Overbeek), microbial adhesion is described as a balance between attractive Lifshitz-van der Waals forces and repulsive or attractive electrostatic forces. An extended DLVO analysis considers the interfacial free-energy ΔGmws between the microorganisms (m) and the silicone rubber with or without an adsorbed biosurfactant layer (s) in aqueous medium (w). According to Van Oss (1989), ΔGmws can be separated into an electrodynamic (LW) and an acid–base (AB) component, i.e.,
while
and
All calculations of interaction energies were made using \(\gamma ^{{{\text{LW}}}}_{{\text{w}}} = 21.8\), \(\gamma ^{ - }_{{\text{w}}} = \gamma ^{ + }_{{\text{w}}} = 25.5\) mJ m−2 for water and the surface free-energy components determined in Table 1 for silicone rubber and microorganisms. If the adhesion is thermodynamically favorable, the values of the free-energy of adhesion will be negative. From the results, summarized in Table 2, it can be observed that thermodynamically:
-
1.
All microorganisms have a favorable free-energy of adhesion (ΔG<0) to bare silicone rubber, the Lifshitz–van der Waals interactions being the most significant.
-
2.
All microorganisms have a unfavorable free-energy of adhesion (ΔG>0) to silicone rubber conditioned with biosurfactant, except for R. dentocariosa GBJ 52/2B but even in this case the ΔG is less negative than that determined for bare silicone rubber.
-
3.
Lifshitz–van der Waals interactions are always favorable and acid–base interactions are always unfavorable (ΔG>0) for silicone rubber with adsorbed biosurfactant.
According to the thermodynamic approach, in experiments done with silicone rubber conditioned with biosurfactant ,there is an unfavorable free energy of adhesion (ΔG>0) for all microorganisms, except R. dentocariosa GBJ 52/2B which had a negative value, thus explaining the less effective reduction in the extent of adhesion observed for this microorganism.
Preventing or retarding biofilm formation implies that it is necessary to interfere in the weakest link, which means in the formation of the conditioning film and adhesion of the first microorganisms (Busscher et al. 1995). The use of biosurfactants adsorbed to silicone rubber is believed to interfere with the formation of this conditioning film, assupported by the observation that the initial deposition rates of all the microorganisms involved in this study decreased. By consequence, biofilm formation can be influenced by adjusting the properties of the voice prostheses’ material or by surface modification.
All of the studied bacteria are hydrophilic except for R. dentocariosa GBJ 52/2B, which exhibits a more hydrophobic character. Normally, an organism will tend to adsorb irreversibly to minimize the free-energy of the system. However, charge repulsion or steric exclusion may prevent a close approach, and even when this has been achieved the ordered water structure associated very closely with the surface could still act as a barrier. Hydrophilic surfaces may be stabilized by ordering of surface-associated water molecules, which would be disrupted by the close approach of a bacterial cell. This releases the ordered water molecules into the bulk phase, increasing their free-energy. Hydrophobic surfaces should attract strongly because water molecules moving away from the surface into the bulk will decrease their free-energy, as their level of hydrogen bonding will increase. Alternatively, the approach of a hydrophilic cell to a hydrophobic surface would yield a stronger interaction (Chamberlain 1992). This could explain why the microorganisms studied adhered strongly to the bare hydrophobic silicone rubber but hardly to the hydrophilic silicone rubber with the adsorbed biosurfactant layer.
The most encompassing theory to explain the adhesion of microorganisms to solid surfaces is the concept of interfacial free-energy as the controlling force in cell adhesion. According to this theory, cell adhesion is favored when the interfacial tension between the solid, the microorganism, and the suspending liquid is reduced. For most microorganisms, this translates into greater microbial adhesion to solid surfaces in direct proportion to the hydrophobicity of the surface, provided that the suspending medium is water or a simple buffer. Surface-active agents reduce hydrophobic interactions and by doing so reduce microbial adhesion to silicone rubber (Klotz 1990).
In conclusion, this study may be a step in developing strategies to prevent microbial colonization of silicone-rubber voice prostheses, i.e. changing the surface properties of silicone rubber by adsorbing the biosurfactant obtained from L. lactis 53. Therewith, the lifetime of the voice prostheses would be extended.
References
Bos R, Van der Mei HC, Busscher HJ (1999) Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods to study. FEMS Microbiol Rev 23:179–230
Busscher HJ, Neu TR, Van der Mei HC (1994) Biosurfactant production by thermophilic dairy streptococci. Appl Microbiol Biotechnol 41:4–7
Busscher HJ, Bos R, Van der Mei HC (1995) Initial microbial adhesion is a determinant for the strength of biofilm adhesion. FEMS Microbiol Lett 128:229–234
Busscher HJ, Geertsema-Doornbusch GI, Everaert EP, Verkerke GJ, Van de Belt-Gritter B, Kalicharan R, Van der Mei HC (1996) Biofilm formation and silicone rubber surface modification in the development of a total artificial larynx. In: Algaba J (ed) Surgery and prosthetic voice restoration after total and subtotal laryngectomy. Elsevier, Amsterdam, pp 47–52
Busscher HJ, Van Hoogmoed CG, Geertsema-Doornbusch GI, Van der Kuijl-Booij M, Van der Mei HC (1997) Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl Environ Microbiol 63:3810–3817
Busscher HJ, Bruinsma G, Van Weissenbruch R, Leunisse C, Van der Mei HC, Dijk F, Albers FW (1998) The effect of buttermilk consumption on biofilm formation on silicone rubber voice prostheses in an artificial throat. Eur Arch Otorhinolaryngol 255:410–413
Busscher HJ, Van de Belt-Gritter B, Westerhof M, Van Weissenbruch R, Albers FW, Van der Mei HC (1999) Microbial interference in the colonization of silicone rubber implant surfaces in the oropharynx: Streptococcus thermophilus against a mixed fungal/bacterial biofilm. In: Rosenberg E (ed) Microbial ecology and infectious disease. American Society for Microbiology, Washington, pp 66–74
Chamberlain AH (1992) The role of adsorbed layers in bacterial adhesion. In: Melo LF, Bott TR, Fletcher M, Capdeville B (eds) Biofilms—science and technology. NATO ASI Series, Series E: applied sciences, vol 223. Kluwer, The Netherlands, pp 59–67
Desai J, Banat I (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Farber BF, Wolff AG (1993) Salicylic acid prevents the adherence of bacteria and yeast to silastic catheters. J Biomed Mater Res 27:599–602
Fiechter A (1992) Biosurfactants: moving towards industrial application. Tibtech 10:208–217
Foley I, Gilbert P (1996) Antibiotic resistance of biofilms. Biofouling 10:334–346
Holly FJ, Owen MJ (1983) In: Mittal KL (ed) Physicochemical aspects of polymer surfaces. Plenum, New York
Hommel RK, Ratledge C (1993) Biosynthetic mechanisms of low molecular weight surfactants and their precursor molecules. In: Kosaric N (ed) Biosurfactants: production, properties and applications. Dekker, New York, pp 3–63
Klotz SA (1990) Role of hydrophobic interactions in microbial adhesion to plastics used in medical devices. In: Doyle RJ, Rosenberg M (eds) Microbial cell surface hydrophobicity. American Society of Microbiology, Washington, pp 107–136
Mahieu HF, Van Saene JJ, Den Besten J, Van Saene HK (1986) Oropharynx decontamination preventing Candida vegetation on voice prostheses. Arch Otolaryngol Head Neck Surg 112:1090–1092
Neu TR, Van der Mei HC, Busscher HJ, Dijk F, Verkerke GJ (1993) Biodeterioration of medical-grade silicone rubber used for voice prostheses: a SEM study. Biomaterials 14:459–464
Pratt-Terpstra IH, Weerkamp AH, Busscher HJ (1989) Microbial factors in a thermodynamic approach of oral streptococcal adhesion to solid substrata. J Colloid Interface Sci 129:568–574
Reid G (1999) Biofilms in infectious disease and on medical devices. Int J Antimicrob Agents 11:223–226
Rodrigues L, Teixeira J, Oliveira R (2003) Response surface optimization of the medium components for the production of biosurfactant by lactic acid bacteria. In: Abstracts of 11th European congress on biotechnology, 24–29 August, Basel, Switzerland, p 76
Sjollema J, Busscher HJ, Weerkamp A (1989) Real-time enumeration of adhering microorganisms in a parallel plate flow cell using automated image analysis. J Microbiol Methods 9:73–78
Van der Mei HC, Van de Belt-Gritter B, Van Weissenbruch R, Dijk F, Albers FW, Busscher HJ (1999) Effect of consumption of dairy products with probiotic bacteria on biofilm formation on silicone rubber implant surfaces in an artificial throat. Trans IChemE 77:156–158
Van der Vegt W, Van der Mei HC, Noordmans J, Busscher HJ (1991) Assessment of bacterial biosurfactant production through axisymmetric drop shape analysis by profile. Appl Microbiol Biotechnol 35:766–770
Van Oss CJ (1989) Hydrophobicity and hydrophilicity of biosurfaces. Curr Opin Colloid Interface Sci 2:503–510
Van Oss CJ, Gillman CF (1972) Phagocytosis as a surface phenomenon. I. Contact angles and phagocytosis of non-opsonized bacteria. J Reticulo Soc 12:283–292
Van Oss CJ, Good R, Chaudhury M (1988) Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 4:884–891
Van Weissenbruch R, Bouckaert S, Remon J, Nelis HJ, Aerts R, Albers FW (1997) Chemoptophylaxis of fungal deterioration of the Provox prostheses. Ann Otol Rhinol Laryngol 106:329–337
Velraeds MC, Van der Mei HC, Reid G, Busscher HJ (1996) Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol 62:1958–1963
Volpe C, Siboni S (1997) Some reflections on acid–base solid surface free energy theories. J Colloid Interface Sci 195:121–136
Acknowledgments
The FCT provided financial support for L. Rodrigues through a doctoral research grant SFRH/BD/4700/2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, L., van der Mei, H., Teixeira, J.A. et al. Biosurfactant from Lactococcus lactis 53 inhibits microbial adhesion on silicone rubber. Appl Microbiol Biotechnol 66, 306–311 (2004). https://doi.org/10.1007/s00253-004-1674-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1674-7